Abstract
Glycosylphosphatidylinositol anchored proteins (GPI-APs) represent a class of soluble proteins attached to the external leaflet of the plasma membrane by a post-translation modification, the GPI anchor. The 28 genes currently involved in the synthesis and remodelling of the GPI anchor add to the ever-growing class of congenital glycosylation disorders. Recent advances in next generation sequencing technology have led to the discovery of Mabry disease and CHIME syndrome genetic aetiology. Moreover, with each described mutation known phenotypes expand and new ones emerge without clear genotype-phenotype correlation. A protein database search was made for human GPI-APs with defined pathology to help building-up a physio-pathological mechanism from a clinical perspective. GPI-APs function in vitamin-B6 and folate transport, nucleotide metabolism and lipid homeostasis. Defining GPI-APs role in disease bears significant clinical implications.
Keywords: Congenital glycosylation disorders, GPI anchored proteins, Vitamin B6, FOLR, Ecto 5′nucleotidase, GPIHBP1, Vanin 1, Urokinase plasminogen activator receptor
1. Introduction
In 1970, Mabry et al. made the first observation of the Hyperphosphatasia with mental retardation syndrome (HPMRS) with the following findings: severe mental retardation, seizures, various neurologic abnormalities, and elevated serum levels of alkaline phosphatase. With recent advances in the next generation sequencing technology both hypomorphic and loss of function mutations of the genes involved in synthesis or remodelling of the GPI-APs have been described.
This helped expanding HPMRS clinical phenotype to multi-system involvement. PIGA gene defect, previously associated with paroxysmal nocturnal haemoglobinuria (PNH), was also attributed multiple congenital anomalies, hypotonia and seizures X-linked syndrome. Moreover, PNH was also described in a PIGT gene mutation. Other clinical phenotypes associated with molecular defects in the biosynthesis of the GPI anchor include: CHIME syndrome (coloboma, heart defects, ichthyosiform dermatosis, intellectual disability, and either ear defects or epilepsy), MCAHS syndrome (multiple congenital anomalies, hypotonia and seizures, type 1–3) and early-onset epileptic encephalopathies (Table 1).
Table 1.
Syndromic clinical presentation of GPI-AP defects (https://www.omim.org).
| Clinical presentation |
HPMRS (6 types) |
CHIME OMIM # 280000 |
MCAHS 1 OMIM # 614080 |
MCAHS 2 OMIM # 300868 |
|---|---|---|---|---|
| Inheritance | AR | AR | AR | X-linked |
| Dysmorphism | Secondary microcephaly Plagiocephaly Coronal synostosis Midface hypoplasia Prognathism Hypertelorism Long palpebral fissures Broad nasal bridge Broad nasal tip Short philtrum Large fleshy earlobes Downturned mouth corners Tented mouth Cleft palate |
Brachycephaly Hypertelorism Broad, flat nasal root Short philtrum Full lips Anomalous dentition Cleft palate Epicathic folds Macrosomia at birth |
Macrocephaly Macrosomia at birth Poor growth Coarse facial features Light hair Bitemporal narrowing Depressed nasal bridge High arched palate with wide alveolar ridge Frontal bossing Long philtrum Micrognathia Large, fleshy ears Overfolded helices Low-set ears Posteriorly rotated ears Cupped ears Epicanthal folds Hypertelorism Depressed nasal bridge Small nose Upturned nares Thin lips Open mouth High-arched palate Cleft palate |
Macrosomia at birth Accelerated linear growth Obesity Macrocephaly Microcephaly Micrognathia Coarse facies Overfolded helix Upslanting palpebral fissures Widely spaced eyes Depressed nasal bridge Short, anteverted nose Small mouth Downturned corners of the mouth Triangular mouth High-arched palate Gingival hyperplasia Microdontia Pointed teeth Widely-spaced teeth Short neck |
| Cardiovascular | ASD VSD Peripheral pulmonary stenosis Tetralogy of Fallot |
VSD Tetralogy of Fallot Transposition of great arteries Peripheral pulmonary stenosis |
VSD ASD Over-riding aorta Hypoplastic pulmonary trunk Non-compacting cardiomyopathy |
ASD Right ventricular hypertrophy and arrhythmia |
| Respiratory | Lung hypoplasia (in some patients) Diaphragmatic hernia Poor respiratory drive requiring tracheostomy and assisted ventilation. |
Obstructive apnoea | ||
| Gastrointestinal | Feeding difficulties requiring tube feeding Esophageal atresia Intestinal malrotation Hirschsprung disease Anorectal abnormalities (anal stenosis, atresia anovestibular fistula, anteriorly displaced anus) |
Upper GI dysmotility | Feeding difficulties requiring tube feeding Congenital diaphragmatic hernia Gastroesophageal reflux Hepatomegaly Intestinal malrotation Anal stenosis or atresia |
Upper GI dysmotility Hepatomegaly Cirrhosis Iron deposition (1 family) |
| Genitourinary | Dilated / mega ureter Ectopia of ureter/urethra Hydronephrosis Kidney duplication Vesicoureteral reflux |
Duplicated renal collecting system Hydronephrosis Ureteropelvic junction obstruction Bicornuate uterus |
Dysplastic kidney Hydrocele Hydronephrosis Hypoplasia of the ureter Vesicoureteral reflux Duplicated collecting system Microphallus, cryptorchidism |
Multicystic kidneys Unilateral hydronephrosis Duplicated collecting system Vesicoureteral reflux Microphallus |
| Skeletal | Pectus excavatum Joints contractures Brachytelephalangy Tapered fingers Broad halluces Hypoplastic toes Clinodactyly/syndactily Osteopenia Hip dysplasia Proximal limb shortening |
Hip dysplasia Pectus excavatum Nipples small, low set Brachydactyly Fifth finger camptodactily and clinodactily |
Hip dysplasia Narrow inferior iliacs Fifth finger clinodactyly Hypoplastic distal phalanx Hypoplastic nails, anonychia |
Joint contractures |
| Skin, nail, hair | Hypertrichosis Supernumerary nipples Hypoplastic or absent nails Bilateral hypoplastic fifth fingernail Severe ichtyosis Inguinal hernia |
Migratory ichthyosiform dermatosis Thickened palms and soles Light coloured, fine and spars scalp hair |
Deep plantar crease Supranumery nipples |
Ichthyosis Seborrheic dermatitis Linear plaque-like scales Pigmentation abnormalities Hypoplastic nails |
| Muscular | Hypotonia Muscular atrophy |
Hypotonia | Hypotonia | Hypotonia |
| Neurology | Intellectual disability (moderate - severe) Developmental regression Seizures (myoclonic, tonico-clonic, infantile spasms, absence) Movement disorder (chorea, athetoid and dystonic hand movements, ataxia) Delayed myelinisation Cerebral atrophy Dandy-Walker malformation Atrophy of temporal lobes, thin corpus callosum, dilated lateral ventricles Cerebellar vermis atrophy Sleep disturbance Bruxism ADHD Aggressive outbursts |
Intelectual disability (moderate – severe) Seizure disorder exacerbated by high environmental temperature or fever (myoclonic, Cerebral atrophy Aggressive behaviour Wide base gait Violent and self-abusive behaviour |
Delayed psychomotor development Intelectual disability (moderate - severe) Developmental regression Hyporeflexia / Hyperreflexia Spasticity Tremor Choreoathetosis Cerebellar atrophy Cerebral atrophy Epilepsy Ataxia |
Delayed psychomotor development Intelectual disability (moderate - severe) Developmental regression Epileptic encephalopathy (hypsarrhythmia, burst-suppression pattern, myoclonic seizures) Limb spasticity Brain abnormalities (thin corpus callosum, absent septum pellucidum, absent olfactory bulbs and tracts, hypoplastic cerebellum, cortical atrophy, spongy gliosis) Scant iron deposition in the basal ganglia (1 family) Hyperreflexia |
| Ophthalmology | Congenital cataracts Strabismus Esostropia Myopia Cortical visual impairment Oculomotor apraxia |
Retinal coloboma | Cloudy cornea Nystagmus Wandering eye movements Strabismus Esotropia Cortical visual impairment |
Cortical visual impairment |
| Hearing | Sensorineural hearing loss |
Ear anomalies (over folded helices) Conductive hearing loss |
Conductive and sensorineural hearing loss | |
| Infectious | Recurrent infections in infancy and childhood |
Fatal pneumonia | Fatal pneumonia | Fatal pneumonia Fatal sepsis |
| Blood Work-up |
Hyperphosphatasia | Acute lymphoblastic leukemia (1 patient) | Low or fluctuating alkaline phosphatase | Fluctuating or high alkaline phosphatase levels in some patients Dyslipidemia |
Pending on the cell polarisation type, the GPI-anchor is required for trafficking proteins to certain domains of the plasma membrane. There, the GPI-APs oligomerize in the rich cholesterol/sphingolipid areas by saturated fatty acids interactions within the GPI anchor and with other molecules building up “signalling platforms”. Several studies showed that GPI-APs oligomerize/cluster at the cell surface of different cell types such as fibroblasts, immune T and epithelial cells [[1], [2], [3], [4], [5]]. Importantly, GPI-AP clustering at the cell surface is cholesterol-sensitive and dynamically regulated by the cortical cytoskeleton. Crucial for their biological role, GPI-AP clustering at the membrane rafts permits interaction with side partners such as enzymes, adaptors, co-factors and scaffolding proteins, initiating spatio-temporal compartmentalization and context-specific activation of downstream signalling cascades [6, 7].
