Abstract
Aim:
The aim of the study was to evaluate the effect of staining solutions, remineralizing agent, and antioxidant on color stability of tooth during and after bleaching.
Materials and Methods:
Ninety human central incisors were bleached using 35% hydrogen peroxide (Pola office) and allocated to three groups (n = 30). Group I stained with cola-based soft drinks, Group II stained with pomegranate juice, and Group III stained with turmeric. The groups were then divided into three subgroups (n = 10): subgroup A – control (artificial saliva), subgroup B – remineralizing agent (Vantej), and subgroup C - antioxidant (grape seed extract [GSE]). Specimens were bleached according to the manufacturer's recommendations. Color variation measurement was performed using a photoreflectance spectrophotometer before bleaching, during each bleaching session (2-times/week), and after (7 and 15 days) the cessation of bleaching. Artificial saliva was used as the storage medium for the specimens except when measurements were to be recorded. The results were then subjected to statistical analysis.
Results:
Freshly bleached teeth exposed to Vantej and GSE and significantly lowered the stain absorption.
Conclusion:
Among the experimental agents, Vantej performed statistically better at all time intervals.
Keywords: Antioxidants, bleaching, remineralizing agent, staining solution
INTRODUCTION
Tooth bleaching has become one of the most esthetic dental treatments, which is rather conservative and cost-effective for improving a person's smile.[1] There is no agreement regarding the time necessary for the patient to wait before the use of food containing colorants as it could disturb dental bleaching potency.
Discoloration of the tooth during or just after the bleaching is possible if pigments of food and beverages are consumed during that period. Hence, it was thought that freshly bleached tooth subjected to surface treatment was an effective way to reduce the uptake of stains and retain the outcome of bleaching for a prolonged time.
One potential way to reverse this damage is by the use of mineralizing agents namely, Novamin. Novamin is calcium sodium phosphosilicate bioactive glass (BAG) which when exposed to body fluids deposit hydroxycarbonate crystals, a mineral that is analogous to natural tooth mineral.[2]
Recently, grape seed extract (GSE) has been introduced in many fields of dentistry. Proanthocyanidins (PAs) in GSE augment the production of collagen, decline the deterioration of the collagen matrix, and promote the transformation of insoluble collagen into soluble collagen.[3,4]
Bedran-Russo et al.[5] reported that 6.5% GSE solution in contact with demineralized dentin can raise its modulus of elasticity, strength, and the bulk of cross-linked collagen. Moreover, another in vitro study found that PAs are fundamental antioxidants for calcium absorption.[6] In a study by Subramonian et al.,[7] it was found that the use of GSE before bonding procedures on bleached enamel completely neutralizes the deleterious effects of bleaching and increases the bond strength significantly. However, no studies can be found in the literature describing the effects of proanthocyanidin on bleached enamel to prevent restaining.
Considering these factors, our study has been undertaken to evaluate and compare the effect of potential remineralizing agent and antioxidants on color stability of bleached tooth exposed to different staining solutions.
The null hypothesis was that there is no effect of remineralizing agent and antioxidant on prevention of staining during and after bleaching.
MATERIALS AND METHODS
Sample size
Ninety extracted maxillary central incisors which on observation were free from caries, cracks, or hypoplastic defects were chosen and stored in 0.5% chloramine T. The roots were sectioned from the cementoenamel junction using a diamond disc (Shofu, Kyoto, Japan). Each specimen with the labial surface exposed was separately immersed in rectangle molds constructed using chemically cured acrylic resin. Then, the enamel surfaces were polished using prophylaxis paste and a polishing brush and washed. Thereafter, the teeth were placed in distilled water at 37°C for no more than 3 days before bleaching procedures.
Color evaluation
The Commission Internationale de I'Eclairage (CIE) L*a*b* color scale was used for baseline color measurement relative to the standard illuminant C over a white base using a reflectance spectrophotometer (UV-2450, Shimadzu Corp.). The CIE L*a*b* color setup is a three-dimensional color analysis: L* specifies lightness coordinate, and its value ranks from 0 for perfect black to 100 for perfect white, a* is chromaticity coordinates on the green–red (−a*¼ green and +a*¼ red), axes and b* is for blue − yellow (−b*¼ blue and +b*¼ yellow) axes. The complete change in color (dE*) was determined using the following formula:
ΔE*=[(ΔL*)2(Δa*)2(Δb*)2]½
Where dL*, da*, and db* shows the variation in L*, a*, and b* values, respectively.
