Abstract
Introduction:
The aim of this study was to investigate the microhardness and microstructural features of newer tricalcium silicate materials: TheraCal LC, mineral trioxide aggregate (MTA), biodentine (BD), and Endosequence Root Repair Material (ERRM) putty, after exposure to acidic environments in comparison with distilled water.
Materials and Methods:
A total of 80 extracted single-rooted premolars were collected. All the selected specimens were sectioned vertically, and cavities were prepared on the root surface. Specimens were divided into four groups of 20 each, i.e., Group 1: (n = 15) MTA (ProRoot, Dentsply Tulsa Dental, Tulsa, OK, USA), Group 2: (n = 15) BD (Septodont, France), Group 3: (n = 15) ERRM putty (Brasseler, USA), and Group 4: (n = 15) TheraCal LC (Bisco Inc Schaumburg). Materials were placed into prepared cavities. About 10 specimens per each group were exposed to butyric acid buffered at a pH level of 5.5 for 7 days at 37c, and 10 specimens from each group were exposed to distilled water serving as a control group. The surface microhardness was measured after exposure to either acid or distilled water. Scanning electron microscope was used to observe the internal microstructure morphology. Two-way analysis of variance was applied to evaluate the Knoop microhardness value (KHN).
Results:
Results showed that the microhardness values of the materials were significantly higher in the neutral environment of butyric acid at pH 7.4 when compared to those in the acidic condition of pH 5.4 for all groups (P < 0.001). TheraCal LC had higher microhardness values than BD, MTA, ERRM putty at 5.5 pH levels (P < 0.001).
Conclusion:
The microhardness values of TheraCal LC, BD, ERRM Putty, and MTA were reduced in an acidic environment, which resulted in these materials having more porous and less crystalline microstructures. TheraCal LC seems the most suitable material for application to an area of inflammation where a low pH value may exist.
Keywords: Biodentine, butyric acid, endosequence root repair putty, microhardness, microstructure, mineral trioxide aggregate, TheraCal LC
INTRODUCTION
The outcomes of endodontic procedures are influenced by the chemical and physical properties of the materials used. An ideal root repair material should be biocompatible, nonresorbable, radiopaque, dimensionally stable, and insoluble in tissue fluids and should have sufficient sealing property. Over the years, various root repair materials, such as amalgam, Super-EBA, Intermediate Restorative Material, glass ionomer cement, and calcium phosphate cement, were used.[1]
Mineral trioxide aggregate (MTA), a calcium silicate-based hydraulic cement, has shown promising results over the past years.[2] Despite the various beneficial properties of MTA such as biocompatibility, sealing ability, and antibacterial effects, it has a few disadvantages such as difficulty in handling and long setting time.[3]
To overcome the disadvantages of MTA, a calcium silicate-based material, biodentine (BD) (Septodont, Saint Maur Des FOSSES, France) was introduced.[4] BD consists of calcium carbonate, zirconium oxide, tricalcium silicate, and a water-based liquid-containing calcium chloride which is used as setting accelerator and water-reducing agent. BD with increased physical properties and reduced setting time claimed to be used as a dentin restorative material as well as root repair material.
It has been reported that the hardness, pushout bond strength to dentin, and sealing ability of MTA and BD were decreased after placing in an acidic environment.[5,6]
Hence, to overcome the above drawbacks, a new tricalcium silicate material Endosequence Root Repair material putty (ERRM; Brasseler, Savannah, GA) has been introduced.[7] According to the manufacturer, it is composed of calcium silicates, monobasic calcium phosphate, zirconium oxide, tantalum oxide, proprietary fillers, and thickening agents.[8] The material is biocompatible, hydrophilic, insoluble, radiopaque, and aluminum free. It is having high pH and working time of nearly 30 min. ERRM is available in a moldable putty form to facilitate placement in clinical situations with physical properties comparable to MTA and BD.
TheraCal LC (Bisco Inc, Schaumburg, IL, USA) is a new light-cured resin-modified calcium silicate-filled material designed for direct and indirect pulp capping. It consists of approximately 45% wt mineral material (type III Portland cement), 10% wt radiopaque component, 5% wt hydrophilic thickening agent (fumed silica), and approximately 45% resin. It also shows physiochemical bonding to dentin, good sealing abilities, and it is well tolerated by immortalized odontoblast cells.[9]
An acidic pH value, mostly as a result of bacterial-induced local metabolic acidosis or tissue inflammation, might probably affect the physical and chemical properties of calcium silicate-based material.[10] As of now, no studies have been conducted regarding the effect of acidic pH comparing TheraCal LC, endosequence putty root repair with MTA and BD.