The role of the GPI-APs at the plasma membrane is even more complex, considering their recruitment to exosomes, release in the extracellular environment by proteolysis or further trafficking via endocytic pathway [[8], [9], [10], [11]]. Broadly, a defect in the later stages of biosynthesis / remodelling of the GPI anchor results in shedding of the soluble protein in the extracellular environment, with or without the abnormal GPI signal; in defective early GPI anchor biosynthesis, the soluble protein will be subjected to intracellular degradation [12]. This mechanism explains high plasma alkaline phosphatase levels in patients with HPMRS [13].
Abnormal surface expression of the GPI-APs was demonstrated by transfection of patients' DNA to GPI-AP defective cell lines. In vitro functional analysis has shown variable degrees of GPI-APs restoration compared to wild gene transfection [[14], [15], [16], [17], [18], [19], [20], [21], [22], [23]].
2. Materials and method
A protein database search was made (www.uniprot.org and www.omim.org) for human GPI-APs with defined pathologic role. Then, literature was reviewed to describe each GPI-AP's timing and tissue expression, individual pathology and available treatment. The GPI-APs meeting these criteria are summarised in Table 2.
Table 2.
GPI-APs function and physio-pathologic roles.
| GPI-AP | Other post-translational modifications | Function | Pathology† | Tissue specificity⁎ |
|---|---|---|---|---|
| TNAP | N-glycosylated Phosphorilated Disulphide bond |
Phosphatase, nucleotidase | Hypophosphatasia | Widely expressed |
| FOLR1 | N-glycosylated Disulphide bond |
Folate transport across choroid plexus | Cerebral folate deficiency | Choroid plexus Kidney Lung Cerebellum |
| 5′ ecto-nucleotidase | N-glycosylated Disulphide bond |
Ecto-nucleotidase | Defective purinergic signalling Calcification of joints and big arteries (CALJA) |
Widely expressed |
| GPIHBP1 | N-glycosylated Disulphide bond |
Lipoprotein lipase transport | Hyperlipoproteinemia 1D | Adipose tissue Lung Endothelial cell |
| Vanin 1 | N-glycosylated | Inflammatory response and innate immunity Coenzyme A biosynthesis |
Impaired cyto-protective role Defective coenzyme A biosynthesis associates:
|
Widely expressed |
| Carboxy-peptidase M | N-glycosylated Disulphide bond |
Peptidase | Not yet defined | Lung Kidney Adipose tissue Female tissues |
| Urokinase plasminogen activator receptor | N-glycosylated Disulphide bond |
Cell surface fibrinolysis Via interactome |
|
Bone marrow Immune tissue Lung |
| Alpha and Beta tectorin | N-glycosylated Disulphide bond |
Tectorial membrane component |
|
Ear Oocyte Bone marrow and immune system |
| Otoancorin | N-glycosylated | Interface between epithelia and tectorial membrane |
Non-syndromic deafness (DFNB22) | Ear Bone marrow and immune system |
| CD59 | N-glycosylated O-glycosylated Disulphide bond |
Potent inhibitor of the complement membrane attack complex action |
|
Widely expressed |
| CD55 | Disulphide bond N-glycosylated |
Regulates complement activation |
|
Widely expressed |
| Prion protein | N-glycosylated Disulphide bond |
Its primary physiological function is unclear. |
|
Brain |
3. Results
3.1. Tissue non-specific alkaline phosphatase (TNAP)
TNAP belongs to the large family of ecto-phosphatases, being strongly expressed in embryo and early postnatal period, however its expression declines during postnatal development. TNAP hydrolyses extracellular pyridoxal phosphate (PLP, the major active form of vitamin B6) into pyridoxal, which is transferred through cell membranes and the blood–brain barrier. In cells, pyridoxal is trapped by re-phosphorylation to PLP that serves as co-factor to more than 70 enzymatic reactions (B6 database - http://bioinformatics.unipr.it/cgi-bin/bioinformatics/B6db/home.pl). B6-dependent enzymes are involved in the metabolism of aminoacids (serine, threonine, tryptophan-kynurenine pathway, glycine), neurotransmitters (dopamine, serotonin, GABA, glutamate, d-serine, histamine), molybdenum cofactor and sphingolipids.
Patients with hypophosphatasia, caused by mutations in TNAP gene, present high PLP in plasma across childhood [24]. Infantile presentation is clinically associated with PLP dependent-seizures and biochemically with high plasma and cerebrospinal fluid PLP levels [25, 26]. This abnormal distribution could be explained by a normal maternal-dependent trans-placental B6 transport but deficient cellular uptake. Unable to convert the PLP to pyridoxal, the TNAP-deficient patient becomes intracellularly B6 depleted. Applying metabolomics, Cruz et al. aimed to identify the biochemical changes associated with B6-deficient metabolism in several mouse models with null TNAP, heterozygote and normal TNAP expressing brain tissues. The amplitude of the concentration variation between the TNAP-knockout mice and the other genotypes grouped together reached −40% for GABA, −80% for adenosine and + 450% for cystathionine [27]. Dysregulation of the transulfuration pathway and of purinergic signalling represents the biochemical expression of the TNAP deficient brain. The CSF PLP levels were showed to have a negative age correlation [28]. This seems to follow the postnatal decline in TNAP expression, with an expected steep in patients with GPI-AP deficiency.
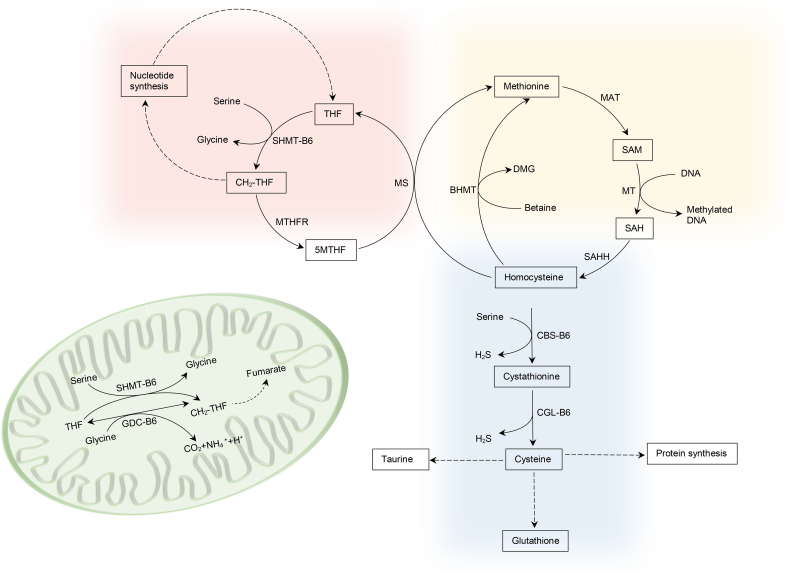
The pathways of 1-carbon metabolism, transsulfuration and glutathione synthesis are critical for nucleotide synthesis, DNA and histone methylation, and antioxidant defense. PLP acts as co-factor of 5 enzymes in these metabolic pathways: cystathionine β-synthase (CBS), cystathionine γ-lyase (CGL), cytoplasmic and mitochondrial serine hydroxymethyltransferase (cSHMT and mSHMT), and glycine decarboxylase (GDC) in the mitochondria (see Fig. 1).
Fig. 1.
Schematic representation of 1-carbon metabolism and transsulfuration pathway. BHMT betaine-homocysteine methyltransferase, MAT methionine adenosyltransferase, MS methionine synthase, MTHFR methylene-tetrahydrofolate reductase, MT methyl-transferase, SAHH S-adenoylhomocysteine hydrolase.
The transsulfuration pathway acts in sulfur amino acid metabolism contributing to the regulation of cellular homocysteine, cysteine production (required for synthesis of proteins, glutathione and taurine) and generation of H2S for signalling functions. Kinetic studies, on animals and humans, have shown that although PLP serves as coenzyme for both CBS and CGL enzymes, CGL is more sensitive to B6-deficiency [29].