ΔE >3.7 – easily visible difference.
ΔE between 3.7 and 1 – clinically acceptable difference
ΔE <1 – difference clinically not visible.
All the specimens were examined under the spectrophotometer at the following stages,
Prebleaching
During each bleaching session – 2 times/week
Postbleaching – After 7 and 15 days of bleaching.
Bleaching procedure
Labial surfaces of ninety specimens were bleached with Pola office (SDI, Victoria, Australia) according to manufacturer instruction. A thick layer of the bleaching paste was applied on the tooth surface and cured with a light-emitting diode source for three 2.5 min light irradiation cycles and with 10 min resting time. Three repeated application was done at the same sitting followed by rinsing the specimens with distilled water, and the procedure was repeated after 1 week and 2 weeks.
Surface treatments
After bleaching, the specimens were randomly divided into three Groups (I, II, and III) (n = 30) concordant to the staining solutions used (cola-based soft drink [CBSD], pomegranate juice, and turmeric solution). Each day fresh turmeric solution was prepared by adding 5 g of turmeric (MDH, Haldi powder, India) in 500 ml of distilled water and boiling for 10 min. The groups were then divided into three subgroups (n = 10) according to the surface treatment. Subgroup A – control (artificial saliva), subgroup B – remineralizing agent (Vantej toothpaste, India), and subgroup C – antioxidant (GSE). For the preparation of 10% antioxidant solutions, 10 g GSE powder (Biovea, USA) was dissolved in 100 ml of distilled water.
Group IA: Subsequent to bleaching, the samples were immersed in artificial saliva for 2 h, subsequently placed in staining solution (CBSD) for 10 min, followed by washing and storing in artificial saliva for the rest day, for a period of 24 h. This process was repeated every day until the next bleaching session (1 week and 2 weeks)
Group IB: Application of a remineralizing agent was done for 5 min immediately after bleaching followed by storage in saliva and staining in CBSD same as Group IA
Group IC: Application of an antioxidant was done for 10 min immediately after bleaching followed by storage in saliva and staining in CBSD same as Group IA
Group IIA: Similar to Group IA except the staining solution used was pomegranate juice
Group IIB: Application of a remineralizing agent and staining in pomegranate juice same as Group IB
Group IIC: Application of an antioxidant and staining in pomegranate juice same as Group IC
Group IIIA: Similar to Group IA except the staining solution used was turmeric solution
Group IIIB: Application of a remineralizing agent and staining in turmeric solution same as Group IB
Group IIIC: Application of an antioxidant and staining in turmeric solution same as Group IC.
During the experimental period, the teeth were stored in artificial saliva at 37°C simulating oral conditions. The artificial saliva was prepared and changed each day, and a digital pH meter was used to check the pH of the solution.
Statistical analysis
Mean and standard deviation for each group and subgroup are shown in Tables 1 and 2. One-way analysis of variance was applied to see the difference between the groups. The Bonferroni post hoc test was performed to confirm the results of ANOVA test between each group. P value of 0.05 has been considered statistically significant. Data analysis was done using SPSS version 15.0 statistical analysis software (SPSS Inc. Chicago, IL).
Table 1.
One-way analysis of variance test for intergroup comparison
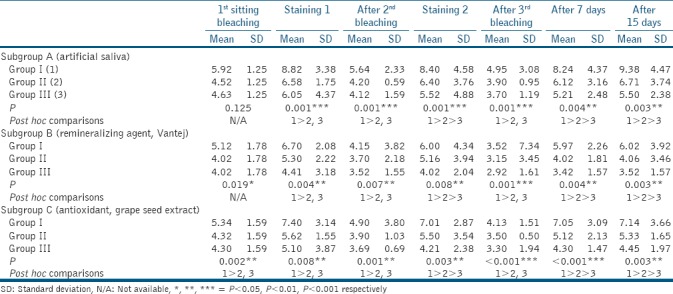
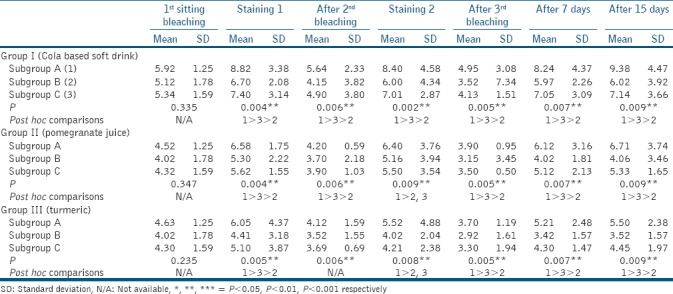
Table 2.