Hence, the aim of the present study is to evaluate the microhardness and microstructural changes of ERRM putty, TheraCal LC, MTA, and BD after exposure to the acidic environment.
MATERIALS AND METHODS
A total of 80 extracted human mandibular premolar teeth were selected. Selection criteria included the presence of a single root canal, mature apex, the absence of root filling, and without fractures or caries. After extraction, the teeth were kept in 5% sodium hypochlorite for 30 min to eliminate residual soft tissues. The collected samples were then vertically sectioned buccolingually using low-speed diamond disc under water coolant. On the cut section of each specimen, a size of 2 mm × 2 mm dimension cavity marked at the junction of a cervical and middle third of the root surface to standardize the size of perforation. The cavities were then prepared using high-speed super-torque handpiece from the inner root surface of the vertically sectioned specimen to outer surface. The significance of vertical sectioning of the tooth structure is to simulate the clinical situation of root perforation.
All the collected samples (n = 80) were randomly divided into four groups such that 20 specimens per each group (n = 20).
Group I (n = 20): One gram of Proroot MTA (Dentsply Tulsa Dental, Tulsa, OK, USA) was mixed with 0.35 ml sterile distilled water and placed into the prepared cavities manually using similar sized hand condenser
Group II (n = 20): BD (Septodont, Saint Maur des Fosses, France) which was available in capsule and liquid form was triturated using amalgamator and packed into prepared cavities of the samples
Group III (n = 20): Endosequece root repair putty (ERRM (Brasseler, Savannah, GA) which was available in premixed paste form, placed directly into prepared cavities using sterile cement carrier under manual pressure
Group IV (n = 20): TheraCal LC (BiscoInc, Schaumburg, IL, USA) which was dispensed into the prepared cavities from the syringe and light cured with a light-emitting diode light-curing unit for 20 s per increment. All the samples were allowed to set at 37c in 100% relative humidity for 7 days.
Each group of 20 specimens was divided into two subgroups according to experimental liquids: (a) exposure to distilled water and (b) exposure to buffered butyric acid (pH = 5.5). Each subgroup was submerged in a petri dish, with its experimental solution, i.e., distilled water and butyric acid (pH = 5.5). Soaked gauze was placed on the bottom surface of samples. The top surface of the samples was covered with moist gauze and placed in an incubator at 37c in 100% relative humidity for about 7 days. The solution-soaked gauze pieces were replenished daily to ensure a constant pH.
Microhardness measurement
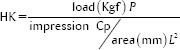
After 7 days, all the specimens were removed from the different solutions. They were washed and gently dried with air spray. The Knoop microhardness test was performed using microhardness tester (FM-100, Futuretech, Kawasaki, Kanagawa Prefecture, Japan) with the diamond-tipped tool. A full load of 100 g was applied for 10 s at room temperature. For microhardness, two indentations were made per specimen using Knoop diamond indenter. Knoop hardness (HK) was calculated according to an equation where Cpis the correction factor related to the shape of the indenter (0.070279), P is the test load (kgf), and L is the length of the longer diagonal (μm).
The data were analyzed using two-way analysis of variance (ANOVA) followed by the post hoc test. The significance level was set at P < 0.05.
Surface analysis with scanning electron microscope
Each specimen from all subgroups was selected for microstructural surface morphology analyses, under scanning electron microscope (SEM). Magnification of ×10,000 was used to evaluate the different topographic microstructure of different set cements.
The rationale of the present study is to evaluate the microhardness and microstructural changes of ERRM putty, TheraCal LC, MTA, and BD after exposure to the acidic environment.
RESULTS
The mean surface hardness ± standard deviation of MTA, BD, Endosequence Root Repair Material putty (ERRM), and TheraCal LC were summarized in Table 1. The data were statistically analyzed using two-way ANOVA. For multiple comparisons, post hoc Tukey test was performed. Results showed that all the specimens exposed to butyric acid (pH = 5.5) had significantly lower microhardness values than those exposed to distilled water (P < 0.05). TheraCal LC and Endosequence putty showed significantly higher microhardness than MTA and BD exposed to the acid solution [Figure 1].
Table 1.
Mean±standard deviations of surface microhardness (Knoop microhardness) of different root repair materials after 7-day setting in distilled water and butyric acid at pH 5.5
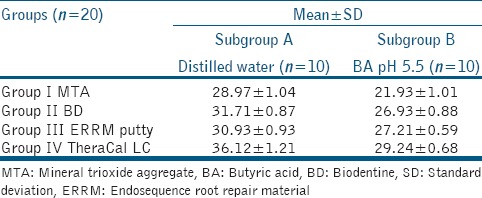
Figure 1.
