Abstract
Alterations in glycosylation are common in cancer and are thought to contribute to disease. Lung cancer and primary malignant brain cancer, most commonly glioblastoma, are genetically heterogeneous diseases with extremely poor prognoses. In this review, we summarize the data demonstrating glycosylation is altered in lung and in brain cancer. We then use specific examples to highlight the diverse roles for glycosylation in these two deadly diseases and illustrate shared mechanisms of oncogenesis. In addition to alterations in glycoconjugate biosynthesis, we also discuss mechanisms of post-synthetic glycan modification in cancer. We suggest that alterations in glycosylation in lung and brain cancer provide novel tumor biomarkers and therapeutic targets.
Keywords: Lung cancer, Glioblastoma, GBM, Glycosylation in cancer, SULF, HSPG, HPSE, Biomarker
1. INTRODUCTION
1.1. Altered glycosylation in cancer.
Glycosylation is one of the most common types of post-translational modification, and it is a critical determinant of protein function. The process of glycosylation refers to the addition of a carbohydrate, or glycan, to a non-carbohydrate structure (aglycone), commonly a protein or lipid. While this process is most common in the ER/Golgi, it can also occur in the cytoplasm and nucleus. Indeed, glycosylation in the cytoplasm can result in rapid alterations in cell signaling. Most secreted and cell-surface proteins are post-translationally modified by glycosylation, including tyrosine kinase receptors and integrins, and the oligosaccharide structure is a critical determinant of biological function (Figure 1). Defined by the nature of the linkage to the aglycone, the major classes of glycans in eukaryotic cells include the N-glycans, O-glycans, glycosphingolipids or glycolipids, and proteoglycans. In cancer, abnormalities in protein glycosylation are common, and they can be a hallmark of carcinogenesis and cancer metastasis (S. Hakomori, 1989; S. I. Hakomori & Cummings, 2012; Ohtsubo & Marth, 2006; Tuccillo et al., 2014). Lung cancer and primary malignant brain cancer, most commonly glioblastoma, are genetically heterogeneous diseases with extremely poor prognoses. Similar to several other malignant diseases, they exhibit striking alterations in glycosylation. These include alterations in gene expression of enzymes that regulate glycan biosynthesis and post-synthetic modification (for a review see (Cohen et al., 2008; Moskal, Kroes, & Dawson, 2009; Rosen & Lemjabbar-Alaoui, 2010; Tuccillo, et al., 2014; Wade et al., 2013). In lung cancer, numerous alterations in glycosylation have been described, including aberrant expression and glycosylation of mucins, altered branching of N-glycans, and increased presence of sialic acid on proteins and glycolipids. In brain tumors, common alterations include N- and O-glycan modifications of integrins and receptor tyrosine kinases and altered sialic acid containing glycoproteins. Common to both cancers are alterations that drive post-synthetic glycan modification. While it is clear that glycosylation is altered in lung and brain cancers, there is limited data on the functional role for these alterations in disease. In this review, we summarize some of the major alterations in glycosylation identified in lung and brain cancers, we draw parallels between these two deadly diseases, and where possible, we highlight examples for which functional data exists.
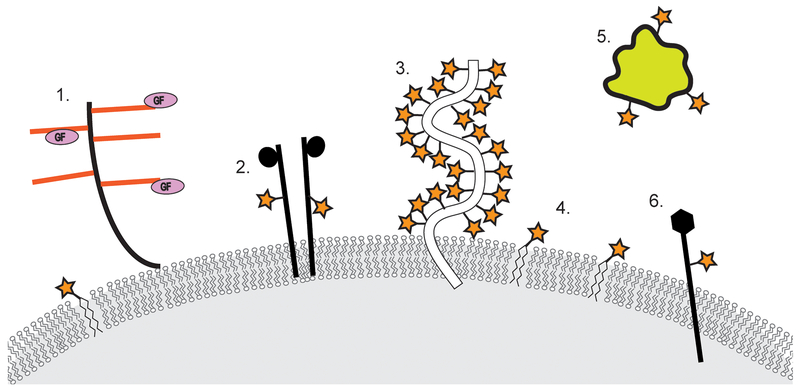
Figure 1. Glycosylation is a common post-translational modification of secreted and cell-surface proteins.
Representative examples of glycosylated molecules include (1) proteoglycans, (2) receptor tyrosine kinases, (3) mucin glycoproteins, (4) glycosphingolipids, (5) secreted proteins, and (6) integrins. Orange bars on proteoglycans denote glycosaminoglycan chains and stars denote glycosylation modifications.
1.2. Lung cancer.
Lung cancer remains the leading cause of cancer mortality in men and women in the U.S. and worldwide, accounting for 1.5 million deaths globally in 2011, up from 1.2 million deaths in 2000, with an estimated 159,260 deaths in the U.S. alone in 2014 (Siegel, Ma, Zou, & Jemal, 2014). About 90 % of lung cancer cases are caused by smoking and use of tobacco products. However, other factors such as radon gas, asbestos and air pollution exposures, as well as chronic infections can contribute to lung carcinogenesis. Lung cancer is divided into two broad histologic classes, which grow and spread differently: small-cell lung carcinomas (SCLC) and non-small cell lung carcinomas (NSCLC). NSCLCs comprise about 80% to 90% of all lung cancers and are further divided into three major histological subgroups: adenocarcinoma, squamous-cell carcinoma, and large cell carcinoma (Travis, Brambilla, & Riely, 2013). As with other cancers (Vogelstein & Kinzler, 2004), there is no single mutation that is responsible for lung cancer, but a succession of molecular changes contributes to tumor formation. Lung cancer is a very heterogeneous disease involving somatic mutations and epigenetic dysregulation of a number of signaling pathways. The identification and characterization of these molecular changes in lung cancer are of critical importance for improving disease prevention and early detection, as well as personalized prognosis and ideal therapy selection for each patient, based on the knowledge of each patient’s tumor characteristics and genetics. There have been considerable advances in our understanding of the molecular genetic changes in lung cancer pathogenesis in recent years and this has led to a vast improvement in the diagnosis and treatment of lung cancers based on the genetic signature of patient’s tumor. Several genetic alterations have been identified in lung cancer, including: 1) Activating mutations in a number of proto-oncogenes such as KRAS, EGFR, BRAF, PI3K, MEK and HER2. 2) Structural rearrangements in ALK, ROS1 and possibly RET. 3) Amplification of proto-oncogenes such as MET in adenocarcinomas, FGFR1 and DDR2 in squamous-cell lung carcinomas. 4) Oncogenic gene overexpression by microRNAs (miRNAs). 5) Inactivation of tumor suppressor genes, including TP53, RB1, CDKN2A, FHIT, RASSF1A, and PTEN. Despite this progress, further understanding of the molecular basis of lung cancer is needed, since current treatment options are frequently inadequate (Osada & Takahashi, 2002; M. Sato, Shames, Gazdar, & Minna, 2007).
1.3. Brain cancer.
Central nervous system (CNS) tumors encompass a diversity of neoplasms, including both primary tumors, derived from components of the normal central nervous system (CNS), and secondary tumors, neoplastic cells that have spread to the CNS from elsewhere in the body. It is estimated that approximately 100,000 new central nervous system tumors are diagnosed each year in the United States. While primary malignant brain tumors comprise less than a third of these cases, they are progressive and often fatal with a five-year relative survival rate of 34% (Hess, Broglio, & Bondy, 2004). In children, CNS tumors are now the most common cause of cancer death.
Infiltrating gliomas, one of the most common primary CNS tumors of adults, include oligodendroglioma, anaplastic oligodendroglioma, diffuse astrocytoma, anaplastic astrocytoma, and glioblastoma. Characterized by diffuse tumor cell invasion into the non-neoplastic brain, they often develop very aggressive biologic behavior with time. Indeed, glioblastoma (GBM), the most common primary malignant brain tumor in adults, is a highly aggressive neoplasm and median survival is less than 15 months (Hess, et al., 2004; Stupp et al., 2009; Stupp et al., 2005). GBM is characterized by aberrant signaling through receptor tyrosine kinase (RTK) signaling pathways and their downstream components. Frequent genetic abnormalities include amplification of the epidermal growth factor receptor (EGFR), loss of heterozygosity (LOH) of 10q, inactivation of PTEN, and loss of CDKN2A. Based on recent expression, genomic and proteomic data (Brennan et al., 2009; Mischel et al., 2003; H. S. Phillips et al., 2006; Verhaak et al., 2010), it is clear that GBM can be further stratified into broad subtypes with different patterns of abnormal RTK signaling pathway activity. Interestingly, expression of a glycosyl hydrolase 18 family member, the secreted glycoprotein CHI3L1 (YKL-40), is upregulated in a subset of GBM and is associated with worse outcome (Colman et al.; Kroes, Dawson, & Moskal, 2007; H. S. Phillips, et al., 2006). Despite advances in our understanding of the disease and its molecular alterations, improved tumor biomarkers and novel therapeutic strategies are needed.
2. N-LINKED GLYCANS.
N-linked glycans, involving the covalent linkage of an oligosaccharide to an asparagine residue of a polypeptide chain, are involved in several important biological processes, including protein folding and oligomerization, targeting proteins to sub-or extracellular locations, and cell–cell interactions. In cancer, alterations in N-glycan structure are common with an increase in highly branched N-glycans (Asada, Furukawa, Segawa, Endo, & Kobata, 1997; Dennis, Laferte, Waghorne, Breitman, & Kerbel, 1987; Dennis, Waller, & Schirrmacher, 1984) and an increase in terminal sialyation (Fogel, Altevogt, & Schirrmacher, 1983; Hedlund, Ng, Varki, & Varki, 2008) being most common. Glycosyltransferases involved in the synthesis of branching N-linked glycans and implicated in cancer include beta1,6-N-acetylglucosaminyl transferase (GnT-V), beta1,4-N-acetylglucosaminyltransferase (GnT-III), and alpha1,6-fucosyltransferase (FUT8).
GnT-V, a key enzyme that increases beta1,6 branching and is encoded by the MGAT5 gene, has increased expression in brain, colon, and breast cancer and has been shown to decrease cell adhesion and promote tumor cell invasion and metastasis (Demetriou, Nabi, Coppolino, Dedhar, & Dennis, 1995; Yamamoto, Oviedo, Sweeley, Saito, & Moskal, 2001; Yamamoto et al., 2000; Zhao et al., 2008). Moreover, in breast and colon carcinoma increased beta1,6-GlcNAc-bearing N-glycans have been shown to be a marker for tumor progression (Dennis, et al., 1987; Fernandes, Sagman, Auger, Demetrio, & Dennis, 1991). In glioma cells, over expression of GnT-V resulted in altered focal adhesions and increased tumor cell invasion (Yamamoto, et al., 2000). Highly branched N-glycans also have essential, yet diverse, roles in growth factor signaling. For example, increased alpha1,6-fucosyltransferase activity, can promote EGF receptor mediated signaling (Wang et al., 2006). Conversely, increased beta1,4-N-acetylglucosaminyltransferase activity can attenuate EGF receptor signaling, promote receptor endocytosis, and increase MAPK signaling in different cell systems (Rebbaa et al., 1997; Y. Sato et al., 2001).
In contrast to glioma, low expression levels of beta1,6-N-acetylglucosaminyl transferase (GnT-V) are associated with relatively short survival time and poor prognosis in NSCLC. Indeed, GnT-V is expressed in the normal lung (Perng, Shoreibah, Margitich, Pierce, & Fregien, 1994), and beta1,6 branching oligosaccharides, synthesized by GnT-V, are found in normal bronchial epithelial cells and alveolar pneumocytes (Li & Roth, 1997). Aberrant expression of GnT-V results from an altered transcription of its gene MGAT5 which can be altered by various mechanisms, including viral and chemical carcinogenesis. A recent study, demonstrated that GnT-V expression is decreased or lost in about half of NSCLCs, while GnT-V is expressed in non-neoplastic bronchial epithelial cells, bronchial gland cells, and alveolar pneumocytes. Histology was significantly correlated with GnT-V expression; low GnT-V expression was more frequently found in squamous-cell carcinomas than in non-squamous-cell carcinomas (Dosaka-Akita et al., 2004). Furthermore, low GnT-V expression was associated with a shorter survival period and was an unfavorable prognostic factor in Stage I resected non-squamous-cell carcinomas. Moreover, the vast majority of NSCLC tumors with a high GnT-V expression level also showed high levels of β1–6 branching oligosaccharides assessed by staining with the plant lectin L-phytohemagglutinin (L-PHA), which preferentially recognizes branched N-glycans bearing β1–6 branched GlcNAc, a product of GnT-V. Interestingly, the Ki-67 labeling index (LI), a marker of proliferation, was significantly lower in NSCLC tumors with high GnT-V expression than in tumors with low GnT-V. It appears that in NSCLCs, which derive from bronchial and alveolar epithelia that normally express GnT-V, GnT-V expression is associated with favorable prognosis. Decreased expression of GnT-V may contribute to altered biological properties of a subset of NSCLCs by decreased synthesis of β1–6 branching oligosaccharides of certain target glycoproteins. In contrast, another study has reported that Mgat5 is highly expressed in CD133-positive lung adenocarcinoma tumors and cell lines when compared to CD133-negative counterparts (X. Zhou, Chen, Wang, Zhang, & Zhao, 2011). Moreover, greater L-PHA staining was observed in CD133-positive cells than in CD133-negative cell. Importantly, knockdown of Mgat5 in CD133-positive cancer cell lines inhibited cancer cell growth both in vitro and in vivo, suggesting increased beta1,6-branching oligosaccharides may play an oncogenic role in CD133-positive lung adenocarcinomas. Of note, the target glycoproteins of GnT-V in the lung and bronchus remain to be determined.
3. O-LINKED GLYCANS.
Another very common covalent modification is the O-linked glycans in which the glycan is linked to the polypeptide by the hydroxyl group of a serine or threonine residue. When covalently alpha-linked via N-acetylgalactosamine (GalNAc) these structures are named mucin-O-glycans. Mucin glycoproteins, glycoproteins that are heavily O-glycosylated, are expressed from the luminal surface of many epithelia (see Mucins, below). Unlike the mucins, when the glycan is covalently beta-linked via N-acetylglucosamine it is termed O-GlcNAc. O-GlcNAcylation is unique as it represents one of the most abundant post-translational modifications in the cytoplasm and nucleus, and it can rapidly change. Thus, O-GlcNAc regulates a number of important biological processes, including cell signaling pathways, gene transcription, cell proliferation, protein degradation, metabolism and insulin sensing. O-GlcNAcylation is regulated by O-GlcNAc transferase (OGT) and its opposing counterpart O-GlcNAcase (OGA). OGT catalyzes the transfer of N-acetylglucosamine from uridine diphospho-N-acetylglucosamine (UDP-GlcNAc) to serine or threonine residues of a wide variety of intracellular proteins, including signaling proteins important for insulin resistance (X. Yang et al., 2008), oncogenes and tumor suppressors (Chou, Hart, & Dang, 1995; W. H. Yang et al., 2006), and transcriptional co-activators that control gluconeogenesis (Dentin, Hedrick, Xie, Yates, & Montminy, 2008).