One-way analysis of variance test for intragroup comparison
RESULTS
On intergroup comparison [Table 1] at all time intervals irrespective of the subgroups, Group I (CBSD) showed significantly higher staining as compared to both Group II (pomegranate juice) and III (turmeric), whereas Group II had significantly higher staining as compared to Group III.
On intragroup comparison [Table 2] at all time intervals irrespective of the groups, restaining was least in subgroup B (Vantej) compared to both subgroup A (artificial saliva) and C (GSE), whereas restaining in subgroup C was less as compared to subgroup A.
DISCUSSION
The tooth color relapse seems to be one of the biggest problems after tooth bleaching. Therefore, the patient should understand that bleaching is not an everlasting treatment and that some intermittent re-bleaching will be required.[8] In dental clinics, generally, practitioners recommend a re-bleaching treatment about 2 years after the first treatment[9] or once a year,[8] but this time can be even shorter depending on restaining conditions.
To examine the teeth under natural conditions, the sample surfaces were not ground flat, which might have led to a greater disparity with respect to stain adsorption and color determination among the specimens.
Middle third of teeth crowns was selected as the zone for the assessment of color to exclude the translucent and worn incisal areas.
To our knowledge, GSE has not been studied with regard to its effect on the color stability of bleached tooth. The biologically active constituents of GSE are polyphenols, mainly proanthocyanidins, which are condensed tannin. Proanthocyanidins are high-molecular-weight polymers comprising of monomeric flavan-3-ol (+)catechin and (−)epicathechins. Grape seed extract has recently been advocated for its beneficial antioxidant, antibacterial and free radical scavenging properties.
The irregularities of the enamel are further boosted with bleaching[10,11] which could also confer to staining after bleaching as suggested by Dahl and Pallensen.[12] Moreover, it was noticed that the susceptibility to staining tends to be greater when the tooth is exposed to 35% hydrogen peroxide.[13]
Teeth exposed to a pH <5.5 for enamel and 6.0 for dentin for an extended period of time can lead to demineralization[14] and erosion of enamel.[15] The cola soft drink (pH 2.60) and pomegranate juice (pH 3.20) used in this investigation are extremely acidic solutions in comparison to turmeric (pH 6.30), showing that the low pH of these solutions may have had a major effect on the structure of the bleached teeth. The erosive loss of enamel is higher with the CBSD which has a low pH because of the presence of phosphoric acid.[16] The pomegranate juice also has a low pH, which may have damaged the surface of the samples and resulted in greater enamel demineralization. Nevertheless, these acidic substances harm enamel and cause more teeth discoloration compared to turmeric.
Turmeric, on the other hand, showed the least staining among all the groups. However, the staining due to turmeric is due to curcumin, its major color constituent. Curcumin is the active ingredient in turmeric which is brightly yellow colored and is commonly used as food coloring agent, which might have resulted in some staining of the bleached tooth. In addition, the pH of turmeric solution was found to be 6.3 which is comparatively greater than coca cola and pomegranate juice.
In the present study, restaining was least in all groups with the application of antioxidant (Vantej) after tooth bleaching as compared to all the other agents. This is because Vantej is a Novamin (5%)-based toothpaste containing BAG which is a synthetic mineral comprising calcium sodium phosphosilicate. It reacts with saliva increasing the pH. At this elevated pH, calcium and phosphate precipitate as calcium phosphate layer and form hydroxycarbonate apatite.[17] These particles have been shown to release ions and remineralize the tooth surface for up to 2 weeks.[18]
The results were in accordance with the study by Mehta et al.[19] and Narayana et al.[20] where highest remineralization was seen by bioactive glass as compared to casein phosphopeptide-amorphous calcium phosphate.
