Abstract
Context:
Remineralizing agents have been recommended to restore the integrity of bleached enamel.
Aims:
The aim of this study was to evaluate the effect of fluoride at high concentration (5000 ppm) applied to bleached enamel.
Materials and Methods:
A total of 30 specimens obtained from newly extracted third molars were divided into two groups (n = 15) as follows: control group and fluoride group. Specimens of both groups received bleaching treatment with 35% hydrogen peroxide, were then submitted to the Vickers hardness number/colorimetric test (VHN/CT) (n = 5) and Ra (n = 10) tests, and stored in artificial saliva. After bleaching, fluoride group received the application of a fluoride-based dentifrice, during 5 min. VHN, RS, and CT analysis of both groups were performed before and after treatments. For VHN, five indentations per specimen were performed, using a microdurometer. Ra analysis was performed with a rugosimeter. The color was analyzed through the CIE L* a* b* system, respectively, using a colorimeter.
Statistical Analysis:
For intergroup statistical analysis, ANOVA with Tukey's posttest was used. All tests were calculated at a significance level of 5%.
Results:
There was statistically significant difference (P < 0.01) between the analyzed groups, on VHN, Ra, and CT evaluations.
Conclusions:
The use of fluoride-based dentifrice at 5000 ppm was effective in minimizing the deleterious effects on bleached enamel.
Keywords: Colorimetry, dentistry, hardness sodium fluoride, tooth bleaching
INTRODUCTION
Several modalities for treating discolored teeth are currently available, such as tooth bleaching, laminates, varnishes, microabrasion, and porcelain-coated crowns.[1] Tooth bleaching remains to be the simplest, most common, less invasive, and low-cost technique.[2] However, previous studies have reported that bleaching peroxides can alter the calcium and phosphate content of enamel.[3,4] Therefore, postbleaching treatment using remineralizing agents has been recommended for restoration of the structural integrity of bleached enamel.[5]
Superficial defects are caused by bleaching treatment, and this generates clinical consequences for patients. For instance, an increased enamel roughness is considered a predisposing factor for bacterial adhesion and pigment absorption.[6]
The repair of microscopic defects reduces hydrogen peroxide diffusion into the pulp chamber, which should theoretically minimize tooth sensitivity.[7] An ideal remineralizing system should provide calcium, phosphate, and fluoride ions that should be capable of affecting the subsurface in addition to forming deposits on the superficial layer of enamel.[8] Previous studies have used products based on different bioactive agents. For instance, a study conducted in 2016 used MI Paste Plus (GC America, Alsip, Illinois, USA), which is based on casein phosphopeptide-amorphous calcium phosphate fluoride,[9] while a 2014 study evaluated the effectiveness of Nano-P (FGM, Joinville, SC, Brazil), which is based on nanohydroxyapatite particles.[10] Furthermore, a study conducted in 2011 tested Mirawhite®tc and Nanosensitive®hca (Hager and Wercken, Dusseldorf, Nordrhein-Westfalen, Germany), which are based on bioactive glass material such as NovaMin®.[11] Among all available methods, fluoride-based therapy is one of the most wellknown.
Fluoride ions favor remineralization by increasing the resistance to acid etching and forming a calcium fluoride (CaF2) layer,[5] which is subsequently incorporated into the enamel as hydroxyfluorapatite or fluorapatite, thus lowering the susceptibility to dissolution.[12] Depending on the features of the remineralizing agent, such as the pH, fluoride concentration, and type of fluoride salt used,[13] the formed CaF2deposit inhibits demineralization.[5] It has already been revealed that agents with a high concentration of fluoride form a thicker CaF2layer, which may provide better protection against erosive processes.[14]
ClinPro™ 5000, a remineralizing dentifrice composed of tricalcium phosphate (TCP) and 1.1% sodium fluoride (5000 ppm; 3M ESPE, Sumaré, SP, Brazil), has been marketed for repairing damage caused to the dental structure. According to the manufacturer, this product may increase dental protection against demineralization through a TCP technology, which allows calcium and phosphate ions to separately coexist with fluoride, making them completely available for the formation of a more resistant mineral layer on the dental surface.
This in vitro study used the Vickers microhardness test, superficial roughness (Ra) test, and colorimetric test (CT) to evaluate the effects of ClinPro™ 5000, a high-concentration fluoride dentifrice (5000 ppm), on human dental enamel subjected to in-office bleaching. Two null hypotheses were tested. The first one states that the topical application of ClinPro™ 5000 does not prevent changes in the Vickers hardness number (VHN) and Ra of enamel. The second one states that the topical application of ClinPro™ 5000 does not influence the effectiveness of bleaching treatment.