Davis et al. experimental study on humans, found high cystathionine but normal homocysteine plasma levels after controlled dietary vitamin B-6 restriction. Interestingly, they have also found an increase in plasma glutathione by approximate 40% [30]. Lima et al. explain, in their experiment on rats, that cellular and plasma cystathionine concentrations increase in B6 deficiency mainly due to the bottleneck caused by reduced CGL activity. The increase in substrate concentration (i.e. cystathionine) yields a nearly proportional increase in v/Vmax due to the high Michaelis-Menten constant of CGL enzyme. This allows cysteine concentrations, cysteine flux, and net transsulfuration flux to be maintained in mild to moderate vitamin B-6 deficiency [31].
Mathematical models, based on experimentally determined kinetic parameters and known regulatory mechanisms, are increasingly used to explain how biological pathways work. Moreover, they can help identifying unexpected system properties or behaviour. Frederik et al., based their mathematical model on the structure and function of the 1‑carbon and glutathione metabolism. They modelled vitamin B6 deficiency by reducing the Vmax of the PLP dependent enzymes, thus simulating a range of vitamin B6 deficiencies. Firstly, they found that cystathionine was by far the most sensitive biomarker for vitamin B-6 deficiency. Secondly, they simulated B6 deficiency induced oxidative stress by increasing H2O2 concentration and confirmed the experimental results that showed increased plasma glutathione (GSH) in humans and hepatic GSH in dietary B6 deficient rats. Interestingly, the effect was independent of B6 deficiency; when B6 deficiency was corrected but oxidative stress remained GSH response was nearly identical [32].
Vitamin B-6 deficiency can be expected to interfere with the metabolic functions that depend critically on SHMT, which catalyses the interconversion of glycine to serine and of THF to CH2-THF in cytoplasm and the mitochondria (see figure). Insufficient CH2-THF, 1-carbon donor for thymidylate synthesis, leads to uracil mis-incorporation into DNA and subsequent strand breaks. CH2-THF is also substrate for the synthesis of 5MTHF, the primary methyl group donor for the methionine cycle that controls DNA and histone methylation and purine synthesis. In rats, vitamin B6 deficiency was found to lower both enzymes abundance. Several experimental studies have shown that SHMT depletion induces glycine auxotrophy. The mathematical model explained this effect. The normal balance between glycine and serine in the absence of SHMT only occurred if there was input of glycine into the system. When glycine input was set to zero, in the absence of SHMT, many metabolites and reaction rates were significantly reduced: the GDC reaction was reduced to nearly zero, the thymidylate synthase reaction was reduced to 59%, and the rate of export of 1‑carbon units from the mitochondria was reduced to 29%. Interestingly, setting the serine input to zero had little effect suggesting that glycine input can completely make up for the lack of serine, but not vice versa [32]. Vanessa et al. targeted metabolomics analysis of 1C metabolites in HepG2 cells showed that methionine cycle flux is maintained by directing 1C units to the re-methylation process rather than purine synthesis explaining higher THF and lower CH2-THF levels in B6 deficient conditions [33].
The presence of CBS in human plasma was confirmed by in-silico search of the proteome database and experimentally evidenced by Krijt et al. [34]. Patients with mild GPI-AP defects that maintain a high PLP plasma level could stimulate CBS activity and lower the homocysteine level within this compartment.
In mammals, 70–80% of the vitamin B6 is located in the skeletal muscle, where it functions as co-factor for glycogen phosphorylase [35]. Further studies are required to appreciate the relation between altered glycogen phosphorylase activity, accumulation of cytoplasmic PAS positive material and clinical hypotonia in GPI-AP defects [19, 36].
3.2. Folate receptor (FOLR)
FOLR defects are associated with neurodegeneration due to cerebral folate transport deficiency. Biochemically, patients present low 5-methyltetrahydrofolate (5MTHF) levels in CSF with normal peripheral folate status. FOLR1 and FOLR2 are anchored at the plasma membrane by the GPI signal, whereas FOLR3 is secreted due to the lack of a signal sequence for GPI-anchor attachment [37]. Within the brain FOLR1 is almost selectively expressed in the choroid plexus. Its high binding affinity for folates perfectly matches the physiological 5MTHF concentration in human plasma. Grapp et al. demonstrate a basolateral to apical sorting of the GPI-anchored FOLR1 that explains receptor-mediated endocytosis and transcytosis of the FOLR1 - 5MTHF complex across the choroid plexus. Then, FOLR1-containing exosomes circulate in the CSF, cross the ependymal cell layer and are distributed in the brain parenchyma [38]. Replacing the GPI-anchor of the FOLR with a trans-membrane domain, in a mouse model, results in endocytosis via clathrin-coated pits and altered intracellular destination of the FOLR with reduced folate transport [39]. In foetal life, FOLR1 is involved in neural tube closure, development of pharyngeal arches and the secondary heart field [40].
3.3. Ecto 5′ nucleotidase (5NTE, CD73)
Purinergic signalling effects are mediated by extracellular nucleotides and nucleosides in virtually all tissues. In the central nervous system, nucleotides mediate neurotransmission and neuron-glia interactions. Elsewhere, they are involved in smooth muscle (e.g. vascular and gut) and myocardial contractility, endocrine secretion, immune response modulation, control of leukocyte trafficking and platelets aggregation at vascular injury sites. Purinergic signalling regulates retinal neurotransmission, blood flow and intraocular pressure. In the inner ear, purine-receptors modulate fluid homeostasis, cochlear blood flow, hearing sensitivity and development. In addition to acute signalling events, there is increasing awareness of purines and pyrimidines potent long-term trophic roles [[41], [42], [43]].
A network of 8 ectoenzyme families, including 5NTE, TNAP and other alkaline or acid phosphatases, govern the duration and magnitude of purinergic signalling by hydrolyzing nucleotides to their corresponding nucleosides. 5NTE hydrolyses a variety of nucleoside 5′-monophosphates including CMP, UMP, IMP, and GMP, whereby AMP generally is the most effectively hydrolysed. The resulted adenosine will act on purinergic receptors (e.g. P1) before being salvage by the cells. Therefore, 5NTE contributes to the termination of the purinergic signalling. Moreover, due to its age-dependent expression, 5NTE has an important recycling role as de-novo synthesis of nucleotides in the adult brain is limited.
It is well-established that adenosine homeostasis is disrupted both in animal models and human epilepsies [44]. Increased 5NTE expression was found in the dentate gyrus of resective surgery specimens from temporal lobe epilepsy patients. Consistent with this data, increased 5NTE activity was also found in a number of rodent models of epilepsy [[45], [46], [47]]. More recently, genetic variants of both 5NTE and ADK were associated with the development of post-traumatic epilepsy in a study involving samples from 162 patients [48]. Thus, increased expression of 5NTE in the epileptic brain might be a compensatory response to inhibit synaptic transmission [44]. Starting from the premise that brain adenosine rises 100-fold under ischemic conditions, Chu et al. demonstrated that 5NTE-knockout mouse astrocyte cultures did not evoke adenosine production or obtained a limited adenosine response in mixed astrocyte-neurons cultures [49].
Wurm et al. demonstrate that adenosine or uracil nucleotides are able to restore purinergic signalling in a 5NTE deficient retina mouse model [50]. Paget et al. conclude that a high level of 5NTE in fibroblast lysate might cause intracellular nucleotide deficiency secondary to decreased salvage in patients presenting with developmental delay, seizures, ataxia, recurrent infections and severe language deficit. Uridine supplementation led to a remarkable improvement in speech and development as well as decreased seizure activity [51]. The anticonvulsant role of uridine, and of nucleotide salvage pathway, is clearly demonstrated in epileptic encephalopathy caused by an inherited defect in de-novo pyrimidine biosynthesis [52].
3.4. GPI-anchored high-density lipoprotein-binding protein 1 (GPIHBP1)
GPIHBP1 is an endothelial cell GPI-AP mainly expressed on the luminal face of capillaries in brown adipose tissue, heart, lung, and liver [55]. GPIHBP1 plays a major role in transporting lipoprotein lipase (LPL) from the subendothelial spaces to the capillary lumen [56]. GPIHBP1 knockout mice cannot transport lipoprotein lipase to the capillary lumen, resulting in mis-localization of lipoprotein lipase within tissues, defective lipolysis of triglyceride-rich lipoproteins and chylomicronemia. The immobilization of LPL in the interstitial space will result in normal tissue stores with very low plasma levels [57]. Few patients with homozygous missense mutations in GPIHBP1 have been reported having a similar phenotype with LPL deficiency [58, 59]. However, defective localisation of GPIHBP1 may have a variable impact on the lipid profile as increased triglyceride uptake by the liver compensates, to a degree, for the loss of LPL-mediated triglyceride delivery to peripheral tissues [60]. Markedly elevated triglycerides and cholesterol with absent post-heparin lipoprotein lipase activity suggesting LPL deficiency was already documented in two patients with GPI-AP defects, one carrying missense PIGA germline mutation and another with a homozygous variant disrupting the PIGH start-codon [61, 62].