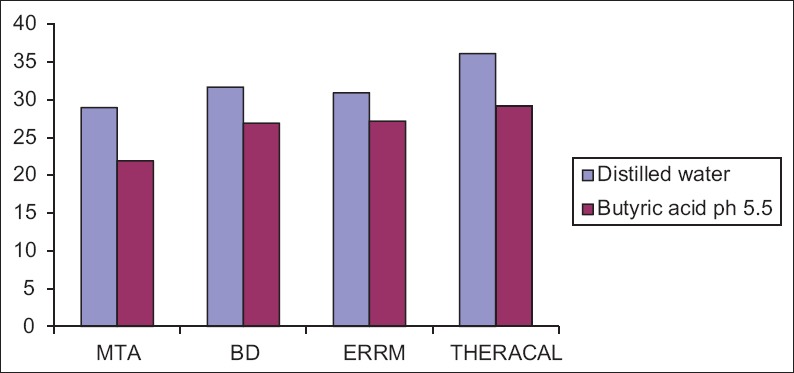
Bar diagram showing mean surface microhardness (KHN) of different root repair and butyric acid of pH 5.4
Scanning electron microscope analysis
The internal microstructure of the four materials exposed to butyric acid or distilled water at various pH levels was observed by SEM. The specimens exposed to pH 5.5 displayed more pores than those stored in distilled water for MTA and ERRM Putty, whereas the BD and TheraCal LC showed less microchannels than MTA and ERRM putty under same magnification [Figure 2].
Figure 2.
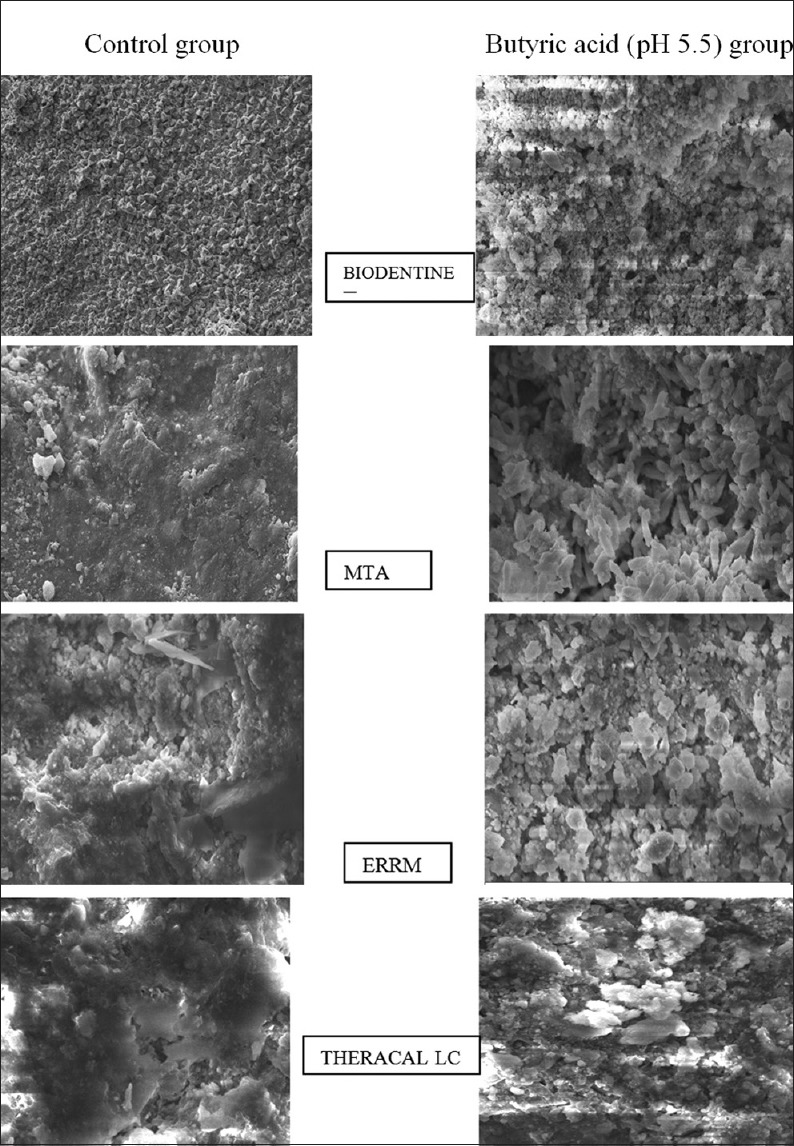
Scanning electron microscope images showing structural topography of all the 4 root repair materials exposed to butyric acid at pH 5.5 (left) and control group (right)
DISCUSSION
Despite of advances in endodontic therapy, root perforation complicates the treatment and deprives the prognosis if not properly managed. Irrespective of location or etiology, root perforation, which is a communication between root canal system and external tooth surface has to be sealed with a material having good physical properties and biocompatibility. In the present study, the effect of acidic environment on the microhardness and microstructural changes of tricalcium silicate materials such as MTA, BD, Endosequence Putty, and TheraCal LC was evaluated.