In human lung squamous cell carcinoma tissues, the O-GlcNAcylation levels and the expression of OGT and OGA were assessed by immunohistochemistry analysis (Mi et al., 2011). This analysis demonstrated that O-GlcNAcylation of lung cancer tissues is significantly elevated compared with that in the adjacent normal tissues. Additionally, OGT expression was markedly enhanced, both at the protein and the transcript levels, in lung cancer tissues compared with that in adjacent normal tissues. In contrast, the level of the opposing enzyme (OGA) was not significantly different between the tumor tissues and their adjacent normal tissues. Thus, OGT expression is probably one of the main causes of O-GlcNAcylation elevation in NSCLC tumor tissues. Further data from this study demonstrated that OGT inhibition in NSCLC cell lines leads to reduction of O-GlcNAcylation. Reduction of O-GlcNAcylation in NSCLC cells, results in a significant decrease of the anchorage-independent growth and in vitro cell invasion abilities of NSCLC cells, two hallmark properties of malignant cells. Together, these results suggest that O-GlcNAcylation might promote lung carcinogenesis.
O-GlcNAc levels can be induced within minutes (Kneass & Marchase, 2004) and accumulate on the minute-to-hours time scale (Rexach et al., 2012). Almost all enzymes involved in the glycolytic pathway are potential substrates for OGT (Clark et al., 2008). Cellular metabolism is significantly altered in rapidly growing cancers. Cancer cells consume glucose avidly and produce elevated levels of lactic acid compared to normal tissues. This phenomenon, known as aerobic glycolysis or the “Warburg effect,” has been observed in almost all aggressive cancers, including lung and brain cancers. O-GlcNAc glycosylation acts as a nutrient sensor of the cellular metabolic state to couple metabolic status to the regulation of many signaling pathways (Hart, Housley, & Slawson, 2007; Love & Hanover, 2005; Rexach, Clark, & Hsieh-Wilson, 2008; X. Yang, et al., 2008). Thereby. O-GlcNAc glycosylation of specific glycolytic enzymes may play a role in regulating glycolysis and contribute to altered metabolic states in lung cancer cells. A Recent study (Yi et al., 2012) has demonstrated that in human NSCLC lines increasing O -GlcNAc levels significantly decrease the activity of phosphofructokinase 1 (PFK1), a major regulatory enzyme that controls the flux through the glycolytic pathway (Sola-Penna, Da Silva, Coelho, Marinho-Carvalho, & Zancan, 2010). This O-GlcNAc glycosylation-induced reduction of PFK1 activity is not associated with a change in the protein expression levels of PFK1. Conversely, enhanced O-GlcNAc levels have minimal effect on other key regulatory points in the glycolytic pathway, including hexokinase, phosphoglycerate kinase and pyruvate kinase. This study has also shown that PFK1 glycosylation occurs at the Ser529 site of the enzyme, and is triggered in NSCLC cell lines in a time-dependent manner under hypoxic conditions or when cells were subjected to glucose deprivation. These conditions are usually associated with tumorigenesis and rapid tumor growth. Noteworthy, under these stress conditions, there was only a modest increase in OGT expression levels but no changes in OGA or PFK1 expression levels. O -GlcNAc glycosylation inhibits PFK1 activity by blocking the binding of fructose-2,6-bisphosphate to the allosteric site of the enzyme, and possibly perturbing the oligomerization of PFK1 subunits. Under conditions of oxidative stress, O-GlcNAc glycosylation of PFK1 redirects the flux of glucose from glycolysis toward the anabolic pentose phosphate pathway (PPP) (Christofk, Vander Heiden, Wu, Asara, & Cantley, 2008; Ralser, Heeren, Breitenbach, Lehrach, & Krobitsch, 2006; Ralser et al., 2007), and the production of precursors necessary for DNA and protein biosynthesis, as well as reducing power in the form of NADPH and GSH to prevent ROS insult. Thus, PFK1 glycosylation confers a selective growth advantage to cancer cells by providing an ability to adapt rapidly to the changing needs and microenvironment of tumor cells. Blocking glycosylation of PFK1 at Ser529 reduced lung cancer cell proliferation in vitro and impaired tumor formation in vivo. Collectively, these findings highlight a novel mechanism for the regulation of metabolic enzymes and pathways by O-GlcNAc glycosylation, and suggest a potential therapeutic approach for treating cancer.
4. MUCINS.
Mucins are large glycoproteins expressed on the luminal epithelial surface and are thought to function as a physical and biological barrier protecting mucous epithelia. The human mucin (MUC) family consists of several secreted (e.g., MUC1 and MUC4) or transmembrane members (e.g., MUC2 to MUC5AC/B). The mucin family consists of proteins that contain tandem repeat structures with a high proportion of prolines, threonine and serines (which constitute the PTS domain). Mucins are characterized by extensive O-glycosylation of the PTS domain through GalNAc O-linkages at the threonine and serine residues. There are four types of mucin-type O-glycans synthesized by different glycosyltransferases: core1, core2, core3, and core4 (Fukuda, 2002). Diverse glycosylation of mucins potentially provides a basis for tissue-specific interaction with the milieu. O-glycosylation is a multistep process in which the carbohydrate chains are synthesized by the sequential addition of sugars in the Golgi compartments where the glycosyltransferases are specifically located (El-Battari et al., 2003). The first epitope attached to the Ser or Thr is N-acetylgalactosamine that corresponds to the Tn antigen. If a neuraminic acid is added, the sialyl-Tn terminal structure appears. The T antigen is formed by the addition of galactose to the Tn epitope (Springer, 1984) and sequential addition of sugar structures elongates the chain. If a fucose residue is also added, to the second to last residue, a Sialyl-Lewis X (SLeX) structure is formed. Lewis antigens are synthesized by the sequential action of fucosyltransferases (see Fucosylation, below). In addition to forming a protective barrier, the transmembrane mucins are implicated in the transduction of growth, and survival signals to maintain the integrity of the epithelial layer. As an integral component of the epithelial stress response, transmembrane mucins contribute to the disruption of polarity and cell–cell interactions, and thereby the epithelial to mesenchymal transition (EMT) (Ponnusamy et al., 2013). While several mucins are expressed in the normal human respiratory epithelium a number of alterations have been observed in neoplastic epithelium (Copin et al., 2000; Jarrard et al., 1998; Lopez-Ferrer et al., 2001). Indeed, human NSCLC tumors express a number of mucins, including MUC1, MUC2, MUC4, MUC5AC, MUC6, and MUC8. Interestingly, in NSCLC tumors (adenocarcinomas and squamous-cell carcinomas) the Tn and sialyl-Tn antigens (truncated O-glycans) are more abundant than the T antigen (Lopez-Ferrer, Barranco, & de Bolos, 2002). Moreover, the expression of sialyl-Tn in adenocarcinomas is greater than in squamous-cell carcinomas (Molinolo, Simpson, Thor, & Schlom, 1990). Furthermore, published reports have shown that most lewis structures are expressed in human NSCLC tumors, and that Lewis type 2 antigens are detected more frequently than Lewis type 1 antigens. In addition, LeY is always the most strongly expressed in NSCLC (Kawai, Suzuki, Kase, & Ozeki, 1993; Longenecker et al., 1984). In addition, higher expression of sialyl-LeA and sialyl-LeX in NSCLC tumors, has been shown to correlate with an up-regulation of fucosyltransferases FUT3 and FUT6 (Togayachi et al., 1999).
Mucin 1 (MUC1) is translated as a single polypeptide that undergoes auto cleavage into N-terminal (MUC1-N) and C-terminal (MUC1-C) subunits (Kufe, 2009). MUC1-N contains the highly glycosylated tandem repeats that are characteristic of the mucin family. In contrast, MUC1-C is a transmembrane protein that functions as a cell-surface receptor (Kufe, 2009). MUC1 has recently emerged as a highly attractive target for the development therapies and vaccines for lung cancer. In NSCLC patients, MUC1 expression assessed with antibodies to less or not glycosylation dependent epitopes, has been shown to correlate with a poor survival outcome (Guddo et al., 1998; Nagai et al., 2006; Tsutsumida et al., 2004). In contrast, tumor-associated MUC1 epitope expression was found to be a favorable prognostic factor in NSCLC patients with lymph node metastases (Kuemmel et al., 2009). MUC1 is highly expressed in type II pneumocytes of the alveolar epithelium and malignant lung cells (Jarrard, et al., 1998). Type II pneumocytes are progenitor cells for normal and neoplastic epithelium during the repair and injury and during carcinogenesis. Several studies have suggested that MUC1 may facilitate epithelial carcinogenesis by a variety of mechanisms.
Immunohistochemical expression of MUC1 has been shown to correlate with increased invasiveness, migration and angiogenesis in lung cancer (Dong et al., 1997). Recent work has shown that MUC1-C induces gene signatures that are highly predictive of overall and disease-free survival of NSCLC patients (Khodarev et al., 2009; MacDermed et al., 2010). Importantly, MUC1 expression in human NSCLC cells is associated with STAT3 activation, PI3K/AKT pathway activation and in vitro growth and in vivo tumorigenesis (Gao et al., 2009; Raina et al., 2011). The MUC1 associated with malignant cells is believed to exhibit unique features with a lower percentage of threonine and serine residues attached to N-acetylgalactosamine and/or without extension through core2 structures. Consequently, MUC1 glycosylation in cancer cells is believed to differ from normal MUC1 by shorter glycan side chains (Cao et al., 1997; Taylor-Papadimitriou, Burchell, Miles, & Dalziel, 1999). The altered MUC1on malignant cells may have an anti-adhesive effect, mediated by a net negative charge of the plasma membrane provided by extra sialic acid residues (Ligtenberg, Buijs, Vos, & Hilkens, 1992) and steric hindrance (Ligtenberg, et al., 1992; Wesseling, van der Valk, Vos, Sonnenberg, & Hilkens, 1995), which interferes with E-cadherin (Wesseling, van der Valk, & Hilkens, 1996), contributing to nodal and distant metastasis (Roy & Baek, 2002). The expression of bulky glycoproteins, such as MUC1, on the surface of cancer cells can also mechanically promote tumor cell growth and survival via the promotion of integrin adhesion and signaling (Paszek et al., 2014).
Tobacco smoke causes about 90 % of all lung cancer cases. A recent study has shown that in cultured normal human bronchial epithelial cells, exposure to cigarette smoke extract (CSE) results in the generation of a variant 400 kDa glycoform of MUC1’s N-terminus (MUC1-N) differing from the 230 kDa and 150 kDa glycoforms in untreated cells (Zhang et al., 2014). The MUC1-N variant promotion by CSE is dependent on the activity of N-acetyl-galactosaminyl transferase-6 enzyme (GALNT6). Subsequently, CSE induces, time-dependent shedding of glycosylated MUC1-N variant and interaction of MUC1-C terminus with EGFR, Src and p120ctn (Zhang, et al., 2014). Consequently, CSE exposure leads to loss of epithelial cadherin, loss of cellular polarity and disruption of cell-cell contact, all of which are major hallmarks of epithelial-mesenchymal transition (EMT), and important characteristics of carcinogenesis. These findings suggest a potential role for the altered MUC1 glycosylation in lung carcinogenesis.
5. SIALIC ACID.
Sialic acids are a group of carbohydrate structures often found in terminating branches of glycan chains that are derived from neuraminic acid. Sialic acids play essential roles in many biological processes, including cell adhesion and immune modulation, and they bind selectins, lectins, and siglecs (Crocker, Paulson, & Varki, 2007; Kelm & Schauer, 1997). Sialic acids may influence tumorigenesis in several ways and the presence of these large negatively charged molecules on the cell surface can attenuate the adhesive property of cells and increase cancer cell motility and invasion (Passaniti & Hart, 1988; R. Schauer, 1982; Roland Schauer, 1985; Yogeeswaran, 1983). Sialylation is a very common and multipurpose terminal glycosylation (R. Schauer, 1982). Elevated sialic acid in glycoproteins has been described in malignant cells (Holmes, Ostrander, & Hakomori, 1986; Vierbuchen, Fruechtnicht, Brackrock, Krause, & Zienkiewicz, 1995; Yogeeswaran & Salk, 1981), and studies suggest that this is an early event in tumorigenesis, sometimes starting years before diagnosis (Gatchev et al., 1993). Sialic acid epitopes are components of many cell-surface receptors and have an ability to mask specific cellular recognition sites involved in the host reaction to foreign cells, including cancer cells (Roland Schauer, 1985). The masking of immune recognition by NK T cells, via binding of sialic acids on tumor cells to Siglec7 on NK cells, may be an important mechanism of immune escape in cancer (Hudak, Canham, & Bertozzi, 2014).
The majority of serum proteins are glycosylated. Cancer-induced alterations in enzymes, such as the sialyltransferase family of enzymes that synthesize sialylated oligosaccharides, can result in slight alterations in serum protein glycosylation. It has been proposed that sialic acid alterations on serum proteins may be a useful tumor biomarker (Kakari et al., 1991; Patel et al., 1995; Patel et al., 1994; Polivkova, Vosmikova, & Horak, 1992; Shamberger, 1986). An investigation of the total serum sialic acid levels in lung cancer patients versus control healthy individuals reported that the levels of total serum sialic acids are considerably elevated in lung cancer patients (with or without metastasis) when compared to healthy controls (Gökmen et al., 2004).
Terminal sialylation of N-glycans can be alpha2,3-linked and alpha2,6-linked. In glioblastoma alpha-2,3-linked terminal sialic acids and their corresponding sialyltransferase are abundant while alpha2,6-linked terminal sialic acids are below the level of detection (Kaneko et al., 1996; Yamamoto, Kaneko, Rebbaa, Bremer, & Moskal, 1997). Interestingly, an increase in alpha2,3-linked terminal sialic acids promoted tumor cell invasion while an increase in alpha2,6-linked terminal sialic acids resulted in decreased cell invasion, altered adhesion-mediated signaling, and decreased tumor growth in vivo (Yamamoto, et al., 2001). As glycosylation is a major determinant of integrin function and changes in the N-glycan structure can alter cell-cell and cell-extracellular matrix interactions, the authors suggest terminal sialylation of N-glycans on integrins, may be an important determinant of tumor cell invasion in GBM.