3.5. Vanin 1 (VNN1)
VNN 1 is a pantetheinase highly expressed in the liver, gut, and kidney, where hydrolyses pantetheine into pantothenic acid (vitamin B5) and cysteamine, a potent antioxidant [63]. Cysteamine modulates cysteine, cystine and GSH levels, playing a significant role in regulating cellular redox status. Indeed, lacking tissue cysteamine, the VNN1 knockout mice displays down-regulated inflammatory response to oxidative stress [64]. Moreover, clinical data support VNN1 biomarker candidacy in auto-inflammatory processes and early diagnosis of tissue injury [[65], [66], [67], [68]]. Pantothenic acid, generated by VNN1 activity, is further recycled to coenzyme A (CoA) through a series of five synthetic reactions. CoA has a major role in mitochondrial and peroxisomal lipid metabolism with neurodegenerative consequences [69, 70]. Thus, VNN1 serves as link between inflammation and metabolism.
Peroxisome Proliferator Activated Receptors (PPAR) are a transcription factor family that modulate acyl-coA synthesis, VNN1 and GPIHBP1 gene expression. Their role can be explored from a therapeutic perspective [71]. Known PPAR agonists, such as fibrates, thiazolidinediones, certain non-steroidal anti-inflammatory drugs (e.g. ibuprofen and indomethacin) and glitazars target PPAR to exercise their metabolic role in hyperlipidaemia, insulin resistance and inflammation modulation in asthma.
3.6. Carboxypeptidase M (CPM)
CPM belongs to the large family of the carboxypeptidases which remove C-terminal amino acids from peptides and proteins, contributing to complement regulation, pro-hormone and neuropeptide processing at the cell surface, and coagulation-fibrinolysis [72]. Its role in human disease is yet to be defined.
3.7. Urokinase plasminogen activator receptor (UPAR)
UPAR was originally identified as the membrane receptor of the serine protease urokinase, thereby implicated in the plasminogen activation and the coagulation cascade. To date, more than 40 proteins, soluble plasma ligands and cell membrane lateral partners, are known to directly interact with UPAR. For example, factor XII and high molecular weight kinin-free kininogen link UPAR with the contact system (which consists of factor XII, kininogen and pre-kallikrein), complement and fibrinolysis system. The fact that UPAR interacts with members of three major families of membrane receptors i.e. G protein-coupled receptors, tyrosine kinases, and integrins implies that the actual number of components constituting UPAR's interacome is high. Indeed, UPAR's expression was found to be up-regulated in various malignancies, inflammatory and infectious conditions. Moreover, UPAR has been found to have a role central nervous system, promoting neuro-repair following ischemic brain injury or language development. Mutations in SRPX2 gene, which acts as an UPAR ligand, have been linked to bilateral perisylvian polymicrogyria, rolandic epilepsy, speech dyspraxia and cognitive disability [[73], [74], [75]].
Paroxysmal nocturnal haemoglobinuria (PNH) is a clonal bone marrow disorder caused by PIGA gene mutation in a hematopoietic stem cell. Deficiency of the GPI-anchored complement regulators CD55 and CD59 on red cells surface leads to intravascular haemolysis upon complement activation. However, in patients with PNH, thrombosis is the most serious clinical complication with a predilection for the hepatic, abdominal and cerebral veins. Sloand and al. showed that GPI-anchored UPAR is decreased or absent on the surface of granulocytes and platelets of patients with PNH while a soluble UPAR, lacking its GPI anchor, is found increased in patient's plasma. Moreover, serum soluble UPAR concentrations correlated with the size of the PNH clone and were highest in patients who later developed thrombosis [76]. Propensity to venous thrombosis was also described by Almeida et al. in three patients with a PIGM hypomorphic promoter mutation and severe clinical phenotype [77].
Mal de Meleda is a rare skin disorder caused by SLURP1 gene mutations, in which affected individuals present palmoplantar keratoderma and ichthyotic changes elsewhere. Human Ly-6/UPAR molecules are a superfamily composed of two subfamilies; one is the GPI-anchor membrane bound proteins (such as CD59, GHPIHBP1 and UPAR) and the other are secreted proteins without the GPI-anchor such as SLURP1 (Secreted Ly6/UPAR Related Protein1). Patients with a clinical diagnosis of CHIME syndrome, and PIGL proven mutations, are usually described having migratory ichthyosiform dermatosis and palmar/plantar hyperkeratosis [[78], [79], [80], [81]]. Moreover, Morren and al. also described a patient with PIGO mutations and psychomotor disability, epilepsy, palmoplantar keratoderma, hyperphosphatasia and platelet dysfunction [82]. The biochemical basis explaining this dermatologic phenotype in patients with GPI-AP defects remains to be established.
3.8. Non-collagenous glycoproteins of the acellular gels of the inner ear - alpha / beta tectorin and otoancorin
The mammalian inner ear consists of the cochlea, the organ of hearing, and the vestibule, which is responsible for balance. The apical surface of each sensory organ is covered by an acellular gel - the tectorial membrane (TM), which contacts the stereocilia bundles of specialized sensory hair cells. Sound induces movement of these hair cells relative to the tectorial membrane, deflects the stereocilia, and leads to fluctuations in the membrane potential, transducing sound into electrical signals. All of the noncollageneous N-glycoproteins of the TM that have been described so far in mammals, namely α-tectorin, β-tectorin are molecules unique to the inner ear. Tectorins are targeted to the apical surface of the inner ear epithelia by the GPI anchor. There, alpha-tectorin forms homomeric filaments after self-association or heteromeric filaments after association with beta-tectorin. Mutations in the gene coding for alpha-tectorin, DFNB21, have been shown to be responsible for autosomal dominant non-syndromic hearing impairment and a recessive form of sensorineural pre-lingual non-syndromic deafness [83]. Localised at the interface between the apical surface of the sensory epithelia and the overlying TM, otoancorin ensures their attachement. Mutations in the otoancorin gene, DFNB22, are associated with autosomal recessive deafness [84].
4. Discussion
Genetic mutations disrupting biosynthesis or GPI-APs remodelling represent an emerging class of congenital glycosylation disorders. Clinically, the pervasive neurologic disability associates multi-system involvement. Estimated to more than 150, these glycosylated proteins play a wide variety of physiological roles in immune response regulation, cellular and intercellular signalling, cell adhesion and migration, neural and embryonic development, and cell metabolism.
The aim of this research was to identify potentially disrupted metabolic pathways, helping the clinician in the pursuit of early diagnosis and management. A combined database search and literature review was employed to identify GPI-APs with known pathology and treatment. GPI-APs have an established role in vitamin-B6, folate and LPL transport. Moreover, cellular signalling is disrupted when 5NTE, tectorin / otoancorin and UPAR's expression at the plasma membrane is impaired.
Patients with TNAP mutations that cause a severe phenotype, present pyridoxine-responsive seizures, undetectable alkaline phosphatase (ALP) and high plasma and CSF PLP. Other, milder presentations, do not associated seizures but persistent low ALP and high plasma PLP across childhood and puberty. Persistent high PLP levels may explain low homocysteine secondary to plasma CBS activation. However, age-dependent CSF PLP decline may be more abrupt and is expected to reach deficient range. Clinical data supports low ALP levels in patients with biosynthetic GPI-APs defects. High ALP plasma levels were also reported in patients with GPI-AP anchor remodelation defects, due to shedding of the protein from the plasma membrane. Other GPI-AP phenotypes describe normal or fluctuant ALP.
The hallmarks of defective PLP transport across blood-brain barrier and cellular membranes is high cystathionine in both CSF and plasma. Intracellular B6 deficiency will disturb transsulfuration pathway, 1‑carbon and nucleotide metabolism. Mitochondrial and nuclear DNA replication, energy metabolism and cell signalling are predictable consequences. Defective 1-carbon metabolism will directly decrease 5MTHF, as shown in other B6-deficient disorders, directing the flow towards re-methylation rather than nucleotide biosynthesis. This will worsen the neural folate metabolism caused by defective folate transport as FOLR1 is also affected.