Microhardness test is not only a measure of strength or resistance to deformation but also it is influenced by the crystal structural stability and has an inverse relationship with porosity. In the present study, HK test was used to evaluate the microhardness as it is insensitive to bulk properties of the cement.[11]
In the current study, the properties of MTA, BD, Endosequence putty, and TheraCal LC were evaluated after exposure to butyric acid at a pH level of 5.5. This study attempted to simulate the actual clinical environment during inflammation using butyric acid at pH 5.5, a byproduct of anaerobic bacteria metabolism.[12]
When considering the clinical situation, the acidotic metabolism of butyric acid may suppress the bone mineralization activity of alkaline phosphatase. Lee et al.[13] stated that pulpal and periapical inflammation typically lowers the tissue pH near the involved tooth to around 5.5.
The present study showed that exposure of the cements to an acidic environment significantly lowers the microhardness value than the control group. These results are consistent with the studies conducted by Bolhari et al.[14,15] and Wang et al.[10] A low pH could potentially inhibit the setting reaction, affect adhesion, or increase the solubility of calcium silicate-based materials, which could affect the mechanical properties of the material including the surface microhardness. TheraCal LC is an example of newly developed, ready-to-use tricalcium silicate materials, and due to its adherence property to a moist substrate, it can be used as root repair material. In the present study, it has shown significantly higher microhardness than MTA, BD, and Endosequence putty in acidic environment.
A substantial change in the microstructure of BD, Endosequence, TheraCal LC, and MTA occurred after exposure to butyric acid at pH of 5.5 compared to distilled water. A needle-shaped structure and cubic crystals-shaped structures of BD were observed. On the other hand, the microstructures of WMTA appeared more eroded with laminated cross-stratified structures and pore formation after exposure to acidic pH. These results were in accordance with the studies conducted by Elnaghy et al.[4] and Namazikhah et al.,[12] who stated that WMTA might be more sensitive to acidic pH environment. Namazikhah et al. reported that the higher porosity of MTA revealed that the environment associated with bacterial colonization that could facilitate the passage of microorganisms or their metabolic products into the periapical tissues.
SEM finding of ERRM putty shows fewer ettringite crystals and high level of porosity due to the low pH environment that inhibited crystallization in hydration reaction of this material which was in agreement with results of Wang et al.[10,15] ERRM materials contain calcium phosphate and tantalum oxide (a radiopacifier) but lacked aluminum. The absence of aluminate phase may result in fewer formed ettringite crystals which interlock cubic crystals. Another possible explanation could be the setting time accelerator in ERRM interfere with the hydration reaction of the cements especially at low pH values when the crystalline structures of the hydrated cement appeared less cohesive.[16]
TheraCal LC is a resin-modified material composed of tricalcium silicate and zirconium oxide. In contrast to other materials, it is light-cured and the initial strength of material is gained immediately because of which it showed more resistance to acidic environment by forming more stable crystals and less microporosities and microhardness value. Camilleri et al.[17] stated that there is no intermediate calcium hydroxide was formed as a by-product of hydration as it does not include water for material hydration. In the present study, TheraCal LC is one of the best used under inflammatory conditions, as it is a light curable material the site of perforation repair and depth of cure has to be considered. Therefore, further in vivo and in vitro research should be done to assess TheraCal LC as root repair material.