Polysialic acid (PSA) is a linear homopolymer of alpha-2-8-linked sialic acid residues. A post-translational modification primarily of neural cell adhesion molecules (NCAM) (Fukuda, 1996), PSA alters cell-cell interactions and tends to promote cell migration. In human lung cancer (SCLC and NSCLC) and astrocytoma, disease is associated with elevated PSA (Petridis, Wedderkopp, Hugo, & Maximilian Mehdorn, 2009; Tanaka et al., 2000). In SCLC, expression of PSA-NCAM correlates with a high metastatic potential and rapid cell proliferation (Komminoth, Roth, Lackie, Bitter-Suermann, & Heitz, 1991; Scheidegger, Lackie, Papay, & Roth, 1994). In NSCLC, PSA was shown to be expressed in 21% of stage I tumors, and 77% of Stage IV tumors. Importantly, PSA expression correlates with nodal metastasis and distant metastasis, but not with the local extent of the primary tumor (Tanaka, et al., 2000). In addition, PSA expression inversely correlates with the degree of tumor differentiation, and is higher in poorly differentiated than in well-differentiated NSCLC tumors. In astrocytoma, PSA expression is also associated with higher grade and the fraction of cells expressing PSA is ten-fold higher in WHO grade III and IV (GBM) tumors as compared to WHO grade II tumors (Petridis, et al., 2009). PSA synthesis is governed by two glycosyltransferases, ST8 alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase 2 (ST8SIA2, also known as STX) and ST8 alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase 4 (ST8SIA4, also known as PST). Both normal lung and tumor tissue express the PST gene (Tanaka, et al., 2000). Conversely, STX expression is unique to NSCLC tumors, it correlates strongly with PSA levels, and it is significantly associated with tumor progression. In keeping, STX gene expression is found only in 4% of stage I NSCLC tumors, and in most (86%) of tumors with stage IV disease. These results suggest a potential role for polysialic acid modification in both lung and brain cancer.
6. FUCOSYLATION.
Fucosylation is one of the most common modifications involving oligosaccharides on glycoproteins and glycolipids. Fucosylation consists of transfer of fucose residue from GDP to N-glycans, O-glycans and glycolipids, and is involved in many of the biological processes, including lymphocyte homing, immune responses, fertilization, and development (Becker & Lowe, 2003). Carcinoma cells are often enriched with sialylated fucosylated lactosaminoglycans such as sialyl-Lewis X (sLeX) and sialyl-Lewis A (sLeA), which are recognized by the endothelial cell adhesion molecule E-selectin (Sawada, Tsuboi, & Fukuda, 1994; Takada et al., 1993; Yamada et al., 1997). E-selectin induces tumor cell binding to endothelium by similar molecular interactions used in leukocyte recruitment to inflammation sites (Bevilacqua & Nelson, 1993; Lowe, 1997; Varki, 1994).
Fucosylation is catalyzed by a family of fucosyltransferase enzymes (FUTs), consisting of 13 members, including FUT1 to 11, protein O-fucosyltransferase 1 (POFUT1), and POFUT2. FUTs promote attachment of fucose to N-, O-, and lipid-linked glycans through an α1,2- (by FUT1 and 2), α1,3- (by FUT3 to 7 and FUT9 to 11), α1,4- (by FUT3 and 5), or α1,6- (by FUT8) linkage, or directly link to the serine/threonine residues of EGF-like or thrombospondin repeats (by POFUT1 and 2, respectively) (Ma, Simala-Grant, & Taylor, 2006; Mollicone et al., 2009). Altered fucosylation may result from deficiency or overexpression of FUTs, and is implicated in several disorders such as cystic fibrosis and cancer (Becker & Lowe, 2003; Miyoshi, Moriwaki, & Nakagawa, 2008). Some fucosylated glycoproteins such as antibodies, which recognize fucosylated sialyl Lewis a/x, have been used as cancer biomarkers. Stage-specific embryonic antigen 1 (SSEA-1/LeX/CD15) is a non-sialylated, fucose-containing trisaccharide that is generated by FUT4. SSEA-1 is highly expressed on embryonic stem cells, in the developing brain, and in the adult subventricular zone (Capela & Temple, 2002, 2006) and it has been proposed as a marker of cancer stem cells in glioblastoma (Son, Woolard, Nam, Lee, & Fine, 2009).
By adding fucose to the innermost GlcNAc residue of an N-linked glycan, FUT8 is the sole enzyme responsible for the α1,6-linked (core) fucosylation and is central for regulating many protein functions. As mentioned above, FUT8 modification is particularly important for cell signaling even in non-neoplastic cells. Lack of core fucosylation on the TGF-β1 receptor (Wang et al., 2005), the EGF receptor (Wang, et al., 2006), or integrin α3β1 (Zhao et al., 2006) results in a dramatic decrease in their ligand binding affinity and inhibition of the corresponding downstream signaling pathways. In both brain and lung cancer, aberrant EGFR pathway activity and integrin mediated signaling are thought to be important oncogenic drivers. Thus, alterations in core fucosylation could have a major impact on cancer development and progression. The specificity of glycan modification, however, is important to note and is well illustrated for EGFR. Core fucosylation of the EGF receptor can clearly promote EGFR signaling, yet other forms of glycosylation inhibit EGFR signaling. Site-directed mutagenesis of EGFR glycosylation sites, N420 and N579, inhibit EGF-independent receptor dimerization (Takahashi et al., 2008; Tsuda, Ikeda, & Taniguchi, 2000; Whitson et al., 2005). Furthermore, overexpression of sialyltransferases and α1,3-fucosyltransferases (FUT4 or FUT6) in lung cancer cells, suppress EGF-induced receptor dimerization and phosphorylation. Treatment with sialidase or fucosidase corroborated the above-mentioned effect on EGFR dimerization (Y. C. Liu et al., 2011). In GBM, POFUT1, was identified in a screen for highly expressed glycoconjugates in tumors (Kroes, et al., 2007). POFUT1 mediated O-fucose modification is thought to be important for the normal function a number of signaling pathways and enzymes including Notch (Shi & Stanley, 2003). Together, these results demonstrate the important role for specific glycosylation events in cell signaling and cancer growth.
Remarkably, FUT 8 upregulation correlates with tumor metastasis, disease recurrence, and poor survival in NSCLC patients. In accordance with these observations, FUT8 silencing in aggressive lung cancer cell lines significantly inhibits their malignant behaviors, including in vitro invasion and cell proliferation, as well as in vivo metastasis and tumor growth (Chen et al., 2013). Furthermore, FUT8 silencing induces alterations in glycosylation of several surface antigens, receptors, and adhesion molecules, including EGFR and integrins implicated in brain and lung tumorigenesis, and regulates the expression of numerous genes associated with malignancy. These results highlight a multifaceted role of FUT8 in tumor progression. The overexpression of FUT8 in NSCLC cells, appears to occur during EMT process, through suppression of E-cadherin and translocation of β-catenin to the nucleus, where it complexes with LEF-1 to transactivate FUT8 expression. Core fucosylation may also be a critical determinant of antibody-dependent cell-mediated cytotoxicity, as deletion of the core fucose from the Fc region of IgG1, enhances its binding affinity to Fcγ receptor, and greatly improves (over 50 fold) antibody-dependent cell-mediated cytotoxicity (Okazaki et al., 2004; Shinkawa et al., 2003). Core fucosylation of E-cadherin is associated with enhanced cell–cell adhesion (Osumi et al., 2009).
Lung cancer cells express a wide array of sialylated or fucosylated glycans on the surface, including sLeX, sLeA, sTn, Ley, and polysialic acid (PSA) (Dube & Bertozzi, 2005). Previous reports have demonstrated that expression of FUT4 and FUT7 genes is associated with a worst survival outcome in lung cancer patients and that FUT7 is a greater indicator of a poor prognosis than FUT4 through its involvement in sLeX synthesis (Ogawa, Inoue, & Koide, 1996). In keeping, metastatic human lung adenocarcinoma cell lines have enhanced expression of FUT4 and FUT7 genes compared with their non-metastatic counterpart. In addition, These FUTs overexpression in metastatic lines correlates with an increased surface expression of lex- and Lea-related molecules and an in vitro enhanced adhesive capacity to E-selectin-expressing endothelial cells. It has been further demonstrated that FUT7 overexpression in a human lung adenocarcinoma cell line induces overexpression of Lewis X (LeX) residues, which in turn is sufficient to endow these cells with a metastatic behavior both in vitro and in vivo (Martin-Satue, de Castellarnau, & Blanco, 1999).
7. HEPARAN SULFATE PROTEOGLYCANS AND THEIR MODIFYING ENZYMES.
Many, if not most of the molecular events associated with tumor growth, neovascularization, and metastasis are influenced by interactions between cells and their extracellular matrix (ECM). Heparan sulfate proteoglycans (HSPGs), present on the cell surface and in the extracellular microenvironment, bind to and regulate signaling of diverse protein ligands, such as growth factors, morphogens, chemokines, and cytokines. Composed of a core protein and modified by the covalent addition of HS carbohydrate chains, interaction with ligand depends largely on the pattern and density of the sulfation modifications of HS, particularly the 6-O-sulfation of glucosamine (6OS) (Esko & Lindahl, 2001; Rosen & Lemjabbar-Alaoui, 2010). Based on these interactions HSPGs play fundamental roles in cell growth, differentiation, adhesion and motility.
Two major families of cell-surface–associated HSPGs are the syndecans and the glypicans. The syndecan glycoproteins, named syndecan1-4, represent a group of membrane-spanning proteoglycans with diverse extracellular and intracellular functions (Bernfield et al., 1992; Liang, Haring, Roughley, Margolis, & Margolis, 1997). In contrast, the glypicans lack a cytoplasmic signaling component and are attached to the cell membrane by means of a glycosyl phosphatidylinositol (GPI) anchor (David, 1993). A third family of membrane-associated HSPGs, possibly with more restricted expression, is represented by particular splice variants of the highly polymorphic CD44 (Jackson et al., 1995). The extracellular matrix, however, contains still other forms of HSPG, primarily the basement membrane proteoglycan perlecan, or HSPG2 (Iozzo, Cohen, Grassel, & Murdoch, 1994).
Most cells express multiple forms of HSPG and in brain cancer (Wade, et al., 2013) and lung cancer (Nackaerts et al., 1997) there is altered expression of multiple HSPGs. The functional role for many of these alterations in HSPG expression in cancer, however, remains largely unknown. Moreover, HSPG function is complex and is partially dependent on the oncogenic signaling pathways for a given tumor. For example, while the expression of syndecan-1 appears to be suppressed by malignant transformation in chemically induced mouse skin tumors (Inki & Jalkanen, 1996), it is critical for Wnt-1 induced tumorigenesis of the mouse mammary gland (Alexander et al., 2000)). In human pancreatic cancer cells, glypican-1 is overexpressed and is crucial for efficient tumor growth, metastasis and angiogenesis (Aikawa et al., 2008; Capurro, Xiang, Lobe, & Filmus, 2005; Kleeff et al., 1998). In hepatocellular carcinoma, glypican-3 is able to stimulate canonical Wnt signaling and promote tumor growth (Capurro, et al., 2005). In contrast, glypican-3 has been shown to inhibit cell proliferation and suppress tumor growth in lung cancer (Kim et al., 2003).
CD44, a heavily N- and O-glycosylated transmembrane molecule, is a multifunctional receptor involved in cell-cell and cell-matrix interactions. CD44 is the principal receptor for hyaluronic acid (HA), a major constituent of the brain ECM, and changes in CD44 glycosylation have a dramatic impact on HA binding. Interestingly, CD44 is overexpressed in a significant subset of human GBM (H. S. Phillips, et al., 2006; Wade, et al., 2013) and CD44 expression is used to help define a subset of human GBM with particularly poor survival (Bhat et al., 2013; Colman, et al.). Over-expression of CD44 splice variant forms has also been related to metastatic cell behavior (Hofmann et al., 1991). Together these data suggest differential, and possibly tumor-specific involvement of various HSPGs.
In human lung cancer HSPG expression is markedly altered compared to normal epithelia (Nackaerts, et al., 1997). For example, non-small-cell lung carcinomas, particularly poorly differentiated tumors, often express reduced amounts of the major cell-surface–associated HSPGs (most consistently, syndecan-1). CD44 or CD44-variant proteins, in contrast, were found on all tumor cells, irrespective of their differentiation. Interestingly, staining reactions for native HS were consistently reduced in squamous-cell lung carcinomas, in the cells in contact with the stroma and in the less differentiated areas of these tumors. However, reactions for D-HS (terminal desaturated glucuronates on the HS stubs resulting from heparinase pre-treatment), were not reduced, suggesting a structural change in the HS of these tumor cells.
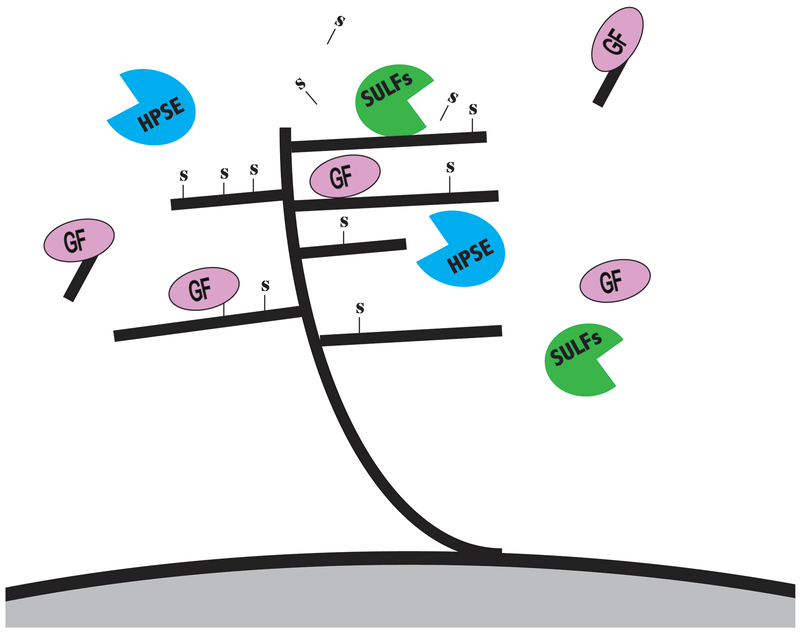
HSPGs bind to and interact with diverse ligands and a major determinant of this interaction is the glycosaminoglycan side chains, in particular the 6O-sulfation of glucosamine (6OS). Two recently discovered sulfatases (SULF1 and SULF2) provide a novel mechanism for the regulation of HSPG-dependent signaling by removing 6OS post-synthetically in the extracellular environment. SULFs are neutral pH, extracellular enzymes, which remove 6OS from intact heparin/HSPGs; they promote key signaling pathways by mobilizing protein ligands (e.g., Wnt, GDNF, PDGF-B, BMP-4) from HSPG sequestration, thus liberating the ligands for binding to signal transduction receptors (Morimoto-Tomita, Uchimura, Werb, Hemmerich, & Rosen, 2002) (Figure 2).
Figure 2. Post-synthetic enzymatic modification of heparan sulfate proteoglycans (HSPGs) helps regulate signaling of diverse protein ligands, including growth factors, morphogens, chemokines, and cytokines.
SULFs are neutral pH, extracellular enzymes, which remove 6OS from intact HS, and can promote key signaling pathways by mobilizing protein ligands (e.g., Wnt, GDNF, PDGF-B, BMP-4) from HSPG sequestration. The heparanase (HPSE) enzyme, an endo-beta-D-glucuronidase, cleaves HS chains to produce bioactive HS fragments that retain growth factor-binding activity.