MATERIALS AND METHODS
This in vitro study was conducted after gaining approval from the Institutional Ethics Committee (approval number, 1.61.622.292) and followed the principles of the Helsinki Declaration for biomedical research. Informed consent was obtained from all tooth donors.
All teeth used in this experimental study were donated by patients after they provided written informed consent. Teeth with cracks, stains, or any other enamel defects were excluded from the study. In total, 30 third molars were used for this study. All selected teeth were transversely sectioned across the cementoenamel junction, and the roots were discarded. One specimen per tooth crown was prepared by cutting the labial surface with a double-sided steel disc (KG Sorensen, Cotia, SP, Brazil), followed by smoothening with diamond tips (n° 4138; KG Sorensen, Cotia, SP, Brazil). The cut portion always corresponded to the central area of the labial surface; thus, enamel prisms with the same inclinations were obtained. The surfaces were polished using 600-grit, 1200-grit, and 2000-grit wet sandpaper in succession under running water, followed by enamel polishing using the polisher APL-4 (AROTEC, Cotia, SP, Brazil), which had a felt disc, and aluminum paste (Union Carbide, Greensburg, LA, EUA). The specimens were stored in distilled water until the beginning of the experiment.
For the experiment, all specimens were divided into two groups (n = 15 each) as follows: A control group, where only bleaching with 35% hydrogen peroxide (White and Brite Advanced; 3M/ESPE, Sumaré, SP, Brazil) was performed, and a fluoride group, where bleaching with 35% hydrogen peroxide was followed by the topical application of Clinpro™ 5000 (3M ESPE, Sumaré, SP, Brazil) for 5 min. All analyses were performed before and after bleaching treatment.
The bleaching agent was applied according to the manufacturer's recommendations in three 45 min sessions at an interval of 7 days. In each session, the agent was applied three times for 15 min. After each bleaching session, the samples were washed using running water. The surfaces were polished with a felt disc and diamond excel polishing paste (FGM, Joinville, SC, Brazil) coupled with a low-speed handpiece (Dabi Atlante, Ribeirão Preto, SP, Brazil). The specimens were stored in artificial saliva (500 ml; potassium chloride, sodium chloride, magnesium chloride, potassium phosphate, calcium chloride, preservatives, CMC, and sorbitol; Aqueous Solution QSP; Apis Mell Pharmacy, Belém, PA, Brazil), which was changed daily and kept in a biological stove at 37°C (Quimis, Diadema, São Paulo, Brazil).
For VHN measurements, five specimens in each group were tested. On each specimen, five indentations separated by 100 μm were made using a load of 25 gf for 5 s in a microdurometer (Shimadzu, Barueri, São Paulo, Brazil). Ocular lens with a ×100 and ×400 increase objective was used. Penetrations were created to hypothetically map the total area of the specimens (lateral left, right, top, bottom, and central extremities). The percentage change in VHN (VHN%) was calculated using the following formula:
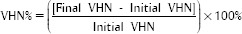
For the Ra test, 10 specimens from each group were analyzed using a rugosimeter (Mitutoyo, Suzano, SP, Brazil). The tip of the rugosimeter touched the specimen and explored 4 mm diagonally to derive three diametrically opposed measurements. The superficial Ra value was obtained using the machine's own program, which was previously established and calibrated. The program compensated for changes in the surface anatomy of the specimen without affecting the Ra value. The mean Ra value was calculated as the arithmetic average of the sum of the absolute values of the roughness deviation profile from the central line along the evaluated path. As a complementary analysis, the percentage change in Ra (%RaS) was calculated for each group using the following formula:
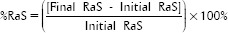
CT was performed using a tristimulus colorimeter (Konica Minolta/Tecnal Indústria, Piracicaba, SP, Brazil) based on the CIE L* a* b* system. To evaluate the color change between the initial and final results, ΔE was calculated using the following formula:
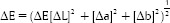
All data were analyzed using BioEstat 5.0 software (Mamirauá Civil Society, Tefé, AM, Brazil). Two-factor ANOVA was performed, considering it is the test for assessing the effects of independent variables (control or fluoride) on each dependent variable (VHN, Ra, and ΔE) at two-time points. In case of significant differences, Tukey's post hoc test was adopted to identify differences between two groups or two-time points. A value of P < 0.05 was considered statistically significant.