Multi-antiepileptic resistant seizures were virtually described in all patients with GPI-APs defects. High dose pyridoxine was attempted in patients with HPMR phenotype with clinical benefit [[85], [86], [87]]. However, combined pyridoxine and folinic supplementation to overcome the transport defects has not been reported yet. Furthermore, abnormal expression of the 5NTE at the plasma membranes disrupts nucleotide salvage pathway and purinergic signalling. Uridine supplementation is a potential therapeutic tool in rescuing both pathways as it acts on P2Y4/6 purinergic receptors.
Hyperlipidemia is already shown to be associated with GPI-APs defects, however the onset may be dependent on the lipid metabolism regulatory complexity. The VNN role in coenzyme-A biosynthesis and disease is yet to be defined.
GPI-AP gene defects will only modify the protein expression targeted to the plasma membrane by the GPI-anchor. However, most GPI-AP are also described in trans-membrane and soluble form (intra or extra-cellular) without the attached GPI-anchor. Their role and expression need to be considered together with tissue specificity when attempting to resolve genotype-phenotype correlations. For example, complex regulation across cell types and tissues govern coagulation or complement activation and lipid homeostasis. A systems biology approach, using mathematical modelling, can provide a significant insight into GPI-APs role in modulating particular cellular functions.
Multiple post-translational modifications implicate GPI-APs in the pathophysiology of other types of glycosylation disorders and beyond. Modified plasma membrane lipid composition, in lysosomal disorders, disturbs GPI-APs membrane distribution in neurons [88]. Moreover, peroxisomal biogenesis defects were shown to perturb GPI anchor remodelling [89]. Therefore, defining GPI-APs role in disease bears significant clinical implications.
References
- 1.Varma R., Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394(6695):798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P., Varma R., Sarasij R.C., Ira Gousset K., Krishnamoorthy G., Rao M., Mayor S. Nanoscale organization of multiple. GPI-anchored proteins in living cell membranes Cell. 2004;116(4):577–589. doi: 10.1016/s0092-8674(04)00167-9. [DOI] [PubMed] [Google Scholar]
- 3.Brameshuber M., Weghuber J., Ruprecht V., Gombos I., Horváth I., Vigh L., Eckerstorfer P., Kiss E., Stockinger H., Schütz G.J. Imaging of mobile long-lived nanoplatforms in the live cell plasma membrane. J. Biol. Chem. 2010;285(53):41765–41771. doi: 10.1074/jbc.M110.182121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paladino Simona, Lebreton Stéphanie, Tivodar Simona, Formiggini Fabio, Ossato Giulia, Gratton Enrico, Tramier Marc, Coppey-Moisan Maïté, Zurzolo Chiara. Golgi sorting regulates organization and activity ofGPI-proteins at apical membranes. Nat. Chem. Biol. 2014;10(5):350–357. doi: 10.1038/nchembio.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki K.G., Kasai R.S., Hirosawa K.M., Nemoto Y.L., Ishibashi M., Miwa Y., Fujiwara T.K., Kusumi A. Transient GPI-anchored protein homodimers are units for raft organization and function. Nat. Chem. Biol. 2012;8(9):774–783. doi: 10.1038/nchembio.1028. [DOI] [PubMed] [Google Scholar]
- 6.Paladino S., Lebreton S., Zurzolo C. Trafficking and membrane organization of GPI-anchored proteins in health and diseases. Curr. Top. Membr. 2015;75:269–303. doi: 10.1016/bs.ctm.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Saha Suvrajit, Anilkumar Anupama Ambika, Mayor Satyajit. GPI-anchored protein organization and dynamics at the cell surface. J. Lipid Res. 2016;57(2):159–175. doi: 10.1194/jlr.R062885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tashima Y., Taguchi R., Murata C., Ashida H., Kinoshita T., Maeda Y. PGAP2 is essential for correct processing and stable expression of GPI-anchored proteins. Mol. Biol. Cell. 2006;17:1410–1420. doi: 10.1091/mbc.E05-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaensch N., Corrêa I.R., Jr., Watanabe R. 15(12) 2014. Stable cell surface expression of GPI-anchored proteins, but not intracellular transport, depends on their fatty acid structure Traffic; pp. 1305–1329. [DOI] [PubMed] [Google Scholar]
- 10.Seong J., Wang Y., Kinoshita T., Maeda Y. Implications of lipid moiety in oligomerization and immunoreactivities of GPI-anchored proteins. J. Lipid Res. 2013;54(4):1077–1091. doi: 10.1194/jlr.M034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zurzolo C., Simons K. Glycosylphosphatidylinositol-anchored proteins Membrane organization and transport\ Biochim Biophys Acta. 2016;1858(4):632–639. doi: 10.1016/j.bbamem.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita T., Fujita M. Biosynthesis of GPI-anchored proteins: special emphasis on GPI lipid remodeling. J. Lipid Res. 2016;57(1):6–24. doi: 10.1194/jlr.R063313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murakami Y., Kanzawa N., Saito K., Krawitz P.M., Mundlos S., Robinson P.N., Karadimitris A., Maeda Y., Kinoshita T. Mechanism for release of alkaline phosphatase caused by glycosylphosphatidylinositol deficiency in patients with hyperphosphatasia mental retardation syndrome. J. Biol. Chem. 2012;287:6318–6325. doi: 10.1074/jbc.M111.331090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jezela-Stanek A., Ciara E., Piekutowska-Abramczuk D., Trubicka J., Jurkiewicz E., Rokicki D., Mierzewska H., Spychalska J., Uhrynowska M., Szwarc-Bronikowska M., Buda P., Said A.R., Jamroz E., Rydzanicz M., Płoski R., Krajewska-Walasek M., Pronicka E. Congenital disorder of glycosylphosphatidylinositol (GPI)-anchor biosynthesis--the phenotype of two patients with novel mutations in the PIGN and PGAP2 genes. Eur. J. Paediatr. Neurol. 2016;20(3):462–473. doi: 10.1016/j.ejpn.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Krawitz P.M. PGAP2 mutations, affecting the GPI-anchor-synthesis pathway, cause hyperphosphatasia with mental retardation syndrome. Am. J. Hum. Genet. 2013;92(4):584–589. doi: 10.1016/j.ajhg.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard M.F., Murakami Y., Pagnamenta A.T., Daumer-Haas C., Fischer B., Hecht J., Keays D.A., Knight S.J., Kölsch U., Krüger U., Leiz S., Maeda Y., Mitchell D., Mundlos S., Phillips J.A., 3rd, Robinson P.N., Kini U., Taylor J.C., Horn D., Kinoshita T., Krawitz P.M. Mutations in PGAP3 impair GPI-anchor maturation, causing a subtype of hyperphosphatasia with mental retardation. Am. J. Hum. Genet. 2014;94(2):278–287. doi: 10.1016/j.ajhg.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen L., Tawamie H., Murakami Y., Mang Y., Ur Rehman S., Buchert R., Schaffer S., Muhammad S., Bak M., Nöthen M.M., Bennett E.P., Maeda Y., Aigner M., Reis A., Kinoshita T., Tommerup N., Baig S.M., Abou Jamra R. Hypomorphic mutations in PGAP2, encoding a GPI-anchor-remodeling protein, cause autosomal-recessive intellectual disability. Am. J. Hum. Genet. 2013;92(4):575–583. doi: 10.1016/j.ajhg.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiyonobu T., Inoue N., Morimoto M., Kinoshita T., Murakami Y. Glycosylphosphatidylinositol (GPI) anchor deficiency caused by mutations in PIGW is associated with west syndrome and hyperphosphatasia with mental retardation syndrome. J. Med. Genet. 2014;51(3):203–207. doi: 10.1136/jmedgenet-2013-102156. [DOI] [PubMed] [Google Scholar]