One or both SULF transcripts are broadly overexpressed in many human cancers, including non-small cell lung cancer (NSCLC), glioblastoma, hepatocellular carcinoma, breast cancer, head and neck cancer, pancreatic adenocarcinoma, multiple myeloma, and gastric carcinoma (Bret, Moreaux, Schved, Hose, & Klein; Rosen & Lemjabbar-Alaoui, 2010; Wade, et al., 2013). SULF2 has been directly implicated as a driver of carcinogenesis in NSCLC (H Lemjabbar-Alaoui et al., 2010), malignant glioma, including glioblastoma and oligodendroglioma (Johansson et al., 2004; Johansson, Goransson, & Westermark, 2005; J. J. Phillips et al., 2012), pancreatic cancer (Nawroth et al., 2007)), and hepatocellular carcinoma (Lai et al., 2010). In GBM, there is robust expression of SULF2 in an important subset of human tumors, and, using knockdown and transgenic approaches, we demonstrated SULF2 promotes tumor cell proliferation, tumor growth in vivo, and the activity of multiple RTKs, including PDGFRα(J. J. Phillips, 2012; J. J. Phillips, et al., 2012). Abnormal PDGFR signaling is thought to be an early driver alteration in the vast majority of GBM (Ozawa et al., 2014) and associated with worse outcome in a subset of GBM (J. J. Phillips et al., 2013). In NSCLC, we found ((H Lemjabbar-Alaoui, et al., 2010) and unpublished work): 1) upregulation of both SULFs at the transcript level; 2) SULF2 protein expression in 96/113 (85%) human NSCLC tumors with minimal levels in normal lung; 3) SULF2 protein promotes the in vitro malignant phenotype, and the tumorigenicity in mice of SULF-2 positive human NSCLC cell lines; and 4) SULF2 promotes human lung carcinogenesis by regulation of cells surface HSPGs 6-O-sulfation status, regulation of Wnt signaling and the kinase activity of three critical receptors (i.e., EGFR, IGF-1R and cMet) (unpublished observations, H. Lemjabbar-Alaoui). Dysregulation of each of these three receptors has been causally linked to lung cancer development, progression, and increased resistance to chemotherapy (Engelman & Janne, 2008; Engelman et al., 2007; Lei, Mayotte, & Levitt, 1999). Together, these findings demonstrate that SULF2, and the corresponding alterations in HS sulfation, can regulate multiple signaling pathways important in diverse cancers.
Further evidence supporting the role for the HS side chain composition (degree of sulfation) in tumorigenicity comes from a lung metastasis model (D. Liu, Shriver, Venkataraman, El Shabrawi, & Sasisekharan, 2002) in which tumor cells had markedly reduced tumorigenicity after heparinase III but not heparinase I treatment. Saccharide fragments derived from heparinase III treatment have more of tri- and di-sulfated disaccharides, whereas the heparinase I-treated HS GAGs have more mono- and un-sulfated disaccharides. In GBM, expression of the HS biosynthetic enzyme HS3ST3a1 is increased in human GBM (Wade, et al., 2013) and in GBM cell lines (Su et al., 2006). Moreover, size exclusion chromatography/mass spectrometry (SEC-MS) demonstrated lower average sulfation levels in HS disaccharides derived from GBM versus anaplastic astrocytoma (Shao, Shi, Phillips, & Zaia, 2013).
Consistent with an oncogenic role for SULF2, methylation of the SULF2 promoter is associated with better overall survival of NSCLC patients receiving chemotherapy for advanced disease (Tessema, Yingling, & Thomaa, 2011). Additionally, advanced lung adenocarcinoma patients (Stages II–IV) with methylated SULF2 survive significantly longer than those with unmethylated SULF2. Interestingly, SULF2 methylation in lung adenocarcinoma patients is associated with a high expression of interferon-inducible gene ISG15, a marker for increased sensitivity to topoisomerase-1 inhibitors. Thus, SULF2 methylation may sensitize lung tumors to topoismerase-1 inhibitors. In keeping, SULF2 silencing dramatically enhanced sensitivity to topoismerase-1 inhibitors in SULF2 positive human lung cancer cell lines, both in vitro and in vivo (Tessema, et al., 2011). Of note, high expression of SULF1 transcript has also been reported to correlate with poorer prognosis in a cohort of 127 patients with lung adenocarcinoma compared to patients with low SULF1 expression (Bret, et al., 2011).
Similar to the SULFS, the heparanase (HPSE) enzyme modifies the GAG chains of HSPGs post-synthetically and can alter cell signaling (Figure 2). An endo-beta-D-glucuronidase, HPSE can act both at the cell-surface and within the extracellular matrix to cleave HS chains at several sites and produce large fragments that retain growth factor-binding activity. The importance of HSPGs in cancer is highlighted by the extensive literature on this subject (Parish, Freeman, & Hulett, 2001; Vlodavsky et al., 1999); Vlodavsky, 2007 #104;(McKenzie, 2007). HPSE action can influence cell signaling in several ways and the increased expression of HPSE in many cancers has been associated with promotion of tumor cell invasion, tumor cell proliferation, metastases, angiogenesis, and poor outcome (Cohen, et al., 2008; Parish, et al., 2001; Vlodavsky, et al., 1999; Vlodavsky, Ilan, Naggi, & Casu, 2007). HPSE may also promote chronic inflammation as has been demonstrated in colitis-associated colon cancer (Lerner et al., 2011).
In lung cancer, several studies have shown overexpression of HPSE in tumors (NSCLC and SCLC) (Cohen, et al., 2008; Fernandes dos Santos et al., 2014). Interestingly, depending on the cellular localization HPSE protein expression inversely correlates with lung cancer patients’ survival. Cytoplasmic heparanase is associated with poor prognosis, whereas nuclear heparanase correlates with a better survival outcome (Cohen, et al., 2008). Significant HPSE expression has also been observed in lung tumor microenvironment associated cells, such as fibroblasts, epithelial cells, and inflammatory cells. Additionally, HPSE expression correlates with tumor node metastasis (TNM) staging, invasion, metastasis and prognosis in non-small cell lung carcinoma (Fernandes dos Santos, et al., 2014). HSPGs bind to and assemble extracellular matrix (ECM) proteins (i.e., laminin, fibronectin, collagen type IV) and thereby contribute significantly to the ECM self-assembly and integrity. In carcinoma, it has been reported that HPSE activity correlates with the metastatic potential of tumor-derived cells via enhanced cell dissemination as a result of HS cleavage and remodeling of the ECM barrier (Parish, et al., 2001; Vlodavsky & Friedmann, 2001). Increased HPSE expression levels have also been identified in gliomas, including GBM (Hong, Nelson, deCarvalho, & Kalkanis, 2010).
Collectively, these data suggest that alterations in glycosylation of intracellular and extracellular molecules can promote oncogenic signaling and invasive cell behaviors in cancer, and thus may represent attractive therapeutic targets and prognostic biomarkers.
8. CLINICAL SIGNIFICANCE
As reviewed above, in both lung and brain cancer, aberrant glycosylation is common and specific changes have been associated with more aggressive disease. While additional functional studies are needed, the data suggest altered glycosylation contributes to disease and has a great diagnostic and prognostic potential. Lung cancer and brain cancer are two of the most deadly cancers, and, in those with very advanced disease, median survival from diagnosis is less than one year. Earlier diagnosis of disease using a robust biomarker would improve survival. Perhaps this is most evident in non-small cell lung cancer (NSCLC) where more than 60% of patients having lung cancer are diagnosed at late-stages when a cure is unlikely (Douillard et al., 2006). The five-year survival rate for patients with advanced disease remains dismal at less than 10%, whereas the 5-year survival rate for patients with stage I disease is greater than 70% (Siegel, et al., 2014). This is largely due to the late stage of diagnosis and the lack of effective treatments for late-stage disease. Similar to lung cancer, malignant astrocytoma, the most common of which is glioblastoma (GBM), often presents late in disease. At diagnosis, the tumor is often large and there is extensive, diffuse invasion into the surrounding brain parenchyma. Additional tumor-specific biomarkers, particularly those with a known functional relevance in disease, in lung and brain cancer would be expected to improve diagnosis and provide a needed tool to measure therapeutic response. Below we highlight some of the glycan alterations currently being pursued as potential disease biomarkers and therapeutic targets.
8.1. Biomarkers.
HSPGs and their modifying enzymes.
In NSCLC and malignant astrocytoma, the levels of specific and of total HSPGs are altered (Aviel-Ronen et al., 2008; Joensuu et al., 2002; Steck et al., 1989). For example, Syndican 1 (SDC1) levels are elevated in malignant astrocytoma (Watanabe et al., 2006) and in NSCLC (Joensuu, et al., 2002), glypican 3 levels are increased in NSCLC (Aviel-Ronen, et al., 2008), and glypican 1 levels are increased in malignant astrocytoma (Su, et al., 2006). As HSPGs are commonly shed into blood (and other body fluids), these changes can be detected in the plasma, as demonstrated for SDC1 where plasma levels in NSCLC patients are significantly greater than in controls (44 ng/ml (n=184) vs 16 ng/ml, n=100, respectively) (Joensuu, et al., 2002).
As extracellular enzymes that are both tethered to the cell membrane and secreted, the SULFs also have great potential as novel biomarkers for early detection of cancer in body fluids. Moreover, we have recently shown that SULFs are present in serum and plasma (unpublished data, S. Rosen, J. Phillips, and H. Lemjabbar-Alaoui). SULF2 alters the 6OS status of HSPGs, and we have recently demonstrated a SULF2-dependent alteration in the 6OS status of HSPGs on tumor cells (NSCLC) and in actual tumor tissues (murine model for malignant astrocytoma) (H Lemjabbar-Alaoui, et al., 2010; H. Lemjabbar-Alaoui et al., 2010; J. J. Phillips, et al., 2012). Thus, the 60S status of tumor-derived HSPGs in blood may also serve as a novel biomarker for the detection of SULF expressing cancers.
Post-synthetic modification of HS by the extracellular SULFs modulates the binding of a multiplicity of signaling molecules, including Wnts, VEGF, GDNF, and FGF-1.(Uchimura et al., 2006). It is plausible that one consequence of SULF overexpression in tumors could be the mobilization of multiple protein ligands (growth factors and chemokines) from their association with HSPGs (Uchimura, et al., 2006). These released ligands may include factors associated with tumorigenesis, such as VEGF, IL-8, and PDGF. In NSCLC, increased blood levels of VEGF and IL-8 are associated with disease progression (Laack et al., 2002; Orditura et al., 2002). In malignant astrocytoma, blood levels of VEGF are elevated relative to controls (Takano et al., 1996). While no one factor may be specific for a SULF-expressing tumor, a combination of SULF enzyme levels, altered HSPG, and altered growth factor levels might have the sensitivity and specificity to be used clinically. As described above, SULF2 expression may also have prognostic value in predicting lung cancer patients’ response to already approved topoisomerase-1 inhibitors, and in selecting patients who may benefit the most from this type of therapy (Tessema, et al., 2011).
Similar to SULFs and as reviewed above, HPSE and its activity-associated byproducts may have a clinical diagnostic and prognostic value in the treatment of lung and brain cancer. From a prognostic standpoint, the presence of cytoplasmic HPSE in tumor cells correlates with poor prognosis, while nuclear HPSE associates with a better survival outcome of lung cancer patients (Cohen, et al., 2008). Furthermore, an investigation of the relationship of the serum heparanase and VEGF levels with the clinicopathological characteristics of NSCLC showed that serum HPSE and VEGF levels are significantly higher In NSCLC patients compared to healthy individuals. Moreover, levels were highest in patients with poorly differentiated, clinical stage III and IV tumors with lymph node-positive disease (ChuanMing G, 2009). High serum levels of HPSE and VEGF correlated with increased NSCLC tumor growth, invasion and metastasis while gender, age, and pathological type were not associated with HPSE serum levels.
Mucins.
Several studies have established an altered status of mucin glycosylation in lung cancer tumors, as well as the potential use of mucins as prognostic indicators in lung cancer patients (Guddo, et al., 1998; Nagai, et al., 2006; Tsutsumida, et al., 2004). One such example is MUC1 whose overexpression in squamous-cell cancer of the lung is associated with poor survival (Guddo, et al., 1998). Interestingly, expression of the aberrantly glycosylated tumor-associated MUC1 is a positive prognostic factor in patients with metastatic NSCLC (Kuemmel, et al., 2009). MUC4 is another mucin with a diagnostic potential for lung cancer. Positive MUC4 protein expression has been shown in 72% of Lung carcinoma tissues (Hanaoka et al., 2001). Additionally, MUC4 expression may be an indicator of poor prognosis in lung adenocarcinoma. Moreover, MUC4 overexpression can differentiate lung adenocarcinoma from malignant mesothelioma, with sensitivity and specificity, 91.4% s and 100% respectively (Llinares et al., 2004).
Fucosylation.
Increased expression of sialyl Lewis X or A antigens on metastatic cancer cells leads to their selectin-mediated extravasation (Borsig, Wong, Hynes, Varki, & Varki, 2002). In addition, overexpression of sialylated and fucosylated glycans on tumor cells is associated with poor prognosis in several cancers, including lung adenocarcinoma (Nangia-Makker, Conklin, Hogan, & Raz, 2002). Profound fucosylation of the serum microenvironment, probably caused by tumor shedding of fucosylated glycoproteins, may disrupt adhesion and contribute to metastases. An increase (about 2 fold) in fucosylated glycans in NSCLC and SCLC sera, compared to sera from healthy individuals, has been documented in several investigations (Kossowska, Ferens-Sieczkowska, Gancarz, Passowicz-Muszynska, & Jankowska, 2005). Furthermore, it appears that the fucosylation status of sera glycans may serve as a predictive factor for patient survival (Kossowska, et al., 2005). Together, these results suggest that the fucosylation pattern of serum proteins may be promising in the search for prognostic factors, which might aid in selecting the most suitable therapy and predicting the probable course of lung cancer.
Glycosylation of serum proteins.
Most serum proteins are glycosylated, and cancer-associated alterations in serum protein glycosylation could be a useful biomarker for disease. IgG, one of the most important components of serum proteins, is known to facilitate a vast repertoire of blood immunological processes and responses through the interactions of its Fc region with other proteins. The Fc region of IgG molecules is N-glycosylated, and interactions of IgG Fc region with Fc receptors are augmented by N-linked glycosylation at Asn-297. These interactions are critical to fundamental processes such as the antibody-dependent cell-mediated cytotoxicity (ADCC), antibody-dependent cellular phagocytosis, release of inflammation-associated mediator molecules (Arnold, Wormald, Sim, Rudd, & Dwek, 2007), and complement dependent cytotoxicity (Chan & Carter, 2010). Indeed, changes in Fc-glycosylation can affect the biological activity of the IgG. Lack of core fucose can promote enhanced ADCC activity and elevated bisecting N-acetylglucosamine (GlcNAc) can increase ADCC activity (Hodoniczky, Zheng, & James, 2005). The specificity of these interactions is highlighted by the functional shift from a pro-inflammatory to an anti-inflammatory effect with an increase in IgG Fc-sialylation with 2, 6-linkage to penultimate galactose (Anthony et al., 2008). In lung cancer, a marked increase in sera IgG1 Fc-agalactosylation (decrease in galactosylation) compared to healthy individuals has been reported (Chen, et al., 2013). Additionally, the incidence of galactosyl-related Fc-glycosylation of sera IgG1 is significantly lower in the lung cancer patients compared to controls. These observed changes in IgG Fc-glycosylation in lung cancer are age and gender dependent. Importantly, the results of this study showed that Fc-glycosylation has the potential to discriminate patients with lung cancer from healthy controls (Chen, et al., 2013). In addition, this sex- and age-dependent diagnostic ability of IgG1 Fc-glycosylation is more significant in young (age ≤ 51 years) and senior (age ≥79 years) people. These changes in IgG Fc-glycosylation may echo the human immunological and pathological states in lung cancer patients.