RESULTS
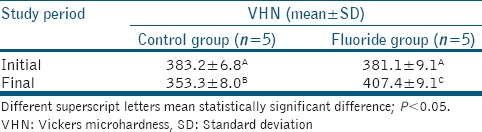
Table 1 shows the mean VHNs at baseline and after the intervention. In the intergroup assessment, the initial means did not present statistically significant differences (P = 0.22). However, the final mean VHNs differed statistically significant (P < 0.001). After the intervention, the control group exhibited a significant decrease in the mean VHN, whereas the fluoride group exhibited a significant increase in the mean VHN after ClinPro™ 5000 application.
Table 1.
Mean and standard deviation of Vickers microhardness values of the groups treated at the different evaluation times. ANOVA with Tukey’s posttest
The mean Ra values at baseline and after intervention are shown in Table 2. In the intergroup evaluation, the initial mean Ra values showed no significant differences between groups (P = 0.39), whereas the final mean Ra value showed a significant difference (P < 0.001), being lower in the fluoride group than in the control group. After the intervention, both groups presented significant differences relative to the baseline values, with an increase in the control group and a decrease in the fluoride group.
Table 2.
Mean and standard deviation of superficial roughness values of the treated groups at the different evaluation times. ANOVA with Tukey’s posttest

Finally, the mean ΔE was higher in the fluoride group (12.42 ± 1.3) than in the control group (9.96 ± 2.1; P < 0.01).
DISCUSSION
Tooth bleaching has been reported to be the most conservative method for treating discolored teeth when compared to more invasive restorative modalities such as laminates or porcelain-coated crowns.[1] However, several studies have associated the use of whitening chemical agents with alterations in the mineral content of enamel.[3,4,5] In this sense, the present investigation evaluated the effects of a high-concentration fluoride dentifrice (5000 ppm), ClinPro™ 5000, on human dental enamel subjected to in-office bleaching. It was observed that VHN significantly decreased and Ra significantly increased after the application of 35% hydrogen peroxide in the control group. This finding confirmed that the bleaching agent caused certain deleterious effects to the enamel surface and changed the biomechanical properties of the dental tissues, as previously mentioned in the literature.[15] Coceska et al.[15] evaluated these changes using three-dimensional scanning electron microscopy and reported the loss of interprismatic substance and sodium (Na) and magnesium (Mg) ions in addition to morphological changes, including increased porosity, depressions, and erosions.
Previous studies have shown that substantial hardness recovery or mineral deposition on damaged dental tissues may be primarily caused by supplemental ions in the storage medium (artificial or human saliva).[3,16] However, in the present study, the use of artificial saliva only was not able to prevent or suppress the demineralization process, as revealed by the decrease in VHN and increase in Ra after bleaching in the control group. This finding suggests the need for additional therapies such as the use of bioactive remineralizing agents, for example, fluoride, which was the bioactive agent used in the present study.
Several topical fluoride-based products are currently available for dental practice.[17] The different fluoride formulae and concentrations in these products represent options for the treatment of several problems in dental tissues, such as caries, dentine sensitivity, and erosion.[18] It is the clinician's responsibility to select the most efficient formula and concentration for resolving the problem. Attin et al.[5] tested the application of highly concentrated solutions of fluoride on bleached teeth and observed that VHNs were restored to those observed for unbleached teeth in approximately 99% of specimens.
In the present study, the postintervention VHN was significantly higher in the fluoride group than in the control group; this revealed the efficacy of 5000 ppm fluoride for remineralization and rejected the first null hypothesis of this investigation regarding VHN. The solubility of CaF2crystals decreases with an increase in their size.[19] This increase in the size of the crystals seems to be dependent on the concentration of the fluoride agent, among other factors.[20] Therefore, it is suggested that smaller crystals of CaF2dissolve during the demineralization process, while the bigger ones present better resistance.[21] In the present study, the postintervention Ra was significantly lower in the fluoride group than in the control group; this evidenced the restorative potential of fluoride at the concentration of 5000 ppm. Therefore, the first null hypothesis of this investigation was also rejected regarding Ra. The presence of fluoride may minimize enamel changes through CaF2deposition, mitigating demineralization effects, and preventing changes that may be harmful to the bleached enamel structure.[22]
Statistical analysis showed that ΔE was significantly greater in the fluoride group than in the control group. Thus, the second null hypothesis of this study was rejected. Moreover, this finding provided evidence that fluoride not only improved the superficial morphology of the enamel but also contributed to the optimization and maintenance of the bleaching effect. The role of tissue repair in the efficacy of the bleaching treatment is also highlighted, considering that some superficial features of the bleached enamel, such as increased roughness, porosity, and depression, may contribute to the retention of pigments.[23] Fluoride contributes to the repair of these microstructural defects through absorption and precipitation of calcium and phosphate, which are present in saliva. Yamagishi et al. used a fluoride-hydroxyapatite acidic solution and revealed that a new hydroxyapatite layer is formed in the presence of this compound.[24] This action of fluoride on the microstructure of enamel damaged by bleaching decreased the damage and consequently, may have reduced the retention of pigments, which contributed to the increased effectiveness of bleaching. However, no scientific evidence corroborating this result has been found.