- 19.Ilkovski B., Pagnamenta A.T., O'Grady G.L., Kinoshita T., Howard M.F., Lek M., Thomas B., Turner A., Christodoulou J., Sillence D., Knight S.J., Popitsch N., Keays D.A., Anzilotti C., Goriely A., Waddell L.B., Brilot F., North K.N., Kanzawa N., Macarthur D.G., Taylor J.C., Kini U., Murakami Y., Clarke N.F. Mutations in PIGY: expanding the phenotype of inherited glycosylphosphatidylinositol deficiencies. Hum. Mol. Genet. 2015;24(21):6146–6159. doi: 10.1093/hmg/ddv331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanigawa J., Mimatsu H., Mizuno S., Okamoto N., Fukushi D., Tominaga K., Kidokoro H., Muramatsu Y., Nishi E., Nakamura S., Motooka D., Nomura N., Hayasaka K., Niihori T., Aoki Y., Nabatame S., Hayakawa M., Natsume J., Ozono K., Kinoshita T., Wakamatsu N., Murakami Y. Phenotype-genotype correlations of PIGO deficiency with variable phenotypes from infantile lethality to mild learning difficulties. Hum. Mutat. 2017;38(7):805–815. doi: 10.1002/humu.23219. [DOI] [PubMed] [Google Scholar]
- 21.Kuki I., Takahashi Y., Okazaki S., Kawawaki H., Ehara E., Inoue N., Kinoshita T., Murakami Y. Vitamin B6-responsive epilepsy due to inherited GPI deficiency. Neurology. 2013;81(16):1467–1469. doi: 10.1212/WNL.0b013e3182a8411a. [DOI] [PubMed] [Google Scholar]
- 22.Hong Y., Ohishi K., Kang J.Y., Tanaka S., Inoue N., Nishimura J., Maeda Y., Kinoshita T. Human PIG-U and yeast Cdc91p are the fifth subunit of GPI Transamidase that attaches GPI-anchors to proteins. Mol. Biol. Cell. 2003;14(5):1780–1789. doi: 10.1091/mbc.E02-12-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skauli Nadia, Wallace Sean, Chiang Samuel C.C., Barøy Tuva, Holmgren Asbjørn, Stray-Pedersen Asbjørg, Bryceson Yenan T., Strømme Petter, Frengen Eirik, Misceo Doriana. Novel PIGT variant in two brothers: expansion of the multiple congenital anomalies-Hypotonia seizures syndrome 3 phenotype. Gene. 2016;7(12):108. doi: 10.3390/genes7120108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whyte M.P., Coburn S.P., Ryan L.M., Ericson K.L., Zhang F. Vol. 110. 2018. Hypophosphatasia: Biochemical hallmarks validate the expanded pediatric clinical nosology Bone; pp. 96–106. (2018 Jan 31) [DOI] [PubMed] [Google Scholar]
- 25.Belachew Dina, Kazmerski Traci, Libman Ingrid, Goldstein Amy C., Stevens Susan T., Deward Stephanie, Vockley Jerry, Sperling Mark A., Balest Arcangela L. Infantile Hypophosphatasia Secondary to a Novel Compound Heterozygous Mutation Presenting with Pyridoxine-Responsive Seizures. JIMD Rep. 2013;11:17–24. doi: 10.1007/8904_2013_217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Roo M.G.A., Abeling N.G.G.M., Majoie C.B., Bosch A.M., Koelman J.H.T.M., Cobben J.M., Duran M., Poll-The B.T. Infantile hypophophatasia without bone deformities presenting with severe pyridoxine-resistant seizures. Mol. Genet. Metab. 2014;111(3):404–407. doi: 10.1016/j.ymgme.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Cruz T., Gleizes M., Balayssac S., Mornet E., Marsal G., Millán J.L., Malet-Martino M., Nowak L.G., Gilard V., Fonta C. Identification of altered brain metabolites associated with TNAP activity in a mouse model of hypophosphatasia using untargeted NMR-based metabolomics analysis. J. Neurochem. 2017;140(6):919–940. doi: 10.1111/jnc.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ormazabal Aida, Oppenheim Marcus, Serrano Mercedes, García-Cazorla Angels, Campistol Jaume, Ribes Antonia, Ruiz Angeles, Moreno Juan, Hyland Keith, Clayton Peter, Heales Simon, Artuch Rafael. Pyridoxal 5′-phosphate values in cerebrospinal fluid: reference values and diagnosis of PNPO deficiency in paediatric patients. Mol. Genet. Metab. 2008;94:173–177. doi: 10.1016/j.ymgme.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Gregory Jesse F., Deratt Barbara N., Ralat Luisa Rios-Avila Maria, Stacpoole Peter W. Vitamin B6 nutritional status and cellular availability of pyridoxal 5’-phosphate govern the function of the Transsulfuration Pathway's canonical reactions and hydrogen sulfide production via side reactions. Biochimie. 2016;126:21–26. doi: 10.1016/j.biochi.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis S.R., Quinlivan E.P., Stacpoole P.W., Gregory J.F., 3rd. Plasma glutathione and cystathionine concentrations are elevated but cysteine flux is unchanged by dietary vitamin B-6 restriction in young men and women. J. Nutr. 2006;136(2):373–378. doi: 10.1093/jn/136.2.373. [DOI] [PubMed] [Google Scholar]
- 31.Lima C.P., Davis S.R., Mackey A.D., Scheer J.B., Williamson J., Gregory J.F., 3rd. Vitamin B-6 deficiency suppresses the hepatic transsulfuration pathway but increases glutathione concentration in rats fed AIN-76A or AIN-93G diets. J. Nutr. 2006;136(8):2141–2147. doi: 10.1093/jn/136.8.2141. [DOI] [PubMed] [Google Scholar]
- 32.Nijhout H.F., Gregory Jesse F., Fitzpatrick Courtney, Eugenia Cho, Yvonne Lamers K., Ulrich Cornelia M., Reed Michael C. A Mathematical Model Gives Insights into the Effects of Vitamin B-6 Deficiency on 1-Carbon and Glutathione Metabolism. J. Nutr. 2009;139(4):784–791. doi: 10.3945/jn.109.104265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.da Silva Vanessa R., Ralat Maria A., Quinlivan Eoin P., Deratt Barbara N., Garrett Timothy J., Yueh-Yun Chi, Frederik Nijhout H., Reed Michael C., Gregory Jesse F. Targeted metabolomics and mathematical modeling demonstrate that vitamin B-6 restriction alters one-carbon metabolism in cultured HepG2 cells. Am. J. Physiol. Endocrinol. Metab. 2014;307(1):E93–E101. doi: 10.1152/ajpendo.00697.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krijt Jakub, Kopecká Jana, Hnízda Aleš, Moat Stuart, Kluijtmans Leo A.J., Mayne Philip, Kožich Viktor. Determination of cystathionine beta-synthase activity in human plasma by LC-MS/MS: potential use in diagnosis of CBS deficiency. J. Inherit. Metab. Dis. 2011;34(1):49–55. doi: 10.1007/s10545-010-9178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coburn Vitamin B6 Metabolism and Interactions with TNAP. Subcell. Biochem. 2015;76:207–238. doi: 10.1007/978-94-017-7197-9_11. [DOI] [PubMed] [Google Scholar]
- 36.Thompson M.D., Nezarati M.M., Gillessen-Kaesbach G., Meinecke P., Mendoza-Londono R., Mornet E., Brun-Heath I., Squarcioni C.P., Legeai-Mallet L., Munnich A., Cole D.E. Hyperphosphatasia with seizures, neurologic deficit, and characteristic facial features: five new patients with Mabry syndrome. Am. J. Med. Genet. A. 2010;152A:1661–1669. doi: 10.1002/ajmg.a.33438. [DOI] [PubMed] [Google Scholar]
- 37.Wibowo Ardian S., Singh Mirage, Reeder Kristen M., Carter Joshua J., Kovach Alexander R., Meng Wuyi, Ratnam Manohar, Zhang Faming, Dann Charles E. Structures of human folate receptors reveal biological trafficking states and diversity in folate and antifolate recognition. PNAS. 2013;110(38):15180–15188. doi: 10.1073/pnas.1308827110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grapp Marcel, Wrede Arne, Schweizer Michaela, Hüwel Sabine, Galla Hans-Joachim, Snaidero Nicolas, Simons Mikael, Bückers Johanna, Low Philip S., Urlaub Henning, Gärtner Jutta, Steinfeld Robert. Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat. Commun. 2013;4:2123. doi: 10.1038/ncomms3123. [DOI] [PubMed] [Google Scholar]
- 39.Ritter T.E., Fajardo O., Matsue H., Anderson R.G., Lacey S.W. Folate receptors targeted to clathrin-coated pits cannot regulate vitamin uptake. Proc. Natl. Acad. Sci. U. S. A. 1995;92(9):3824–3828. doi: 10.1073/pnas.92.9.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu H., Wlodarczyk B.J., Scott M., Yu W., Merriweather M., Gelineau-van Waes J., Schwartz R.J., Finnell R.H. Cardiovascular abnormalities in Folr1 knockout mice and folate rescue Birth Defects Res A. Clin Mol Teratol. 2007;79(4):257–268. doi: 10.1002/bdra.20347. [DOI] [PubMed] [Google Scholar]