8.2. Therapeutics
HSPGs modifying enzymes (i.e., HPSE and SULFs) are promising therapeutic targets for cancer. By regulating the bioactivity of multiple HSPG-binding ligands, these modifying enzymes may be a hub in the network of signaling pathways critical for cancer development and progression. A substantial body of literature has supported the potential of heparanase as a viable therapeutic target, and thus led to the development of a number of HPSE inhibitors, including small molecules, carbohydrate-based HS mimetics, natural product inhibitors and neutralizing antibodies. Several compounds have been shown to inhibit HPSE activity and its associated protumorigenic activities in preclinical settings (McKenzie, 2007). For example, Suramin (a polysulphonated napthylurea) and its analogues have been shown to inhibit purified HPSE activity as well as the cellular invasiveness, angiogenesis and the metastatic potential of cancer cells (Marchetti, Reiland, Erwin, & Roy, 2003). However, it has been suggested that suramin may have a dual inhibitory role by also binding growth factors and subsequently competitively blocking the interactions of these factors with respective receptors (Pesenti, Sola, Mongelli, Grandi, & Spreafico, 1992).
Another example of targeted anti-HPSE therapies is sulfated phosphomannopentaose (PI-88), a heparin mimetic, which consists of chemically sulfated yeast oligosaccharides (≈2000 daltons) (Khachigian & Parish, 2004; Vlodavsky, et al., 2007). While PI-88 was initially characterized as an inhibitor of HSPE, it has also been found to inhibit the enzymatic activity of both SULFs at comparable potencies found for HPSE (Hossain et al., 2010). Similar to Suramin, PI-88 has been shown to interfere with the binding or action of HS-bound growth factors (Ferro, Hammond, & Fairweather, 2004). In general, the heparan sulfate mimetics, which are highly sulfated oligosaccharides, can inhibit heparanase enzymatic activity, sequester HSPG-binding factors, and inhibit SULF2 (Dredge et al., 2011; Hossain, et al., 2010; Johnstone et al., 2010). In pre-clinical studies, HS mimetics have effectively targeted multiple HSPG-dependent phenotypes and have resulted in decreased tumor growth, tumor invasion, tumor metastasis, and angiogenesis making them attractive therapeutic agents (Joyce, Freeman, Meyer-Morse, Parish, & Hanahan, 2005; H. Zhou et al., 2011). PI-88 has been tested in clinical trials for advanced melanoma (Phase II) and post-resection liver cancer (Phase III) (http://www.progen.com.au/pipeline/) (McKenzie, 2007);(C. J. Liu et al., 2009). Clinical trials in NSCLC and prostate cancer have also been carried out (Kudchadkar, Gonzalez, & Lewis, 2008). However, a recurring and forbidding problem with PI-88 is the development of thrombocytopenia in recipients (Kudchadkar, et al., 2008). Recent pre-clinical studies of a new rationally engineered HS mimetic, M402, suggest potential as a therapeutic agent (H. Zhou, et al., 2011).
Heparan sulfate mimetics have the potential to inhibit both HPSE and SULF function in cancer. Select targeting of the SULFs may also be a useful therapeutic strategy. From our published work on Wnt signaling and PDGFRA signaling, and our unpublished work on EGFR signaling, SULF-2 regulates a number of pathways, which are subject to aberrant activation in cancer (H. Lemjabbar-Alaoui, et al., 2010; Nawroth, et al., 2007; Wade, et al., 2013). This regulation is exerted proximal to the interaction of growth factors with receptor tyrosine kinases (RTKs) and the activation of intracellular kinases. Thus, the SULFs are upstream of the receptor tyrosine kinases, which dominate current approaches to cancer therapeutics, and therefore, represent a highly novel cancer target. As an extracellular enzyme, the SULFs are amenable to inhibition by either small molecules or functional neutralization by antibodies.
Altered glycosylation patterns in tumor may also generate tumor-specific antigens that can be exploited for therapy. Particular examples include vaccines against the mucin MUC1. TG4010 is an anti-MUC1 vaccine that uses a recombinant vaccinia virus (modified vaccinia virus of Ankara, MVA), combining the human MUC1 and interleukin-2 coding sequences (De Pas et al., 2012). A randomized phase II study for advanced NSCLC patients with confirmed MUC1 expression compared the use of TG4010 combined with chemotherapy (cisplatin and vinorelbine), to TG4010 as monotherapy and subsequent treatment with TG4010 plus chemotherapy (Ramlau et al., 2008). The median survival rates in the two groups were 12.7 vs. 14.9 months, respectively. Further analysis showed that a subgroup of patients with a detectable CD8+ T-cell response generated an immune response against MUC1 and had longer median survival. A small randomized phase II study for advanced NSCLC patients compared combining this vaccine with chemotherapy (cisplatin and vinorelbine) (Quoix et al., 2011). The results from this study showed that the progression-free survival after 6 months was slightly improved for the vaccine group (43.2 vs. 35.1 %, P= 0.307), albeit not significantly. Additionally, the effect on median overall survival (10.7 vs. 10.3 months) did not reach statistical significance (Quoix, et al., 2011). It is hoped that additional tumor-specific glycan epitopes will be targeted with this type of approach.
REFERENCES
- Aikawa T, Whipple CA, Lopez ME, Gunn J, Young A, Lander AD, & Korc M (2008). Glypican-1 modulates the angiogenic and metastatic potential of human and mouse cancer cells. Journal of Clinical Investigation, 118(1), 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander CM, Reichsman F, Hinkes MT, Lincecum J, Becker KA, Cumberledge S, & Bernfield M (2000). Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nature Genetics, 25(3), 329–332. [DOI] [PubMed] [Google Scholar]
- Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, & Ravetch JV (2008). Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science, 320(5874), 373–376. doi: 10.1126/science.1154315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JN, Wormald MR, Sim RB, Rudd PM, & Dwek RA (2007). The impact of glycosylation on the biological function and structure of human immunoglobulins. Annual Review of Immunology, 25, 21–50. doi: 10.1146/annurev.immunol.25.022106.141702 [DOI] [PubMed] [Google Scholar]
- Asada M, Furukawa K, Segawa K, Endo T, & Kobata A (1997). Increased expression of highly branched N-glycans at cell surface is correlated with the malignant phenotypes of mouse tumor cells. Cancer Research, 57(6), 1073–1080. [PubMed] [Google Scholar]
- Aviel-Ronen S, Lau SK, Pintilie M, Lau D, Liu N, Tsao MS, & Jothy S (2008). Glypican-3 is overexpressed in lung squamous cell carcinoma, but not in adenocarcinoma. Modern Pathology, 21(7), 817–825. [DOI] [PubMed] [Google Scholar]
- Becker DJ, & Lowe JB (2003). Fucose: biosynthesis and biological function in mammals. Glycobiology, 13(7), 41R–53R. doi: 10.1093/glycob/cwg054 [DOI] [PubMed] [Google Scholar]
- Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, & Lose EJ (1992). Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annual Review of Cell Biology, 8, 365–393. doi: 10.1146/annurev.cb.08.110192.002053 [DOI] [PubMed] [Google Scholar]
- Bevilacqua MP, & Nelson RM (1993). Selectins. Journal of Clinical Investigation, 91(2), 379–387. doi: 10.1172/JCI116210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat KP, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, Aldape K (2013). Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer cell, 24(3), 331–346. doi: 10.1016/j.ccr.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsig L, Wong R, Hynes RO, Varki NM, & Varki A (2002). Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proceedings of the National Academy of Sciences of the United States of America, 99(4), 2193–2198. doi: 10.1073/pnas.261704098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan C, Momota H, Hambardzumyan D, Ozawa T, Tandon A, Pedraza A, & Holland E (2009). Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS One, 4(11), e7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bret C, Moreaux J, Schved JF, Hose D, & Klein B (2011). SULFs in human neoplasia: implication as progression and prognosis factors. J Transl Med, 9, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Blohm D, Ghadimi BM, Stosiek P, Xing PX, & Karsten U (1997). Mucins (MUC1 and MUC3) of gastrointestinal and breast epithelia reveal different and heterogeneous tumor-associated aberrations in glycosylation. Journal of Histochemistry and Cytochemistry, 45(11), 1547–1557. [DOI] [PubMed] [Google Scholar]
- Capela A, & Temple S (2002). LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron, 35(5), 865–875. [DOI] [PubMed] [Google Scholar]
- Capela A, & Temple S (2006). LeX is expressed by principle progenitor cells in the embryonic nervous system, is secreted into their environment and binds Wnt-1. Developmental Biology, 291(2), 300–313. doi: 10.1016/j.ydbio.2005.12.030 [DOI] [PubMed] [Google Scholar]
- Capurro MI, Xiang YY, Lobe C, & Filmus J (2005). Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Research, 65(14), 6245–6254. [DOI] [PubMed] [Google Scholar]
- Chan AC, & Carter PJ (2010). Therapeutic antibodies for autoimmunity and inflammation. Nature reviews. Immunology, 10(5), 301–316. doi: 10.1038/nri2761 [DOI] [PubMed] [Google Scholar]
- Chen CY, Jan YH, Juan YH, Yang CJ, Huang MS, Yu CJ, Wong CH (2013). Fucosyltransferase 8 as a functional regulator of nonsmall cell lung cancer. Proceedings of the National Academy of Sciences of the United States of America, 110(2), 630–635. doi: 10.1073/pnas.1220425110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TY, Hart GW, & Dang CV (1995). c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. Journal of Biological Chemistry, 270(32), 18961–18965. [DOI] [PubMed] [Google Scholar]
- Christofk HR, Vander Heiden MG, Wu N, Asara JM, & Cantley LC (2008). Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature, 452(7184), 181–186. doi: 10.1038/nature06667 [DOI] [PubMed] [Google Scholar]
- ChuanMing G LQ, Tao HH, Tao Z (2009). Clinical significance of detection of serum heparanase and vascular endothelial growth factor in patients with non-small cell lung cancer. Progress in Modern Biomedicine, 9(21), 4099–4101. [Google Scholar]
- Clark PM, Dweck JF, Mason DE, Hart CR, Buck SB, Peters EC, Hsieh-Wilson LC (2008). Direct in-gel fluorescence detection and cellular imaging of O-GlcNAc-modified proteins. Journal of the American Chemical Society, 130(35), 11576–11577. doi: 10.1021/ja8030467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Doweck I, Naroditsky I, Ben-Izhak O, Kremer R, Best LA, Ilan N (2008). Heparanase is overexpressed in lung cancer and correlates inversely with patient survival. Cancer, 113(5), 1004–1011. doi: 10.1002/cncr.23680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman H, Zhang L, Sulman EP, McDonald JM, Shooshtari NL, Rivera A, Aldape K A multigene predictor of outcome in glioblastoma. Neuro Oncol, 12(1), 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copin MC, Devisme L, Buisine MP, Marquette CH, Wurtz A, Aubert JP, Porchet N (2000). From normal respiratory mucosa to epidermoid carcinoma: expression of human mucin genes. International Journal of Cancer, 86(2), 162–168. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Paulson JC, & Varki A (2007). Siglecs and their roles in the immune system. Nature reviews. Immunology, 7(4), 255–266. doi: 10.1038/nri2056 [DOI] [PubMed] [Google Scholar]
- David G (1993). Integral membrane heparan sulfate proteoglycans. FASEB Journal, 7(11), 1023–1030. [DOI] [PubMed] [Google Scholar]
- De Pas T, Giovannini M, Rescigno M, Catania C, Toffalorio F, Spitaleri G, De Braud F (2012). Vaccines in non-small cell lung cancer: rationale, combination strategies and update on clinical trials. Critical Reviews in Oncology/Hematology, 83(3), 432–443. doi: 10.1016/j.critrevonc.2011.12.005 [DOI] [PubMed] [Google Scholar]
- Demetriou M, Nabi IR, Coppolino M, Dedhar S, & Dennis JW (1995). Reduced contact-inhibition and substratum adhesion in epithelial cells expressing GlcNAc-transferase V. The Journal of cell biology, 130(2), 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis JW, Laferte S, Waghorne C, Breitman ML, & Kerbel RS (1987). Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science, 236(4801), 582–585. [DOI] [PubMed] [Google Scholar]
- Dennis JW, Waller CA, & Schirrmacher V (1984). Identification of asparagine-linked oligosaccharides involved in tumor cell adhesion to laminin and type IV collagen. J Cell Biology, 99(4 Pt 1), 1416–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentin R, Hedrick S, Xie J, Yates J 3rd, & Montminy M (2008). Hepatic glucose sensing via the CREB coactivator CRTC2. Science, 319(5868), 1402–1405. doi: 10.1126/science.1151363 [DOI] [PubMed] [Google Scholar]
- Dong Y, Walsh MD, Cummings MC, Wright RG, Khoo SK, Parsons PG, & McGuckin MA (1997). Expression of MUC1 and MUC2 mucins in epithelial ovarian tumours. Journal of Pathology, 183(3), 311–317. doi: [DOI] [PubMed] [Google Scholar]
- Dosaka-Akita H, Miyoshi E, Suzuki O, Itoh T, Katoh H, & Taniguchi N (2004). Expression of N-acetylglucosaminyltransferase v is associated with prognosis and histology in non-small cell lung cancers. Clinical Cancer Research, 10(5), 1773–1779. [DOI] [PubMed] [Google Scholar]
- Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzales-Larriba JL, Hurteloup P (2006). Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. The Lancet. Oncology, 7(9), 719–727. doi: 10.1016/S1470-2045(06)70804-X [DOI] [PubMed] [Google Scholar]
- Dredge K, Hammond E, Handley P, Gonda TJ, Smith MT, Vincent C, Bytheway I (2011). PG545, a dual heparanase and angiogenesis inhibitor, induces potent anti-tumour and anti-metastatic efficacy in preclinical models. British Journal of Cancer, 104(4), 635–642. doi: 10.1038/bjc.2011.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube DH, & Bertozzi CR (2005). Glycans in cancer and inflammation--potential for therapeutics and diagnostics. Nat Rev Drug Discov, 4(6), 477–488. doi: 10.1038/nrd1751 [DOI] [PubMed] [Google Scholar]