Tooth sensitivity is the primary clinical consequence of bleaching treatment and is associated with microscopic defects on the enamel surface.[5] It has been theorized that these defects allow rapid diffusion of the bleaching agent into the pulp, resulting in an inflammatory process.[17] This clarifies the clinical importance of repair of these deleterious effects on the tooth enamel. Consequently, many manufacturers have launched products based on remineralizing agents such as fluoride, which has been proven effective for the treatment of sensitivity in many studies.[19,20]
Further clinical investigations are necessary to validate the efficacy of 5000 ppm fluoride. However, on the basis of the methodology used in this study, it is safe to infer that the use of a dentifrice containing fluoride at 5000 ppm is effective in minimizing the adverse structural effects associated with bleaching treatment and improving its efficacy.
CONCLUSIONS
The findings of this study suggest that the use of a high-concentration fluoride dentifrice (5000 ppm) such as ClinPro™ 5000 after bleaching with 35% hydrogen peroxide promotes an increase in the microhardness and a decrease in the superficial roughness of enamel without compromising the effectiveness of bleaching treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bollineni S, Janga RK, Venugopal L, Reddy IR, Babu PR, Kumar SS, et al. Role of fluoridated carbamide peroxide whitening gel in the remineralization of demineralized enamel: An in vitro study. J Int Soc Prev Community Dent. 2014;4:117–21. doi: 10.4103/2231-0762.137638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bizhang M, Seemann R, Duve G, Römhild G, Altenburger JM, Jahn KR, et al. Demineralization effects of 2 bleaching procedures on enamel surfaces with and without post-treatment fluoride application. Oper Dent. 2006;31:705–9. doi: 10.2341/05-144. [DOI] [PubMed] [Google Scholar]
- 3.Smidt A, Feuerstein O, Topel M. Mechanical, morphologic, and chemical effects of carbamide peroxide bleaching agents on human enamel in situ. Quintessence Int. 2011;42:407–12. [PubMed] [Google Scholar]
- 4.Joiner A. Review of the effects of peroxide on enamel and dentine properties. J Dent. 2007;35:889–96. doi: 10.1016/j.jdent.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Attin T, Kielbassa AM, Schwanenberg M, Hellwig E. Effect of fluoride treatment on remineralization of bleached enamel. J Oral Rehabil. 1997;24:282–6. doi: 10.1046/j.1365-2842.1997.d01-291.x. [DOI] [PubMed] [Google Scholar]
- 6.Mei L, Busscher HJ, van der Mei HC, Ren Y. Influence of surface roughness on streptococcal adhesion forces to composite resins. Dent Mater. 2011;27:770–8. doi: 10.1016/j.dental.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Loguercio AD, Tay LY, Herrera DR, Bauer J, Reis A. Effectiveness of nano-calcium phosphate paste on sensitivity during and after bleaching: A randomized clinical trial. Braz Oral Res. 2015;29:1–7. doi: 10.1590/1807-3107BOR-2015.vol29.0099. [DOI] [PubMed] [Google Scholar]
- 8.Vashisht R, Kumar A, Indira R, Srinivasan MR, Ramachandran S. Remineralization of early enamel lesions using casein phosphopeptide amorphous calcium phosphate: An ex-vivo study. Contemp Clin Dent. 2010;1:210–3. doi: 10.4103/0976-237X.76385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heshmat H, Ganjkar MH, Miri Y, Fard MJ. The effect of two remineralizing agents and natural saliva on bleached enamel hardness. Dent Res J (Isfahan) 2016;13:52–7. doi: 10.4103/1735-3327.174713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Costa Soares MU, Araújo NC, Borges BC, Sales Wda S, Sobral AP. Impact of remineralizing agents on enamel microhardness recovery after in-office tooth bleaching therapies. Acta Odontol Scand. 2013;71:343–8. doi: 10.3109/00016357.2012.681119. [DOI] [PubMed] [Google Scholar]