- 41.Kovacs Z., Dobolyi A., Kekesi K.A., Juhasz G. 5'-Nucleotidases Nucleosides and their Distribution in the Brain: Pathological and Therapeutic Implications. Curr. Med. Chem. 2013;20(34):4217–4240. doi: 10.2174/0929867311320340003. [DOI] [PubMed] [Google Scholar]
- 42.Yegutkin G.G. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: functional implications and measurement of activities. Crit. Rev. Biochem. Mol. Biol. 2014;49(6):473–497. doi: 10.3109/10409238.2014.953627. [DOI] [PubMed] [Google Scholar]
- 43.Burnstock Geoffrey. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol. Rev. 2006;58(1):58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- 44.Boison Detlev. Adenosinergic signaling in epilepsy. Neuropharmacology. 2016;104:131–139. doi: 10.1016/j.neuropharm.2015.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonan C.D., Walz R., Pereira G.S., Worm P.V., Battastini A.M., Cavalheiro E.A., Izquierdo I., Sarkis J.J. Changes in synaptosomal ectonucleotidase activities in two rat models of temporal lobe epilepsy. Epilepsy Res. 2000;39(3):229–238. doi: 10.1016/s0920-1211(00)00095-4. [DOI] [PubMed] [Google Scholar]
- 46.Cognato Gde P., Bruno A.N., da Silva R.S., Bogo M.R., Sarkis J.J., Bonan C.D. Antiepileptic drugs prevent changes induced by pilocarpine model of epilepsy in brain ecto-nucleotidases. Neurochem. Res. 2007;32(6):1046–1055. doi: 10.1007/s11064-006-9272-y. [DOI] [PubMed] [Google Scholar]
- 47.Barros-Barbosa A.R., Ferreirinha F., Oliveira Â., Mendes M., Lobo M.G., Santos A., Rangel R., Pelletier J., Sévigny J., Cordeiro J.M., Correia-De-Sá P. 12(4) 2016. Adenosine A2A receptor and ecto-5′-nucleotidase/CD73 are upregulated in hippocampal astrocytes of human patients with mesial temporal lobe epilepsy (MTLE) Purinergic Signal; pp. 719–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diamond M.L., Ritter A.C., Jackson E.K., Conley Y.P., Kochanek P.M., Boison D., Wagner A.K. Genetic variation in the adenosine regulatory cycle is associated with posttraumatic epilepsy development. Epilepsia. 2015;56(8):1198–1206. doi: 10.1111/epi.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chu Stephanie, Xiong Wei, Parkinson Fiona E. Effect of ecto-5′-nucleotidase (eN) in astrocytes on adenosine and inosine formation. Purinergic Signal. 2014;10(4):603–609. doi: 10.1007/s11302-014-9421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wurm A., Lipp S., Pannicke T., Linnertz R., Krügel U., Schulz A., Färber K., Zahn D., Grosse J., Wiedemann P., Chen J., Schöneberg T., Illes P., Reichenbach A., Bringmann A. Endogenous purinergic signaling is required for osmotic volume regulation of retinal glial cells. J. Neurochem. 2010;112(5):1261–1272. doi: 10.1111/j.1471-4159.2009.06541.x. [DOI] [PubMed] [Google Scholar]
- 51.Page Theodore, Yu Alice, Fontanesi John, Nyhan William L. Developmental disorder associated with increased cellular nucleotidase activity. Proc. Natl. Acad. Sci. U. S. A. 1997;94(21):11601–11606. doi: 10.1073/pnas.94.21.11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johannes Koch, Johannes A. Mayr, Bader Alhaddad, Christian Rauscher, Jo rgen Bierau, Reka Kovacs-Nagy, Karlien L. M. Coene, Ingrid Bader, Monika Holzhacker, Holger Prokisch, Hanka Venselaar, Ron A. Wevers, Felix Distelmaier, Tilman Polster, Steffen Leiz, Cornelia Betzler, Tim M. Strom, Wolfgang Sperl, Thomas Meitinger, Saskia B. Wortmann and Tobias B. Haack (2017) CAD mutations and uridine-responsive epileptic encephalopathy Brain 140; 279–286. [DOI] [PubMed]
- 55.Loughner Chelsea L., Bruford Elspeth A., McAndrews Monica S., Delp Emili E., Swamynathan Sudha, Shivalingappa K., Swamynathan Organization, evolution and functions of the human and mouse Ly6/uPAR family genes. Hum Genomics. 2016;10:10. doi: 10.1186/s40246-016-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young S.G., Davies B.S.J., Voss C.V., Gin P., Weinstein M.M., Tontonoz P., Reue K., Bensadoun A., Fong L.G., Beigneux A.P. GPIHBP1, an endothelial cell transporter for lipoprotein lipase. J. Lipid Res. 2011;52(11):1869–1884. doi: 10.1194/jlr.R018689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franssen R., Young S.G., Peelman F., Hertecant J., Sierts J.A., Schimmel A.W., Bensadoun A., Kastelein J.J., Fong L.G., Dallinga-Thie G.M. Chylomicronemia with low postheparin lipoprotein lipase levels in the setting of GPIHBP1 defects. Circ. Cardiovasc. Genet. 2013;3(2):169–178. doi: 10.1161/CIRCGENETICS.109.908905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Charriere S., Peretti N., Bernard S., Di Filippo M., Sassolas A., Merlin M., Delay M., Debard C., Lefai E., Lachaux A., Moulin P., Marcais C. GPIHBP1 C89F Neomutation and hydrophobic C-terminal domain G175R mutation in two pedigrees with severe hyperchylomicronemia. J. Clin. Endocrinol. Metab. 2011;96(10):E1675–E1679. doi: 10.1210/jc.2011-1444. [DOI] [PubMed] [Google Scholar]
- 59.Coca-Prieto I., Kroupa O., Gonzalez-Santos P., Magne J., Olivecrona G., Ehrenborg E., Valdivielso P. Childhood-onset chylomicronaemia with reduced plasma lipoprotein lipase activity and mass Identification of a novel GPIHBP1 mutation. J. Intern. Med. 2011;270(3):224–228. doi: 10.1111/j.1365-2796.2011.02361.x. [DOI] [PubMed] [Google Scholar]
- 60.Weinstein Michael M., Goulbourne Christopher, Davies Brandon S.J., Tu Yiping, Barnes Richard H., II, Watkins Steven M., Davis Ryan, Reue Karen, Tontonoz Peter, Beigneux Anne P., Fong Loren G., Young Stephen G. Reciprocal Metabolic Perturbations in the Adipose Tissue and Liver of GPIHBP1-deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2012;32(2):230–235. doi: 10.1161/ATVBAHA.111.241406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tarailo-Graovac Maja, Sinclair Graham, Stockler-Ipsiroglu Sylvia, Van Allen Margot, Rozmus Jacob, Shyr Casper, Biancheri Roberta, Tracey Oh., Sayson Bryan, Lafek Mirafe, Ross Colin J., Robinson Wendy P., Wasserman Wyeth W., Rossi Andrea, van Karnebeek Clara D.M. The genotypic and phenotypic spectrum of PIGA deficiency. Orphanet J Rare Dis. 2015;10:23. doi: 10.1186/s13023-015-0243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pagnamenta Alistair T., Murakami Yoshiko, Anzilotti Consuelo, Titheradge Hannah, Oates Adam J., Morton Jenny, The DDD Study, Kinoshita Taroh, Kini Usha, Taylor Jenny C. A homozygous variant disrupting the PIGH start-codon is associated with developmental delay, epilepsy, and microcephaly. Hum. Mutat. 2018;39(6):822–826. doi: 10.1002/humu.23420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boersma Y.L., Newman J., Adams T.E., Cowieson N., Krippner G., Bozaoglu K., Peat T.S. The structure of vanin 1: a key enzyme linking metabolic disease and inflammation. Acta Crystallogr D Biol Crystallogr. 2014;70(Pt 12):3320–3329. doi: 10.1107/S1399004714022767. [DOI] [PubMed] [Google Scholar]
- 64.Pitari G., Malergue F., Martin F., Philippe J.M., Massucci M.T., Chabret C., Maras B., Duprè S., Naquet P., Galland F. Pantetheinase activity of membrane-bound Vanin-1: lack of free cysteamine in tissues of Vanin-1 deficient mice. FEBS Lett. 2000;483(2–3):149–154. doi: 10.1016/s0014-5793(00)02110-4. [DOI] [PubMed] [Google Scholar]