It is clear from the results of this study that the use of GSE showed some remineralizing potential which was less than Vantej but better than artificial saliva.
In agreement with this, Cheng et al.[21] reported that gallic acid, found in GSE, certainly affects the remineralization process resulting in a mineral deposition by the PA collagen interaction, maintaining the exposed collagen matrix.
Despite the fact that mature tooth enamel is devoid of collagen, Açil et al.[22] reported that type I collagen is found in enamel though the concentration is remarkably less as related to that in dentin. In addition, Felszeghy et al.[23] showed that type X collagen is present in the enamel matrix, which might be involved in enamel mineralization. It is notable that the terminal carboxyl and amine groups mainly confer to the fusion of collagen peptides to the hydroxyapatite surfaces and aid in hydroxyapatite growth.[24]
Artificial saliva (control group) showed some amount of remineralization though it was the least among all the groups. This was due to the calcium and phosphate saturation and buffering capacity characteristics of saliva.[25]
There were significant differences in the ΔE values calculated at various time intervals in all the groups with ΔE >1 unit. These results should be illustrated as a caution for exercising complete restraint on substances containing the studied colorant, especially during dental bleaching.
CONCLUSION
In the current study, application of Vantej paste and use of GSE immediately after tooth bleaching resulted in decreased staining of the tooth. Further analysis, especially, clinical assessment is required to elucidate the success of this approach.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.American Dental Association Council on Scientific Affairs. Tooth Whitening/Bleaching: Treatment Considerations for Dentists and Their Patients. American Dental Association. 2010:1–12. [Google Scholar]
- 2.Burwell AK, Litkowski LJ, Greenspan DC. Calcium sodium phosphosilicate (NovaMin): Remineralization potential. Adv Dent Res. 2009;21:35–9. doi: 10.1177/0895937409335621. [DOI] [PubMed] [Google Scholar]
- 3.Aldini G, Carini M, Piccoli A, Rossoni G, Facino RM. Procyanidins from grape seeds protect endothelial cells from peroxynitrite damage and enhance endothelium-dependent relaxation in human artery: New evidences for cardio-protection. Life Sci. 2003;73:2883–98. doi: 10.1016/s0024-3205(03)00697-0. [DOI] [PubMed] [Google Scholar]
- 4.Walter R, Miguez PA, Arnold RR, Pereira PN, Duarte WR, Yamauchi M, et al. Effects of natural cross-linkers on the stability of dentin collagen and the inhibition of root caries in vitro. Caries Res. 2008;42:263–8. doi: 10.1159/000135671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedran-Russo AK, Pashley DH, Agee K, Drummond JL, Miescke KJ. Changes in stiffness of demineralized dentin following application of collagen crosslinkers. J Biomed Mater Res B Appl Biomater. 2008;86:330–4. doi: 10.1002/jbm.b.31022. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa M, Maki K, Tofani I, Kimura K, Kimura M. Grape seed proanthocyanidins extract promotes bone formation in rat's mandibular condyle. Eur J Oral Sci. 2005;113:47–52. doi: 10.1111/j.1600-0722.2004.00176.x. [DOI] [PubMed] [Google Scholar]
- 7.Subramonian R, Mathai V, Christaine Angelo JB, Ravi J. Effect of three different antioxidants on the shear bond strength of composite resin to bleached enamel: An in vitro study. J Conserv Dent. 2015;18:144–8. doi: 10.4103/0972-0707.153076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein RE, Garber DA. Complete Dental Bleaching. Vol. 14. Chicago: Quintessence Publishing; 1995. pp. 35–56. [Google Scholar]
- 9.Boushell LW, Ritter AV, Garland GE, Tiwana KK, Smith LR, Broome A, et al. Nightguard vital bleaching: Side effects and patient satisfaction 10 to 17 years post-treatment. J Esthet Restor Dent. 2012;24:211–9. doi: 10.1111/j.1708-8240.2011.00479.x. [DOI] [PubMed] [Google Scholar]