- El-Battari A, Prorok M, Angata K, Mathieu S, Zerfaoui M, Ong E, Fukuda M (2003). Different glycosyltransferases are differentially processed for secretion, dimerization, and autoglycosylation. Glycobiology, 13(12), 941–953. doi: 10.1093/glycob/cwg117 [DOI] [PubMed] [Google Scholar]
- Engelman JA, & Janne PA (2008). Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clinical Cancer Research, 14(10), 2895–2899. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Janne PA (2007). MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science, 316(5827), 1039–1043. [DOI] [PubMed] [Google Scholar]
- Esko JD, & Lindahl U (2001). Molecular diversity of heparan sulfate. Journal of Clinical Investigation, 108(2), 169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes B, Sagman U, Auger M, Demetrio M, & Dennis JW (1991). Beta 1-6 branched oligosaccharides as a marker of tumor progression in human breast and colon neoplasia. Cancer Research, 51(2), 718–723. [PubMed] [Google Scholar]
- Fernandes dos Santos TC, Gomes AM, Paschoal ME, Stelling MP, Rumjanek VM, Junior Ado R, … Castelo-Branco MT (2014). Heparanase expression and localization in different types of human lung cancer. Biochimica et Biophysica Acta, 1840(8), 2599–2608. doi: 10.1016/j.bbagen.2014.04.010 [DOI] [PubMed] [Google Scholar]
- Ferro V, Hammond E, & Fairweather JK (2004). The development of inhibitors of heparanase, a key enzyme involved in tumour metastasis, angiogenesis and inflammation. Mini reviews in medicinal chemistry, 4(6), 693–702. [DOI] [PubMed] [Google Scholar]
- Fogel M, Altevogt P, & Schirrmacher V (1983). Metastatic potential severely altered by changes in tumor cell adhesiveness and cell-surface sialylation. The Journal of experimental medicine, 157(1), 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M (1996). Possible roles of tumor-associated carbohydrate antigens. Cancer Research, 56(10), 2237–2244. [PubMed] [Google Scholar]
- Fukuda M (2002). Roles of mucin-type O-glycans in cell adhesion. Biochimica et Biophysica Acta, 1573(3), 394–405. [DOI] [PubMed] [Google Scholar]
- Gao J, McConnell MJ, Yu B, Li J, Balko JM, Black EP, Haura EB (2009). MUC1 is a downstream target of STAT3 and regulates lung cancer cell survival and invasion. International Journal of Oncology, 35(2), 337–345. [PMC free article] [PubMed] [Google Scholar]
- Gatchev O, Rastam L, Lindberg G, Gullberg B, Eklund GA, & Tornberg S (1993). Tumours of the central nervous system and serum sialic acid concentration in men and women. British Journal of Cancer, 68(2), 425–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gökmen SS, Kazezoğlu C, Tabakoğlu E, Altıay G, Güngör O, & Türe M (2004). Serum Total Sialic Acid Levels in Lung Cancer Patients of Different Histological Types with and No Extrapulmonary Metastases. Turkish Journal of Biochemistry, 29(4), 262–267. [Google Scholar]
- Guddo F, Giatromanolaki A, Koukourakis MI, Reina C, Vignola AM, Chlouverakis G, Bonsignore G (1998). MUC1 (episialin) expression in non-small cell lung cancer is independent of EGFR and c-erbB-2 expression and correlates with poor survival in node positive patients. Journal of Clinical Pathology, 51(9), 667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S (1989). Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Advances in Cancer Research, 52, 257–331. [DOI] [PubMed] [Google Scholar]
- Hakomori SI, & Cummings RD (2012). Glycosylation effects on cancer development. Glycoconjugate Journal, 29(8-9), 565–566. doi: 10.1007/s10719-012-9448-4 [DOI] [PubMed] [Google Scholar]
- Hanaoka J, Kontani K, Sawai S, Ichinose M, Tezuka N, Inoue S, Ohkubo I (2001). Analysis of MUC4 mucin expression in lung carcinoma cells and its immunogenicity. Cancer, 92(8), 2148–2157. [DOI] [PubMed] [Google Scholar]
- Hart GW, Housley MP, & Slawson C (2007). Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature, 446(7139), 1017–1022. doi: 10.1038/nature05815 [DOI] [PubMed] [Google Scholar]
- Hedlund M, Ng E, Varki A, & Varki NM (2008). alpha 2-6-Linked sialic acids on N-glycans modulate carcinoma differentiation in vivo. Cancer Research, 68(2), 388–394. doi: 10.1158/0008-5472.CAN-07-1340 [DOI] [PubMed] [Google Scholar]
- Hess KR, Broglio KR, & Bondy ML (2004). Adult glioma incidence trends in the United States, 1977-2000. Cancer, 101(10), 2293–2299. [DOI] [PubMed] [Google Scholar]
- Hodoniczky J, Zheng YZ, & James DC (2005). Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnology Progress, 21(6), 1644–1652. doi: 10.1021/bp050228w [DOI] [PubMed] [Google Scholar]
- Hofmann M, Rudy W, Zoller M, Tolg C, Ponta H, Herrlich P, & Gunthert U (1991). CD44 splice variants confer metastatic behavior in rats: homologous sequences are expressed in human tumor cell lines. Cancer Research, 51(19), 5292–5297. [PubMed] [Google Scholar]
- Holmes EH, Ostrander GK, & Hakomori S (1986). Biosynthesis of the sialyl-Lex determinant carried by type 2 chain glycosphingolipids (IV3NeuAcIII3FucnLc4, VI3NeuAcV3FucnLc6, and VI3NeuAcIII3V3Fuc2nLc6) in human lung carcinoma PC9 cells. Journal of Biological Chemistry, 261(8), 3737–3743. [PubMed] [Google Scholar]
- Hong X, Nelson KK, deCarvalho AC, & Kalkanis SN (2010). Heparanase expression of glioma in human and animal models. Journal of Neurosurgery, 113(2), 261–269. doi: 10.3171/2009.9.JNS09682 [DOI] [PubMed] [Google Scholar]
- Hossain MM, Hosono-Fukao T, Tang R, Sugaya N, van Kuppevelt TH, Jenniskens GJ, Uchimura K (2010). Direct detection of HSulf-1 and HSulf-2 activities on extracellular heparan sulfate and their inhibition by PI-88. Glycobiology, 20(2), 175–186. doi: 10.1093/glycob/cwp159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak JE, Canham SM, & Bertozzi CR (2014). Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nature chemical biology, 10(1), 69–75. doi: 10.1038/nchembio.1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inki P, & Jalkanen M (1996). The role of syndecan-1 in malignancies. Annals of Medicine, 28(1), 63–67. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Cohen IR, Grassel S, & Murdoch AD (1994). The biology of perlecan: the multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochemical Journal, 302 (Pt 3), 625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DG, Bell JI, Dickinson R, Timans J, Shields J, & Whittle N (1995). Proteoglycan forms of the lymphocyte homing receptor CD44 are alternatively spliced variants containing the v3 exon. Journal of Cell Biology, 128(4), 673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard JA, Linnoila RI, Lee H, Steinberg SM, Witschi H, & Szabo E (1998). MUC1 is a novel marker for the type II pneumocyte lineage during lung carcinogenesis. Cancer Research, 58(23), 5582–5589. [PubMed] [Google Scholar]
- Joensuu H, Anttonen A, Eriksson M, Makitaro R, Alfthan H, Kinnula V, & Leppa S (2002). Soluble syndecan-1 and serum basic fibroblast growth factor are new prognostic factors in lung cancer. Cancer Research, 62(18), 5210–5217. [PubMed] [Google Scholar]
- Johansson FK, Brodd J, Eklof C, Ferletta M, Hesselager G, Tiger CF, Westermark B (2004). Identification of candidate cancer-causing genes in mouse brain tumors by retroviral tagging. Proceedings of the National Academy of Sciences of the United States of America, 101(31), 11334–11337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson FK, Goransson H, & Westermark B (2005). Expression analysis of genes involved in brain tumor progression driven by retroviral insertional mutagenesis in mice. Oncogene, 24(24), 3896–3905. [DOI] [PubMed] [Google Scholar]
- Johnstone KD, Karoli T, Liu L, Dredge K, Copeman E, Li CP, Ferro V (2010). Synthesis and biological evaluation of polysulfated oligosaccharide glycosides as inhibitors of angiogenesis and tumor growth. Journal of Medicinal Chemistry, 53(4), 1686–1699. doi: 10.1021/jm901449m [DOI] [PubMed] [Google Scholar]
- Joyce JA, Freeman C, Meyer-Morse N, Parish CR, & Hanahan D (2005). A functional heparan sulfate mimetic implicates both heparanase and heparan sulfate in tumor angiogenesis and invasion in a mouse model of multistage cancer. Oncogene, 24(25), 4037–4051. [DOI] [PubMed] [Google Scholar]
- Kakari S, Stringou E, Toumbis M, Ferderigos AS, Poulaki E, Chondros K, Pavlidis N (1991). Five tumor markers in lung cancer: significance of total and “lipid”-bound sialic acid. Anticancer Research, 11(6), 2107–2110. [PubMed] [Google Scholar]
- Kaneko Y, Yamamoto H, Kersey DS, Colley KJ, Leestma JE, & Moskal JR (1996). The expression of Gal beta 1,4GlcNAc alpha 2,6 sialyltransferase and alpha 2,6-linked sialoglycoconjugates in human brain tumors. Acta Neuropathologica, 91(3), 284–292. [DOI] [PubMed] [Google Scholar]
- Kawai T, Suzuki M, Kase K, & Ozeki Y (1993). Expression of carbohydrate antigens in human pulmonary adenocarcinoma. Cancer, 72(5), 1581–1587. [DOI] [PubMed] [Google Scholar]
- Kelm S, & Schauer R (1997). Sialic acids in molecular and cellular interactions. International Review of Cytology, 175, 137–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachigian LM, & Parish CR (2004). Phosphomannopentaose sulfate (PI-88): heparan sulfate mimetic with clinical potential in multiple vascular pathologies. Cardiovasc Drug Rev, 22(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Khodarev NN, Pitroda SP, Beckett MA, MacDermed DM, Huang L, Kufe DW, & Weichselbaum RR (2009). MUC1-induced transcriptional programs associated with tumorigenesis predict outcome in breast and lung cancer. Cancer Research, 69(7), 2833–2837. doi: 10.1158/0008-5472.CAN-08-4513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Xu GL, Borczuk AC, Busch S, Filmus J, Capurro M, Powell CA (2003). The heparan sulfate proteoglycan GPC3 is a potential lung tumor suppressor. American Journal of Respiratory Cell and Molecular Biology, 29(6), 694–701. doi: 10.1165/rcmb.2003-0061OC [DOI] [PubMed] [Google Scholar]
- Kleeff J, Ishiwata T, Kumbasar A, Friess H, Buchler MW, Lander AD, & Korc M (1998). The cell-surface heparan sulfate proteoglycan glypican-1 regulates growth factor action in pancreatic carcinoma cells and is overexpressed in human pancreatic cancer. Journal of Clinical Investigation, 102(9), 1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneass ZT, & Marchase RB (2004). Neutrophils exhibit rapid agonist-induced increases in protein-associated O-GlcNAc. Journal of Biological Chemistry, 279(44), 45759–45765. doi: 10.1074/jbc.M407911200 [DOI] [PubMed] [Google Scholar]
- Komminoth P, Roth J, Lackie PM, Bitter-Suermann D, & Heitz PU (1991). Polysialic acid of the neural cell adhesion molecule distinguishes small cell lung carcinoma from carcinoids. American Journal of Pathology, 139(2), 297–304. [PMC free article] [PubMed] [Google Scholar]
- Kossowska B, Ferens-Sieczkowska M, Gancarz R, Passowicz-Muszynska E, & Jankowska R (2005). Fucosylation of serum glycoproteins in lung cancer patients. Clinical chemistry and laboratory medicine : CCLM / FESCC, 43(4), 361–369. doi: 10.1515/CCLM.2005.066 [DOI] [PubMed] [Google Scholar]
- Kroes RA, Dawson G, & Moskal JR (2007). Focused microarray analysis of glyco-gene expression in human glioblastomas. Journal of Neurochemistry, 103 Suppl 1, 14–24. doi: 10.1111/j.1471-4159.2007.04780.x [DOI] [PubMed] [Google Scholar]
- Kudchadkar R, Gonzalez R, & Lewis KD (2008). PI-88: a novel inhibitor of angiogenesis. Expert Opin Investig Drugs, 17(11), 1769–1776. [DOI] [PubMed] [Google Scholar]
- Kuemmel A, Single K, Bittinger F, Faldum A, Schmidt LH, Sebastian M, Wiewrodt R (2009). TA-MUC1 epitope in non-small cell lung cancer. Lung Cancer, 63(1), 98–105. doi: 10.1016/j.lungcan.2008.04.005 [DOI] [PubMed] [Google Scholar]
- Kufe DW (2009). Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer, 9(12), 874–885. doi: 10.1038/nrc2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laack E, Kohler A, Kugler C, Dierlamm T, Knuffmann C, Vohwinkel G, Hossfeld DK (2002). Pretreatment serum levels of matrix metalloproteinase-9 and vascular endothelial growth factor in non-small-cell lung cancer. Annals of Oncology, 13(10), 1550–1557. [DOI] [PubMed] [Google Scholar]
- Lai JP, Oseini AM, Moser CD, Yu C, Elsawa SF, Hu C, Roberts LR (2010). The oncogenic effect of sulfatase 2 in human hepatocellular carcinoma is mediated in part by glypican 3-dependent Wnt activation. Hepatology, 52(5), 1680–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Mayotte JE, & Levitt ML (1999). Enhancement of chemosensitivity and programmed cell death by tyrosine kinase inhibitors correlates with EGFR expression in non-small cell lung cancer cells. Anticancer Research, 19(1A), 221–228. [PubMed] [Google Scholar]
- Lemjabbar-Alaoui H, van Zante A, Singer M, Xue Q, Wang Y, & Tsay D (2010). Sulf-2, a heparan sulfate endosulfatase, promotes human lung carcinogenesis. Oncogene, 29, 635 – 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemjabbar-Alaoui H, van Zante A, Singer MS, Xue Q, Wang YQ, Tsay D, Rosen SD (2010). Sulf-2, a heparan sulfate endosulfatase, promotes human lung carcinogenesis. Oncogene, 29(5), 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner I, Hermano E, Zcharia E, Rodkin D, Bulvik R, Doviner V, Elkin M (2011). Heparanase powers a chronic inflammatory circuit that promotes colitis-associated tumorigenesis in mice. The Journal of clinical investigation, 121(5), 1709–1721. doi: 10.1172/JCI43792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WP, & Roth J (1997). Expression of beta 1,6 branched asparagine-linked oligosaccharides in non-mitotic and non-migratory cells of normal human and rat tissues. International Journal of Cancer, 71(3), 483–490. [DOI] [PubMed] [Google Scholar]
- Liang Y, Haring M, Roughley PJ, Margolis RK, & Margolis RU (1997). Glypican and biglycan in the nuclei of neurons and glioma cells: presence of functional nuclear localization signals and dynamic changes in glypican during the cell cycle. The Journal of cell biology, 139(4), 851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligtenberg MJ, Buijs F, Vos HL, & Hilkens J (1992). Suppression of cellular aggregation by high levels of episialin. Cancer Research, 52(8), 2318–2324. [PubMed] [Google Scholar]