- 65.Hosohata K. Role of Oxidative Stress in Drug-Induced Kidney Injury. Int. J. Mol. Sci. 2016:17(11). doi: 10.3390/ijms17111826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang B., Lo C., Shen L., Sood R., Jones C., Cusmano-Ozog K., Park-Snyder S., Wong W., Jeng M., Cowan T., Engleman E.G., Zehnder J.L. The role of vanin-1 and oxidative stress-related pathways in distinguishing acute and chronic pediatric ITP. Blood. 2011;117(17):4569–4579. doi: 10.1182/blood-2010-09-304931. [DOI] [PubMed] [Google Scholar]
- 67.Yamashita N., Yashiro M., Ogawa H., Namba H., Nosaka N., Fujii Y., Morishima T., Tsukahara H., Yamada M. Metabolic pathway catalyzed by Vanin-1 pantetheinase plays a suppressive role in influenza virus replication in human alveolar epithelial A549 cells. Biochem. Biophys. Res. Commun. 2017;489(4):466–471. doi: 10.1016/j.bbrc.2017.05.172. [DOI] [PubMed] [Google Scholar]
- 68.Gensollen T., Bourges C., Rihet P., Rostan A., Millet V., Noguchi T., Bourdon V., Sobol H., Dubuquoy L., Bertin B., Fumery M., Desreumaux P., Colombel J.F., Chamaillard M., Hebuterne X., Hofman P., Naquet P., Galland F. Functional polymorphisms in the regulatory regions of the VNN1 gene are associated with susceptibility to inflammatory bowel diseases. Inflamm. Bowel Dis. 2013;19(11):2315–2325. doi: 10.1097/MIB.0b013e3182a32b03. [DOI] [PubMed] [Google Scholar]
- 69.Theodoulou F.L., Sibon O.C., Jackowski S., Gout I. Coenzyme A and its derivatives: renaissance of a textbook classic. Biochem. Soc. Trans. 2014;42(4):1025–1032. doi: 10.1042/BST20140176. [DOI] [PubMed] [Google Scholar]
- 70.Venco P., Dusi S., Valletta L., Tiranti V. Alteration of the coenzyme A biosynthetic pathway in neurodegeneration with brain iron accumulation syndromes. Biochem. Soc. Trans. 2014;42(4):1069–1074. doi: 10.1042/BST20140106. [DOI] [PubMed] [Google Scholar]
- 71.Kersten S. Integrated physiology and systems biology of PPARα. Molecular Metabolism. 2014;3(4):354–371. doi: 10.1016/j.molmet.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deiteren Kathleen, Hendriks Dirk, Scharpé Simon, Lambeir Anne Marie. Carboxypeptidase M: Multiple alliances and unknown partners. Clin. Chim. Acta. 2009;399:24–39. doi: 10.1016/j.cca.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Eden Gabriele, Archinti Marco, Furlan Federico, Murphy Ronan, Degryse Bernard. The Urokinase Receptor Interactome. Curr. Pharmaceutical Design. 2011;17(19):1874–1889. doi: 10.2174/138161211796718215. [DOI] [PubMed] [Google Scholar]
- 74.D'Alessio S., Blasi F. The urokinase receptor as an entertainer of signal transduction. Front Biosci (Landmark Ed) 2009;14:4575–4587. doi: 10.2741/3550. [DOI] [PubMed] [Google Scholar]
- 75.Smith H.W., Marshall C.J. Regulation of cell signalling by uPAR. Nat. Rev. Mol. Cell Biol. 2010;11(1):23–36. doi: 10.1038/nrm2821. [DOI] [PubMed] [Google Scholar]
- 76.Sloand E.M., Pfannes L., Scheinberg P., More K., Wu C.O., Horne M., Young N.S. Increased soluble urokinase plasminogen activator receptor (suPAR) is associated with thrombosis and inhibition of plasmin generation in paroxysmal nocturnal hemoglobinuria (PNH) patients. Exp. Hematol. 2008;36(12):1616–1624. doi: 10.1016/j.exphem.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Almeida A.M., Murakami Y., Layton D.M., Hillmen P., Sellick G.S., Maeda Y., Richards S., Patterson S., Kotsianidis I., Mollica L., Crawford D.H., Baker A., Ferguson M., Roberts I., Houlston R., Kinoshita T., Karadimitris A. Hypomorphic promoter mutation in PIGM causes inherited glycosylphosphatidylinositol deficiency. Nat. Med. 2006;12(7):846–851. doi: 10.1038/nm1410. [DOI] [PubMed] [Google Scholar]
- 78.Zunich J., Kaye C.I. New syndrome of congenital ichthyosis with neurologic abnormalities. Am. J. Med. Genet. 1983;15(2):331–333. doi: 10.1002/ajmg.1320150217. [DOI] [PubMed] [Google Scholar]
- 79.Tinschert S., Anton-Lamprecht I., Albrecht-Nebe H., Audring H. Neuroectodermal syndrome: migratory ichthyosiform dermatosis, colobomas, and other abnormalities. Pediat Derm. 1996;13(5):363–371. doi: 10.1111/j.1525-1470.1996.tb00702.x. [DOI] [PubMed] [Google Scholar]
- 80.Shashi V., Zunich J., Kelly T.E., Fryburg J.S. Neuroectodermal (CHIME) syndrome: an additional case with long term follow up of all reported cases. J. Med. Genet. 1995;32(6):465–469. doi: 10.1136/jmg.32.6.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ng BG, Hackmann K, Jones MA, Eroshkin AM, He P, Wiliams R, Bhide S, Cantagrel V, Gleeson JG, Paller AS, Rhonda E. Schnur, Sigrid Tinschert, Janice Zunich, Madhuri R. Hegde, and Hudson H. Freeze (2012) Mutations in the glycosylphosphatidylinositol gene PIGL cause CHIME syndrome. Am. J. Hum. Genet. 90(4):685–688. [DOI] [PMC free article] [PubMed]
- 82.Morren M.A., Jaeken J., Visser G., Salles I., Van Geet C., BioResource N.I.H.R., Simeoni I., Turro E., Freson K. PIGO deficiency: palmoplantar keratoderma and novel mutations. Orphanet J Rare Dis. 2017;12(1):101. doi: 10.1186/s13023-017-0654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Verhoeven K., Van Laer L., Kirschhofer K., Legan P.K., Hughes D.C., Schatteman I., Verstreken M., Van Hauwe P., Coucke P., Chen A., Smith R.J., Somers T., Offeciers F.E., Van de Heyning P., Richardson G.P., Wachtler F., Kimberling W.J., Willems P.J., Govaerts P.J., Van Camp G. Mutations in the human alpha-tectorin gene cause autosomal dominant non-syndromic hearing impairment. Nat. Genet. 1998;19(1):60–62. doi: 10.1038/ng0598-60. [DOI] [PubMed] [Google Scholar]
- 84.Zwaenepoel Ingrid, Mustapha Mirna, Leibovici Michel, Verpy Elisabeth, Goodyear Richard, Liu Xue Zhong, Nouaille Sylvie, Nance Walter E., Kanaan Moien, Avraham Karen B., Tekaia Fredj, Loiselet Jacques, Lathrop Marc, Richardson Guy, Petit Christine. Otoancorin, an inner ear protein restricted to the interface between the apical surface of sensory epithelia and their overlying acellular gels, is defective in autosomal recessive deafness DFNB22. Proc. Natl. Acad. Sci. U. S. A. 2002;99(9):6240–6245. doi: 10.1073/pnas.082515999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thompson M.D., Killoran A., Percy M.E., Nezarati M., Cole D.E., Hwang P.A. Hyperphosphatasia with neurologic deficit: a pyridoxine-responsive seizure disorder? Pediatr. Neurol. 2006;34:303–307. doi: 10.1016/j.pediatrneurol.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 86.Kuki I., Takahashi Y., Okazaki S., Kawawaki H., Ehara E., Inoue N., Kinoshita T., Murakami Y. Vitamin B6-responsive epilepsy due to inherited GPI deficiency. Neurology. 2013;81(16):1467–1469. doi: 10.1212/WNL.0b013e3182a8411a. [DOI] [PubMed] [Google Scholar]
- 87.Sakaguchi Tomohiro, Žigman Tamara, Ramadža Danijela Petković, Omerza Lana, Pušeljić Silvija, Hrvaćanin Zrinka Ereš, Miyake Noriko, Matsumoto Naomichi, Barić Ivo. A novel PGAP3 mutation in a Croatian boy with brachytelephalangy and a thin corpus callosum. Hum Genome Var. 2018;5:18005. doi: 10.1038/hgv.2018.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Galvan C., Camoletto P.G., Cristofani F., Van Veldhoven P.P., Ledesma M.D. Anomalous surface distribution of glycosyl phosphatidyl inositol-anchored proteins in neurons lacking acid sphingomyelinase. Mol. Biol. Cell. 2008;19(2):509–522. doi: 10.1091/mbc.E07-05-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Noriyuki Kanzawa, Nobuyuki Shimozawa, Ronald J. A. Wanders, Kazutaka Ikeda, Yoshiko Murakami, Hans R. Waterham, Satoru Mukai, Morihisa Fujita, Yusuke Maeda, Ryo Taguchi, Yukio Fujiki and Taroh Kinoshita (2012) Defective lipid remodeling of GPI anchors in peroxisomal disorders, Zellweger syndrome, and rhizomelic chondrodysplasia punctata J. Lipid Res. 53(4): 653–663. [DOI] [PMC free article] [PubMed]