- 10.Cavalli V, Arrais CA, Giannini M, Ambrosano GM. High-concentrated carbamide peroxide bleaching agents effects on enamel surface. J Oral Rehabil. 2004;31:155–9. doi: 10.1111/j.1365-2842.2004.01138.x. [DOI] [PubMed] [Google Scholar]
- 11.de Vasconcelos AA, Cunha AG, Borges BC, Vitoriano Jde O, Alves-Júnior C, Machado CT, et al. Enamel properties after tooth bleaching with hydrogen/carbamide peroxides in association with a CPP-ACP paste. Acta Odontol Scand. 2012;70:337–43. doi: 10.3109/00016357.2011.654261. [DOI] [PubMed] [Google Scholar]
- 12.Dahl JE, Pallesen U. Tooth bleaching – a critical review of the biological aspects. Crit Rev Oral Biol Med. 2003;14:292–304. doi: 10.1177/154411130301400406. [DOI] [PubMed] [Google Scholar]
- 13.Berger SB, Coelho AS, Oliveira VA, Cavalli V, Giannini M. Enamel susceptibility to red wine staining after 35% hydrogen peroxide bleaching. J Appl Oral Sci. 2008;16:201–4. doi: 10.1590/S1678-77572008000300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Setien V, Roshan S, Cala C, Ramirez R. Pigmentation susceptibility of teeth after bleaching with 2 systems: An in vitro study. Quintessence Int. 2009;40:47–52. [PubMed] [Google Scholar]
- 15.Driessens FC, Theuns HM, Borggreven JM, van Dijk JW. Solubility behaviour of whole human enamel. Caries Res. 1986;20:103–10. doi: 10.1159/000260928. [DOI] [PubMed] [Google Scholar]
- 16.Attin T, Weiss K, Becker K, Buchalla W, Wiegand A. Impact of modified acidic soft drinks on enamel erosion. Oral Dis. 2005;11:7–12. doi: 10.1111/j.1601-0825.2004.01056.x. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds EC. Calcium phosphate-based remineralization systems: Scientific evidence? Aust Dent J. 2008;53:268–73. doi: 10.1111/j.1834-7819.2008.00061.x. [DOI] [PubMed] [Google Scholar]
- 18.Burwell AK, Litkowski L, Greenspan D. Calcium sodium phosphosilicate: Remineralization potential. Adv Dent Res. 2009;21:83–6. doi: 10.1177/0895937409335621. [DOI] [PubMed] [Google Scholar]
- 19.Mehta AB, Kumari V, Jose R, Izadikhah V. Remineralization potential of bioactive glass and casein phosphopeptide-amorphous calcium phosphate on initial carious lesion: An in vitro pH-cycling study. J Conserv Dent. 2014;17:3–7. doi: 10.4103/0972-0707.124085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narayana SS, Deepa VK, Ahamed S, Sathish ES, Meyappan R, Satheesh Kumar KS, et al. Remineralization efficiency of bioactive glass on artificially induced carious lesion an in vitro study. J Indian Soc Pedod Prev Dent. 2014;32:19–25. doi: 10.4103/0970-4388.127047. [DOI] [PubMed] [Google Scholar]
- 21.Cheng L, Li J, Hao Y, Zhou X. Effect of compounds of Galla Chinensis on remineralization of enamel surface in vitro. Arch Oral Biol. 2010;55:435–40. doi: 10.1016/j.archoralbio.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Açil Y, Mobasseri AE, Warnke PH, Terheyden H, Wiltfang J, Springer I, et al. Detection of mature collagen in human dental enamel. Calcif Tissue Int. 2005;76:121–6. doi: 10.1007/s00223-004-0122-0. [DOI] [PubMed] [Google Scholar]
- 23.Felszeghy S, Holló K, Módis L, Lammi MJ. Type X collagen in human enamel development: A possible role in mineralization. Acta Odontol Scand. 2000;58:171–6. doi: 10.1080/000163500429172. [DOI] [PubMed] [Google Scholar]
- 24.Almora-Barrios N, de Leeuw NH. A density functional theory study of the interaction of collagen peptides with hydroxyapatite surfaces. Langmuir. 2010;26:14535–42. doi: 10.1021/la101151e. [DOI] [PubMed] [Google Scholar]
- 25.Cai F, Shan P, Morgan MV, Reynolds EC. Free lozenges containing casein phosphopeptide – Amorphous calcium phosphate (CPP-ACP) Aust Dent J. 2003;48:4. doi: 10.1111/j.1834-7819.2003.tb00037.x. [DOI] [PubMed] [Google Scholar]