- 11.Gjorgievska E, Nicholson JW. Prevention of enamel demineralization after tooth bleaching by bioactive glass incorporated into toothpaste. Aust Dent J. 2011;56:193–200. doi: 10.1111/j.1834-7819.2011.01323.x. [DOI] [PubMed] [Google Scholar]
- 12.Levy FM, Magalhães AC, Gomes MF, Comar LP, Rios D, Buzalaf MA, et al. The erosion and abrasion-inhibiting effect of TiF(4) and NaF varnishes and solutions on enamel in vitro. Int J Paediatr Dent. 2012;22:11–6. doi: 10.1111/j.1365-263X.2011.01151.x. [DOI] [PubMed] [Google Scholar]
- 13.Vieira A, Jager DH, Ruben JL, Huysmans MC. Inhibition of erosive wear by fluoride varnish. Caries Res. 2007;41:61–7. doi: 10.1159/000096107. [DOI] [PubMed] [Google Scholar]
- 14.Yu H, Attin T, Wiegand A, Buchalla W. Effects of various fluoride solutions on enamel erosion in vitro. Caries Res. 2010;44:390–401. doi: 10.1159/000316539. [DOI] [PubMed] [Google Scholar]
- 15.Coceska E, Gjorgievska E, Coleman NJ, Gabric D, Slipper IJ, Stevanovic M, et al. Enamel alteration following tooth bleaching and remineralization. J Microsc. 2016;262:232–44. doi: 10.1111/jmi.12357. [DOI] [PubMed] [Google Scholar]
- 16.Attin T, Schmidlin PR, Wegehaupt F, Wiegand A. Influence of study design on the impact of bleaching agents on dental enamel microhardness: A review. Dent Mater. 2009;25:143–57. doi: 10.1016/j.dental.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Attin T, Albrecht K, Becker K, Hannig C, Wiegand A. Influence of carbamide peroxide on enamel fluoride uptake. J Dent. 2006;34:668–75. doi: 10.1016/j.jdent.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Pai N, McIntyre J, Tadic N, Laparidis C. Comparative uptake of fluoride ion into enamel from various topical fluorides in vitro. Aust Dent J. 2007;52:41–6. doi: 10.1111/j.1834-7819.2007.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 19.Nelson DG, Jongebloed WL, Arends J. Morphology of enamel surfaces treated with topical fluoride agents: SEM considerations. J Dent Res. 1983;62:1201–8. doi: 10.1177/00220345830620120501. [DOI] [PubMed] [Google Scholar]
- 20.Saxegaard E, Rölla G. Fluoride acquisition on and in human enamel during topical application in vitro. Scand J Dent Res. 1988;96:523–35. doi: 10.1111/j.1600-0722.1988.tb01592.x. [DOI] [PubMed] [Google Scholar]
- 21.Murakami C, Bönecker M, Corrêa MS, Mendes FM, Rodrigues CR. Effect of fluoride varnish and gel on dental erosion in primary and permanent teeth. Arch Oral Biol. 2009;54:997–1001. doi: 10.1016/j.archoralbio.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Hattab FN, Qudeimat MA, al-Rimawi HS. Dental discoloration: An overview. J Esthet Dent. 1999;11:291–310. doi: 10.1111/j.1708-8240.1999.tb00413.x. [DOI] [PubMed] [Google Scholar]
- 23.Armênio RV, Fitarelli F, Armênio MF, Demarco FF, Reis A, Loguercio AD, et al. The effect of fluoride gel use on bleaching sensitivity: A double-blind randomized controlled clinical trial. J Am Dent Assoc. 2008;139:592–7. doi: 10.14219/jada.archive.2008.0220. [DOI] [PubMed] [Google Scholar]
- 24.Yamagishi K, Onuma K, Suzuki T, Okada F, Tagami J, Otsuki M, et al. Materials chemistry: A synthetic enamel for rapid tooth repair. Nature. 2005;433:819. doi: 10.1038/433819a. [DOI] [PubMed] [Google Scholar]