- Liu CJ, Lee PH, Lin DY, Wu CC, Jeng LB, Lin PW, Chen PJ (2009). Heparanase inhibitor PI-88 as adjuvant therapy for hepatocellular carcinoma after curative resection: a randomized phase II trial for safety and optimal dosage. Journal of Hepatology, 50(5), 958–968. doi: 10.1016/j.jhep.2008.12.023 [DOI] [PubMed] [Google Scholar]
- Liu D, Shriver Z, Venkataraman G, El Shabrawi Y, & Sasisekharan R (2002). Tumor cell surface heparan sulfate as cryptic promoters or inhibitors of tumor growth and metastasis. Proceedings of the National Academy of Sciences of the United States of America, 99(2), 568–573. doi: 10.1073/pnas.012578299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YC, Yen HY, Chen CY, Chen CH, Cheng PF, Juan YH, Wong CH (2011). Sialylation and fucosylation of epidermal growth factor receptor suppress its dimerization and activation in lung cancer cells. Proceedings of the National Academy of Sciences of the United States of America, 108(28), 11332–11337. doi: 10.1073/pnas.1107385108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinares K, Escande F, Aubert S, Buisine MP, de Bolos C, Batra SK, Copin MC (2004). Diagnostic value of MUC4 immunostaining in distinguishing epithelial mesothelioma and lung adenocarcinoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc, 17(2), 150–157. doi: 10.1038/modpathol.3800027 [DOI] [PubMed] [Google Scholar]
- Longenecker BM, Rahman AF, Leigh JB, Purser RA, Greenberg AH, Willans DJ, et al. (1984). Monoclonal antibody against a cryptic carbohydrate antigen of murine and human lymphocytes. I. Antigen expression in non-cryptic or unsubstituted form on certain murine lymphomas, on a spontaneous murine mammary carcinoma, and on several human adenocarcinomas. International Journal of Cancer, 33(1), 123–129. [DOI] [PubMed] [Google Scholar]
- Lopez-Ferrer A, Barranco C, & de Bolos C (2002). Differences in the O-glycosylation patterns between lung squamous cell carcinoma and adenocarcinoma. American Journal of Clinical Pathology, 118(5), 749–755. doi: 10.1309/LWP3-MFA8-8KX7-60YQ [DOI] [PubMed] [Google Scholar]
- Lopez-Ferrer A, Curull V, Barranco C, Garrido M, Lloreta J, Real FX, & de Bolos C (2001). Mucins as differentiation markers in bronchial epithelium. Squamous cell carcinoma and adenocarcinoma display similar expression patterns. American Journal of Respiratory Cell and Molecular Biology, 24(1), 22–29. doi: 10.1165/ajrcmb.24.1.4294 [DOI] [PubMed] [Google Scholar]
- Love DC, & Hanover JA (2005). The hexosamine signaling pathway: deciphering the “O-GlcNAc code”. Sci STKE, 2005(312), re13. doi: 10.1126/stke.3122005re13 [DOI] [PubMed] [Google Scholar]
- Lowe JB (1997). Selectin ligands, leukocyte trafficking, and fucosyltransferase genes. Kidney International, 51(5), 1418–1426. [DOI] [PubMed] [Google Scholar]
- Ma B, Simala-Grant JL, & Taylor DE (2006). Fucosylation in prokaryotes and eukaryotes. Glycobiology, 16(12), 158R–184R. doi: 10.1093/glycob/cwl040 [DOI] [PubMed] [Google Scholar]
- MacDermed DM, Khodarev NN, Pitroda SP, Edwards DC, Pelizzari CA, Huang L, Weichselbaum RR (2010). MUC1-associated proliferation signature predicts outcomes in lung adenocarcinoma patients. BMC Med Genomics, 3, 16. doi: 10.1186/1755-8794-3-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti D, Reiland J, Erwin B, & Roy M (2003). Inhibition of heparanase activity and heparanase-induced angiogenesis by suramin analogues. International journal of cancer. Journal international du cancer, 104(2), 167–174. doi: 10.1002/ijc.10930 [DOI] [PubMed] [Google Scholar]
- Martin-Satue M, de Castellarnau C, & Blanco J (1999). Overexpression of alpha(1,3)-fucosyltransferase VII is sufficient for the acquisition of lung colonization phenotype in human lung adenocarcinoma HAL-24Luc cells. British Journal of Cancer, 80(8), 1169–1174. doi: 10.1038/sj.bjc.6690482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie EA (2007). Heparanase: a target for drug discovery in cancer and inflammation. British Journal of Pharmacology, 151(1), 1–14. doi: 10.1038/sj.bjp.0707182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X, Yu W (2011). O-GlcNAcylation is a novel regulator of lung and colon cancer malignancy. Biochimica et Biophysica Acta, 1812(4), 514–519. doi: 10.1016/j.bbadis.2011.01.009 [DOI] [PubMed] [Google Scholar]
- Mischel PS, Shai R, Shi T, Horvath S, Lu KV, Choe G, Nelson SF (2003). Identification of molecular subtypes of glioblastoma by gene expression profiling. Oncogene, 22(15), 2361–2373. [DOI] [PubMed] [Google Scholar]
- Miyoshi E, Moriwaki K, & Nakagawa T (2008). Biological function of fucosylation in cancer biology. J Biochem, 143(6), 725–729. doi: 10.1093/jb/mvn011 [DOI] [PubMed] [Google Scholar]
- Molinolo A, Simpson JF, Thor A, & Schlom J (1990). Enhanced tumor binding using immunohistochemical analyses by second generation anti-tumor-associated glycoprotein 72 monoclonal antibodies versus monoclonal antibody B72.3 in human tissue. Cancer Research, 50(4), 1291–1298. [PubMed] [Google Scholar]
- Mollicone R, Moore SE, Bovin N, Garcia-Rosasco M, Candelier JJ, Martinez-Duncker I, & Oriol R (2009). Activity, splice variants, conserved peptide motifs, and phylogeny of two new alpha1,3-fucosyltransferase families (FUT10 and FUT11). Journal of Biological Chemistry, 284(7), 4723–4738. doi: 10.1074/jbc.M809312200 [DOI] [PubMed] [Google Scholar]
- Morimoto-Tomita M, Uchimura K, Werb Z, Hemmerich S, & Rosen SD (2002). Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. Journal of Biological Chemistry, 277(51), 49175–49185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskal JR, Kroes RA, & Dawson G (2009). The glycobiology of brain tumors: disease relevance and therapeutic potential. Expert review of neurotherapeutics, 9(10), 1529–1545. doi: 10.1586/ern.09.105 [DOI] [PubMed] [Google Scholar]
- Nackaerts K, Verbeken E, Deneffe G, Vanderschueren B, Demedts M, & David G (1997). Heparan sulfate proteoglycan expression in human lung-cancer cells. International Journal of Cancer, 74(3), 335–345. [DOI] [PubMed] [Google Scholar]
- Nagai S, Takenaka K, Sonobe M, Ogawa E, Wada H, & Tanaka F (2006). A novel classification of MUC1 expression is correlated with tumor differentiation and postoperative prognosis in non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer, 1(1), 46–51. [PubMed] [Google Scholar]
- Nangia-Makker P, Conklin J, Hogan V, & Raz A (2002). Carbohydrate-binding proteins in cancer, and their ligands as therapeutic agents. Trends in molecular medicine, 8(4), 187–192. [DOI] [PubMed] [Google Scholar]
- Nawroth R, van Zante A, Cervantes S, McManus M, Hebrok M, & Rosen SD (2007). Extracellular sulfatases, elements of the wnt signaling pathway, positively regulate growth and tumorigenicity of human pancreatic cancer cells. PLoS ONE, 2, e392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa J, Inoue H, & Koide S (1996). Expression of alpha-1,3-fucosyltransferase type IV and VII genes is related to poor prognosis in lung cancer. Cancer Research, 56(2), 325–329. [PubMed] [Google Scholar]
- Ohtsubo K, & Marth JD (2006). Glycosylation in cellular mechanisms of health and disease. Cell, 126(5), 855–867. doi: 10.1016/j.cell.2006.08.019 [DOI] [PubMed] [Google Scholar]
- Okazaki A, Shoji-Hosaka E, Nakamura K, Wakitani M, Uchida K, Kakita S, Shitara K (2004). Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcgammaRIIIa. Journal of Molecular Biology, 336(5), 1239–1249. doi: 10.1016/j.jmb.2004.01.007 [DOI] [PubMed] [Google Scholar]
- Orditura M, De Vita F, Catalano G, Infusino S, Lieto E, Martinelli E, Galizia G (2002). Elevated serum levels of interleukin-8 in advanced non-small cell lung cancer patients: relationship with prognosis. Journal of Interferon and Cytokine Research, 22(11), 1129–1135. [DOI] [PubMed] [Google Scholar]
- Osada H, & Takahashi T (2002). Genetic alterations of multiple tumor suppressors and oncogenes in the carcinogenesis and progression of lung cancer. Oncogene, 21(48), 7421–7434. [DOI] [PubMed] [Google Scholar]
- Osumi D, Takahashi M, Miyoshi E, Yokoe S, Lee SH, Noda K, Taniguchi N (2009). Core fucosylation of E-cadherin enhances cell-cell adhesion in human colon carcinoma WiDr cells. Cancer Sci, 100(5), 888–895. doi: 10.1111/j.1349-7006.2009.01125.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa T, Riester M, Cheng YK, Huse JT, Squatrito M, Helmy K, Holland EC (2014). Most Human Non-GCIMP Glioblastoma Subtypes Evolve from a Common Proneural-like Precursor Glioma. Cancer cell, 26(2), 288–300. doi: 10.1016/j.ccr.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish CR, Freeman C, & Hulett MD (2001). Heparanase: a key enzyme involved in cell invasion. Biochimica et Biophysica Acta, 1471(3), M99–108. [DOI] [PubMed] [Google Scholar]
- Passaniti A, & Hart GW (1988). Cell surface sialylation and tumor metastasis. Metastatic potential of B16 melanoma variants correlates with their relative numbers of specific penultimate oligosaccharide structures. Journal of Biological Chemistry, 263(16), 7591–7603. [PubMed] [Google Scholar]
- Paszek MJ, DuFort CC, Rossier O, Bainer R, Mouw JK, Godula K, Weaver VM (2014). The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature, 511(7509), 319–325. doi: 10.1038/nature13535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PS, Raval GN, Rawal RM, Patel GH, Balar DB, Shah PM, & Patel DD (1995). Comparison between serum levels of carcinoembryonic antigen, sialic acid and phosphohexose isomerase in lung cancer. Neoplasma, 42(5), 271–274. [PubMed] [Google Scholar]
- Patel PS, Raval GN, Rawal RR, Patel GH, Balar DB, Shah PM, & Patel DD (1994). Assessing benefits of combining biochemical and immunological markers in patients with lung carcinoma. Cancer Letters, 82(2), 129–133. [DOI] [PubMed] [Google Scholar]
- Perng GS, Shoreibah M, Margitich I, Pierce M, & Fregien N (1994). Expression of N-acetylglucosaminyltransferase V mRNA in mammalian tissues and cell lines. Glycobiology, 4(6), 867–871. [DOI] [PubMed] [Google Scholar]
- Pesenti E, Sola F, Mongelli N, Grandi M, & Spreafico F (1992). Suramin prevents neovascularisation and tumour growth through blocking of basic fibroblast growth factor activity. British Journal of Cancer, 66(2), 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petridis AK, Wedderkopp H, Hugo HH, & Maximilian Mehdorn H (2009). Polysialic acid overexpression in malignant astrocytomas. Acta Neurochirurgica, 151(6), 601–603; discussion 603-604. doi: 10.1007/s00701-009-0324-3 [DOI] [PubMed] [Google Scholar]
- Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Aldape K (2006). Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell, 9(3), 157–173. [DOI] [PubMed] [Google Scholar]
- Phillips JJ (2012). Novel therapeutic targets in the brain tumor microenvironment. Oncotarget, 3(5), 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JJ, Aranda D, Ellison DW, Judkins AR, Croul SE, Brat DJ, Perry A (2013). PDGFRA amplification is common in pediatric and adult high-grade astrocytomas and identifies a poor prognostic group in IDH1 mutant glioblastoma. Brain Pathology, 23(5), 565–573. doi: 10.1111/bpa.12043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JJ, Huillard E, Robinson AE, Ward A, Lum DH, Polley MY, Werb Z (2012). Heparan sulfate sulfatase SULF2 regulates PDGFRalpha signaling and growth in human and mouse malignant glioma. Journal of Clinical Investigation, 122(3), 911–922. doi: 10.1172/JCI58215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polivkova J, Vosmikova K, & Horak L (1992). Utilization of determining lipid-bound sialic acid for the diagnosis and further prognosis of cancer. Neoplasma, 39(4), 233–236. [PubMed] [Google Scholar]
- Ponnusamy MP, Seshacharyulu P, Lakshmanan I, Vaz AP, Chugh S, & Batra SK (2013). Emerging role of mucins in epithelial to mesenchymal transition. Curr Cancer Drug Targets, 13(9), 945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quoix E, Ramlau R, Westeel V, Papai Z, Madroszyk A, Riviere A, Limacher JM (2011). Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. The Lancet. Oncology, 12(12), 1125–1133. doi: 10.1016/S1470-2045(11)70259-5 [DOI] [PubMed] [Google Scholar]
- Raina D, Kosugi M, Ahmad R, Panchamoorthy G, Rajabi H, Alam M, Kufe D (2011). Dependence on the MUC1-C oncoprotein in non-small cell lung cancer cells. Mol Cancer Ther, 10(5), 806–816. doi: 10.1158/1535-7163.MCT-10-1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralser M, Heeren G, Breitenbach M, Lehrach H, & Krobitsch S (2006). Triose phosphate isomerase deficiency is caused by altered dimerization--not catalytic inactivity--of the mutant enzymes. PLoS One, 1, e30. doi: 10.1371/journal.pone.0000030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralser M, Wamelink MM, Kowald A, Gerisch B, Heeren G, Struys EA, … Krobitsch S (2007). Dynamic rerouting of the carbohydrate flux is key to counteracting oxidative stress. J Biol, 6(4), 10. doi: 10.1186/jbiol61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlau R, Quoix E, Rolski J, Pless M, Lena H, Levy E, Velu T (2008). A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV Non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer, 3(7), 735–744. doi: 10.1097/JTO.0b013e31817c6b4f [DOI] [PubMed] [Google Scholar]
- Rebbaa A, Yamamoto H, Saito T, Meuillet E, Kim P, Kersey DS, Moskal JR (1997). Gene transfection-mediated overexpression of beta1,4-N-acetylglucosamine bisecting oligosaccharides in glioma cell line U373 MG inhibits epidermal growth factor receptor function. The Journal of biological chemistry, 272(14), 9275–9279. [DOI] [PubMed] [Google Scholar]
- Rexach JE, Clark PM, & Hsieh-Wilson LC (2008). Chemical approaches to understanding O-GlcNAc glycosylation in the brain. Nat Chem Biol, 4(2), 97–106. doi: 10.1038/nchembio.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexach JE, Clark PM, Mason DE, Neve RL, Peters EC, & Hsieh-Wilson LC (2012). Dynamic O-GlcNAc modification regulates CREB-mediated gene expression and memory formation. Nat Chem Biol, 8(3), 253–261. doi: 10.1038/nchembio.770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen SD, & Lemjabbar-Alaoui H (2010). Sulf-2: an extracellular modulator of cell signaling and a cancer target candidate. Expert Opin Ther Targets, 14(9), 935–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R, & Baek MG (2002). Glycodendrimers: novel glycotope isosteres unmasking sugar coding. case study with T-antigen markers from breast cancer MUC1 glycoprotein. Journal of Biotechnology, 90(3-4), 291–309. [DOI] [PubMed] [Google Scholar]