CONCLUSION
Within the limitations of this in vitro study, microhardness and surface microstructure of MTA and Endosequence root repair putty were highly altered in acidic environment than TheraCal LC and BD. Microhardness of TheraCal LC and BD were statistically higher than MTA and Endosequence putty in acidic solution. Neutral and alkaline solutions may enhance biologic properties of the biomaterials while acidic solution negatively influenced them.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Asgary S, Shahabi S, Jafarzadeh T, Amini S, Kheirieh S. The properties of a new endodontic material. J Endod. 2008;34:990–3. doi: 10.1016/j.joen.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Saghiri MA, Lotfi M, Saghiri AM, Vosoughhosseini S, Fatemi A, Shiezadeh V, et al. Effect of pH on sealing ability of white mineral trioxide aggregate as a root-end filling material. J Endod. 2008;34:1226–9. doi: 10.1016/j.joen.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005;31:97–100. doi: 10.1097/01.don.0000133155.04468.41. [DOI] [PubMed] [Google Scholar]
- 4.Elnaghy AM. Influence of acidic environment on properties of biodentine and white mineral trioxide aggregate: A comparative study. J Endod. 2014;40:953–7. doi: 10.1016/j.joen.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Shokouhinejad N, Nekoofar MH, Iravani A, Kharrazifard MJ, Dummer PM. Effect of acidic environment on the push-out bond strength of mineral trioxide aggregate. J Endod. 2010;36:871–4. doi: 10.1016/j.joen.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 6.Han L, Okiji T. Bioactivity evaluation of three calcium silicate-based endodontic materials. Int Endod J. 2013;46:808–14. doi: 10.1111/iej.12062. [DOI] [PubMed] [Google Scholar]
- 7.Charland T, Hartwell GR, Hirschberg C, Patel R. An evaluation of setting time of mineral trioxide aggregate and EndoSequence root repair material in the presence of human blood and minimal essential media. J Endod. 2013;39:1071–2. doi: 10.1016/j.joen.2013.04.041. [DOI] [PubMed] [Google Scholar]
- 8.Moinzadeh AT, Aznar Portoles C, Schembri Wismayer P, Camilleri J. Bioactivity potential of EndoSequence BC RRM putty. J Endod. 2016;42:615–21. doi: 10.1016/j.joen.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Gandolfi MG, Siboni F, Prati C. Chemical-physical properties of TheraCal, a novel light-curable MTA-like material for pulp capping. Int Endod J. 2012;45:571–9. doi: 10.1111/j.1365-2591.2012.02013.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Ma J, Shen Y, Haapasalo M. Acidic pH weakens the microhardness and microstructure of three tricalcium silicate materials. Int Endod J. 2015;48:323–32. doi: 10.1111/iej.12318. [DOI] [PubMed] [Google Scholar]
- 11.Nekoofar MH, Oloomi K, Sheykhrezae MS, Tabor R, Stone DF, Dummer PM, et al. An evaluation of the effect of blood and human serum on the surface microhardness and surface microstructure of mineral trioxide aggregate. Int Endod J. 2010;43:849–58. doi: 10.1111/j.1365-2591.2010.01750.x. [DOI] [PubMed] [Google Scholar]
- 12.Namazikhah MS, Nekoofar MH, Sheykhrezae MS, Salariyeh S, Hayes SJ, Bryant ST, et al. The effect of pH on surface hardness and microstructure of mineral trioxide aggregate. Int Endod J. 2008;41:108–16. doi: 10.1111/j.1365-2591.2007.01325.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee YL, Lee BS, Lin FH, Yun Lin A, Lan WH, Lin CP, et al. Effects of physiological environments on the hydration behavior of mineral trioxide aggregate. Biomaterials. 2004;25:787–93. doi: 10.1016/s0142-9612(03)00591-x. [DOI] [PubMed] [Google Scholar]
- 14.Bolhari B, Nekoofar MH, Sharifian M, Ghabrai S, Meraji N, Dummer PM, et al. Acid and microhardness of mineral trioxide aggregate and mineral trioxide aggregate-like materials. J Endod. 2014;40:432–5. doi: 10.1016/j.joen.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Poplai G, Jadhav SK, Hegde V. Effect of acidic environment on the surface microhardness of biodentin. World J Dent. 2013;4:100–2. [Google Scholar]
- 16.Giuliani V, Nieri M, Pace R, Pagavino G. Effects of pH on surface hardness and microstructure of mineral trioxide aggregate and aureoseal: An in vitro study. J Endod. 2010;36:1883–6. doi: 10.1016/j.joen.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Camilleri J, Laurent P, About I. Hydration of biodentine, theracal LC, and a prototype tricalcium silicate-based dentin replacement material after pulp capping in entire tooth cultures. J Endod. 2014;40:1846–54. doi: 10.1016/j.joen.2014.06.018. [DOI] [PubMed] [Google Scholar]