- Sato M, Shames DS, Gazdar AF, & Minna JD (2007). A translational view of the molecular pathogenesis of lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer, 2(4), 327–343. [DOI] [PubMed] [Google Scholar]
- Sato Y, Takahashi M, Shibukawa Y, Jain SK, Hamaoka R, Miyagawa J, Taniguchi N (2001). Overexpression of N-acetylglucosaminyltransferase III enhances the epidermal growth factor-induced phosphorylation of ERK in HeLaS3 cells by up-regulation of the internalization rate of the receptors. The Journal of biological chemistry, 276(15), 11956–11962. doi: 10.1074/jbc.M008551200 [DOI] [PubMed] [Google Scholar]
- Sawada R, Tsuboi S, & Fukuda M (1994). Differential E-selectin-dependent adhesion efficiency in sublines of a human colon cancer exhibiting distinct metastatic potentials. The Journal of biological chemistry, 269(2), 1425–1431. [PubMed] [Google Scholar]
- Schauer R (1982). Chemistry, metabolism, and biological functions of sialic acids. Advances in Carbohydrate Chemistry and Biochemistry, 40, 131–234. [DOI] [PubMed] [Google Scholar]
- Schauer R (1985). Sialic acids and their role as biological masks. Trends in Biochemical Sciences, 10(9), 357–360. doi: 10.1016/0968-0004(85)90112-4 [DOI] [Google Scholar]
- Scheidegger EP, Lackie PM, Papay J, & Roth J (1994). In vitro and in vivo growth of clonal sublines of human small cell lung carcinoma is modulated by polysialic acid of the neural cell adhesion molecule. Laboratory Investigation, 70(1), 95–106. [PubMed] [Google Scholar]
- Shamberger RJ (1986). Evaluation of water soluble and lipid soluble sialic acid levels as tumor markers. Anticancer Research, 6(4), 717–720. [PubMed] [Google Scholar]
- Shao C, Shi X, Phillips JJ, & Zaia J (2013). Mass spectral profiling of glycosaminoglycans from histological tissue surfaces. Analytical Chemistry, 85(22), 10984–10991. doi: 10.1021/ac402517s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, & Stanley P (2003). Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proceedings of the National Academy of Sciences of the United States of America, 100(9), 5234–5239. doi: 10.1073/pnas.0831126100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Shitara K (2003). The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. Journal of Biological Chemistry, 278(5), 3466–3473. doi: 10.1074/jbc.M210665200 [DOI] [PubMed] [Google Scholar]
- Siegel R, Ma J, Zou Z, & Jemal A (2014). Cancer statistics, 2014. CA: A Cancer Journal for Clinicians, 64(1), 9–29. doi: 10.3322/caac.21208 [DOI] [PubMed] [Google Scholar]
- Sola-Penna M, Da Silva D, Coelho WS, Marinho-Carvalho MM, & Zancan P (2010). Regulation of mammalian muscle type 6-phosphofructo-1-kinase and its implication for the control of the metabolism. IUBMB Life, 62(11), 791–796. doi: 10.1002/iub.393 [DOI] [PubMed] [Google Scholar]
- Son MJ, Woolard K, Nam DH, Lee J, & Fine HA (2009). SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell stem cell, 4(5), 440–452. doi: 10.1016/j.stem.2009.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer GF (1984). T and Tn, general carcinoma autoantigens. Science, 224(4654), 1198–1206. [DOI] [PubMed] [Google Scholar]
- Steck PA, Moser RP, Bruner JM, Liang L, Freidman AN, Hwang TL, & Yung WK (1989). Altered expression and distribution of heparan sulfate proteoglycans in human gliomas. Cancer Research, 49(8), 2096–2103. [PubMed] [Google Scholar]
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Mirimanoff RO (2009). Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet. Oncology, 10(5), 459–466. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Mirimanoff RO (2005). Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England Journal of Medicine, 352(10), 987–996. [DOI] [PubMed] [Google Scholar]
- Su G, Meyer K, Nandini CD, Qiao D, Salamat S, & Friedl A (2006). Glypican-1 is frequently overexpressed in human gliomas and enhances FGF-2 signaling in glioma cells. The American journal of pathology, 168(6), 2014–2026. doi: 10.2353/ajpath.2006.050800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada A, Ohmori K, Yoneda T, Tsuyuoka K, Hasegawa A, Kiso M, & Kannagi R (1993). Contribution of carbohydrate antigens sialyl Lewis A and sialyl Lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Research, 53(2), 354–361. [PubMed] [Google Scholar]
- Takahashi M, Yokoe S, Asahi M, Lee SH, Li W, Osumi D, Taniguchi N (2008). N-glycan of ErbB family plays a crucial role in dimer formation and tumor promotion. Biochimica et Biophysica Acta, 1780(3), 520–524. doi: 10.1016/j.bbagen.2007.10.019 [DOI] [PubMed] [Google Scholar]
- Takano S, Yoshii Y, Kondo S, Suzuki H, Maruno T, Shirai S, & Nose T (1996). Concentration of vascular endothelial growth factor in the serum and tumor tissue of brain tumor patients. Cancer Research, 56(9), 2185–2190. [PubMed] [Google Scholar]
- Tanaka F, Otake Y, Nakagawa T, Kawano Y, Miyahara R, Li M, Wada H (2000). Expression of polysialic acid and STX, a human polysialyltransferase, is correlated with tumor progression in non-small cell lung cancer. Cancer Research, 60(11), 3072–3080. [PubMed] [Google Scholar]
- Taylor-Papadimitriou J, Burchell J, Miles DW, & Dalziel M (1999). MUC1 and cancer. Biochimica et Biophysica Acta, 1455(2–3), 301–313. [DOI] [PubMed] [Google Scholar]
- Tessema M, Yingling C, & Thomaa C (2011). SULF2 methylation is prognostic for lung cancer survival and increases sensitivity to topoisomerase-I inhibitors via induction of ISG15. Oncogene, 12, 1 – 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togayachi A, Kudo T, Ikehara Y, Iwasaki H, Nishihara S, Andoh T, Narimatsu H (1999). Up-regulation of Lewis enzyme (Fuc-TIII) and plasma-type alpha1,3fucosyltransferase (Fuc-TVI) expression determines the augmented expression of sialyl Lewis x antigen in non-small cell lung cancer. International Journal of Cancer, 83(1), 70–79. [DOI] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, & Riely GJ (2013). New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. Journal of Clinical Oncology, 31(8), 992–1001. doi: 10.1200/JCO.2012.46.9270 [DOI] [PubMed] [Google Scholar]
- Tsuda T, Ikeda Y, & Taniguchi N (2000). The Asn-420-linked sugar chain in human epidermal growth factor receptor suppresses ligand-independent spontaneous oligomerization. Possible role of a specific sugar chain in controllable receptor activation. Journal of Biological Chemistry, 275(29), 21988–21994. doi: 10.1074/jbc.M003400200 [DOI] [PubMed] [Google Scholar]
- Tsutsumida H, Goto M, Kitajima S, Kubota I, Hirotsu Y, & Yonezawa S (2004). Combined status of MUC1 mucin and surfactant apoprotein A expression can predict the outcome of patients with small-size lung adenocarcinoma. Histopathology, 44(2), 147–155. [DOI] [PubMed] [Google Scholar]
- Tuccillo FM, de Laurentiis A, Palmieri C, Fiume G, Bonelli P, Borrelli A, Scala G (2014). Aberrant glycosylation as biomarker for cancer: focus on CD43. Biomed Res Int, 2014, 742831. doi: 10.1155/2014/742831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimura K, Morimoto-Tomita M, Bistrup A, Li J, Lyon M, Gallagher J, Rosen SD (2006). HSulf-2, an extracellular endoglucosamine-6-sulfatase, selectively mobilizes heparin-bound growth factors and chemokines: effects on VEGF, FGF-1, and SDF-1. BMC Biochemistry, 7, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A (1994). Selectin ligands. Proceedings of the National Academy of Sciences of the United States of America, 91(16), 7390–7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Hayes DN (2010). Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell, 17(1), 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen MJ, Fruechtnicht W, Brackrock S, Krause KT, & Zienkiewicz TJ (1995). Quantitative lectin-histochemical and immunohistochemical studies on the occurrence of alpha(2,3)- and alpha(2,6)-linked sialic acid residues in colorectal carcinomas. Relation to clinicopathologic features. Cancer, 76(5), 727–735. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I, & Friedmann Y (2001). Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis. The Journal of clinical investigation, 108(3), 341–347. doi: 10.1172/JCI13662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I, Friedmann Y, Elkin M, Aingorn H, Atzmon R, Ishai-Michaeli R, Pecker I (1999). Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nature Medicine, 5(7), 793–802. doi: 10.1038/10518 [DOI] [PubMed] [Google Scholar]
- Vlodavsky I, Ilan N, Naggi A, & Casu B (2007). Heparanase: structure, biological functions, and inhibition by heparin-derived mimetics of heparan sulfate. Current Pharmaceutical Design, 13(20), 2057–2073. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, & Kinzler KW (2004). Cancer genes and the pathways they control. Nature Medicine, 10(8), 789–799. [DOI] [PubMed] [Google Scholar]
- Wade A, Robinson AE, Engler JR, Petritsch C, James CD, & Phillips JJ (2013). Proteoglycans and their roles in brain cancer. FEBS J, 280(10), 2399–2417. doi: 10.1111/febs.12109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Gu J, Ihara H, Miyoshi E, Honke K, & Taniguchi N (2006). Core fucosylation regulates epidermal growth factor receptor-mediated intracellular signaling. Journal of Biological Chemistry, 281(5), 2572–2577. doi: 10.1074/jbc.M510893200 [DOI] [PubMed] [Google Scholar]
- Wang X, Inoue S, Gu J, Miyoshi E, Noda K, Li W, Taniguchi N (2005). Dysregulation of TGF-beta1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proceedings of the National Academy of Sciences of the United States of America, 102(44), 15791–15796. doi: 10.1073/pnas.0507375102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Mabuchi T, Satoh E, Furuya K, Zhang L, Maeda S, & Naganuma H (2006). Expression of syndecans, a heparan sulfate proteoglycan, in malignant gliomas: participation of nuclear factor-kappaB in upregulation of syndecan-1 expression. Journal of Neuro-Oncology, 77(1), 25–32. doi: 10.1007/s11060-005-9010-3 [DOI] [PubMed] [Google Scholar]
- Wesseling J, van der Valk SW, & Hilkens J (1996). A mechanism for inhibition of E-cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Molecular Biology of the Cell, 7(4), 565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling J, van der Valk SW, Vos HL, Sonnenberg A, & Hilkens J (1995). Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. Journal of Cell Biology, 129(1), 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson KB, Whitson SR, Red-Brewer ML, McCoy AJ, Vitali AA, Walker F, Staros JV (2005). Functional effects of glycosylation at Asn-579 of the epidermal growth factor receptor. Biochemistry, 44(45), 14920–14931. doi: 10.1021/bi050751j [DOI] [PubMed] [Google Scholar]
- Yamada N, Chung YS, Takatsuka S, Arimoto Y, Sawada T, Dohi T, & Sowa M (1997). Increased sialyl Lewis A expression and fucosyltransferase activity with acquisition of a high metastatic capacity in a colon cancer cell line. British Journal of Cancer, 76(5), 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Kaneko Y, Rebbaa A, Bremer EG, & Moskal JR (1997). alpha2,6-Sialyltransferase gene transfection into a human glioma cell line (U373 MG) results in decreased invasivity. Journal of Neurochemistry, 68(6), 2566–2576. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Oviedo A, Sweeley C, Saito T, & Moskal JR (2001). Alpha2,6-sialylation of cell-surface N-glycans inhibits glioma formation in vivo. Cancer Research, 61(18), 6822–6829. [PubMed] [Google Scholar]
- Yamamoto H, Swoger J, Greene S, Saito T, Hurh J, Sweeley C, Moskal JR (2000). Beta1,6-N-acetylglucosamine-bearing N-glycans in human gliomas: implications for a role in regulating invasivity. Cancer Research, 60(1), 134–142. [PubMed] [Google Scholar]
- Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, Kim YS, & Cho JW (2006). Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol, 8(10), 1074–1083. doi: 10.1038/ncb1470 [DOI] [PubMed] [Google Scholar]
- Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Evans RM (2008). Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature, 451(7181), 964–969. doi: 10.1038/nature06668 [DOI] [PubMed] [Google Scholar]
- Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA 3rd, Hsieh-Wilson LC (2012). Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science, 337(6097), 975–980. doi: 10.1126/science.1222278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogeeswaran G (1983). Cell surface glycolipids and glycoproteins in malignant transformation. Advances in Cancer Research, 38, 289–350. [DOI] [PubMed] [Google Scholar]
- Yogeeswaran G, & Salk PL (1981). Metastatic potential is positively correlated with cell surface sialylation of cultured murine tumor cell lines. Science, 212(4502), 1514–1516. [DOI] [PubMed] [Google Scholar]
- Zhang L, Gallup M, Zlock L, Chen YT, Finkbeiner WE, & McNamara NA (2014). Pivotal role of MUC1 glycosylation by cigarette smoke in modulating disruption of airway adherens junctions in vitro. Journal of Pathology, 234(1), 60–73. doi: 10.1002/path.4375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Itoh S, Wang X, Isaji T, Miyoshi E, Kariya Y, Gu J (2006). Deletion of core fucosylation on alpha3beta1 integrin down-regulates its functions. Journal of Biological Chemistry, 281(50), 38343–38350. doi: 10.1074/jbc.M608764200 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Sato Y, Isaji T, Fukuda T, Matsumoto A, Miyoshi E, Taniguchi N (2008). Branched N-glycans regulate the biological functions of integrins and cadherins. The FEBS journal, 275(9), 1939–1948. doi: 10.1111/j.1742-4658.2008.06346.x [DOI] [PubMed] [Google Scholar]
- Zhou H, Roy S, Cochran E, Zouaoui R, Chu CL, Duffner J, Kishimoto TK (2011). M402, a novel heparan sulfate mimetic, targets multiple pathways implicated in tumor progression and metastasis. PloS one, 6(6), e21106. doi: 10.1371/journal.pone.0021106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Chen H, Wang Q, Zhang L, & Zhao J (2011). Knockdown of Mgat5 inhibits CD133+ human pulmonary adenocarcinoma cell growth in vitro and in vivo. Clinical and Investigative Medicine. Medecine Clinique et Experimentale, 34(3), E155–162. [DOI] [PubMed] [Google Scholar]