Abstract
Objective:
The purpose of this study was to examine the unique contribution of psychosocial factors including perceived social support, depression, and resilience to communicative participation in adult survivors of head and neck cancer (HNC).
Study Design:
Cross-sectional
Setting:
University-based laboratory and speech clinic
Subjects and Methods:
Adult survivors of HNC who were at least 2 years posttreatment for HNC completed patient-reported outcome measures including those related to communicative participation and psychosocial function. Multiple linear regression analysis was conducted to predict communicative participation. Self-rated speech severity, cognitive function, laryngectomy status, and time since diagnosis were entered first as a block of variables (block 1), and psychosocial factors were entered second (block 2).
Results:
88 adults who were on average 12.2 years post HNC diagnosis participated. The final regression model predicted 58.2% of the variance in communicative participation (full model R2 = 0.58, P < .001). Self-rated speech severity, cognitive function, laryngectomy status, and time since diagnosis together significantly predicted 46.1% of the variance in block 1. Perceived social support, depression, resilience and interactions significantly and uniquely predicted 12.1% of the additional variance in block 2.
Conclusion:
Psychosocial factors such as perceived depression warrant consideration when counseling HNC patients about communication outcomes and when designing future studies related to rehabilitation.
Keywords: head and neck cancer, cancer outcomes, psychosocial outcomes, communication disorders, perceived depression
INTRODUCTION
In 2016, there were 61,760 new cases of head and neck cancer (HNC) diagnosed in the United States.1 Treatments for head and neck cancer (HNC) often result in alterations to the speech and voice mechanism. Difficulties in verbal communication may affect a person’s relationships and participation in everyday roles.2 Consequently, communication in everyday activities, or communicative participation, has emerged as an important patient-reported outcome (PRO) measure.3
Communicative participation has been defined as “taking part in life situations where knowledge, information, ideas, or feelings are exchanged”.4 Changes to voice quality and reduced speech intelligibility are the most obvious barriers to communicative participation in patients with HNC. Yet, beyond voice and speech function, treatment-related variables such as tumor site (e.g., laryngeal), treatment type (e.g., laryngectomy), and symptoms such as fatigue, xerostomia, and poor dentition also may negatively affect communication.5,6
One recent study6 investigated factors that predicted communicative participation in HNC survivors. Results revealed that the strongest predictor among 17 variables was self-rated speech severity, accounting for 22.7% of the variance in scores; better communicative participation was associated with better speech. Better communicative participation also was associated with better cognitive function (19.3%), not having undergone total laryngectomy surgery (3.5%), and longer time since diagnosis (0.7%). Interestingly, variables such as cancer location, age, sex, and self-reported fatigue or pain, among others, did not emerge as significant predictors. Thus, in HNC survivors, many of the contributing factors remain unknown, with 54% of the variance remaining unaccounted for in the model.
In other patient populations, psychosocial factors have been identified as significant predictors of communicative participation.7 For example, factors such as perceived social support have been found to affect communicative participation. This is an important consideration as HNC patients often identify social support as a benefit in their lives.8–10 Other psychosocial factors such as depression, as well as how patients cope in the face of adversity, known as resilience, have been shown to be important predictors of health-related quality of life in HNC.11,12 While health-related quality of life outcomes are not synonymous with communicative participation, the constructs are related.3 Thus, we might hypothesize that decreased depression and increased resilience also might result in better communication outcomes.
The purpose of this study, therefore, was to examine the unique contribution of psychosocial factors including perceived social support, depression, and resilience to communicative participation in adult survivors of HNC. Identifying the novel contribution of these psychosocial variables to communicative participation has implications for counseling and providing targets for rehabilitation.
METHODS
All procedures were approved by the Institutional Review Board at the University of Washington. Participants provided written, informed consent in English.
Participants
A convenience sample was recruited through follow-up clinics at the University of Washington Medical Center, in person and online support groups, and professional contacts from November 14, 2014, through May 10, 2017. All participants were community-dwelling adults, 18 years or older, who had completed treatment for HNC at least 2 years prior to their participation. The 2 year inclusion criterion was selected to ensure that participants had lived with the consequences of HNC long enough to have experienced a wide range of communication situations, and to avoid the fluctuation in scores that may occur immediately posttreatment.13 Participants completed questionnaires in English and reported no additional medical conditions (beyond HNC) that affected speech or voice. Participants were paid $20 for completing questionnaires.
Data Collection
Using a battery of self-report tools, participants answered questions about their medical history and demographic factors. Because of the limitations of self-report, treatment information was collected only for descriptive purposes. Participants also completed validated PRO tools to measure communicative participation and psychosocial factors. PRO measures were administered either using paper forms or online through a secure website as per participant preference. Participants who did not complete the questionnaires within three weeks were contacted once for follow-up.
Primary Outcome Measure
Communicative participation was assessed using the Communicative Participation Item Bank (CPIB) short form, a 10-item PRO measure that asks participants to rate how much their “condition” (i.e., HNC) interferes with communication in everyday situations (e.g., getting your turn in a fast-moving conversation).3,14 Ratings range from not at all to very much on a four point Likert-type scale. Summary scores may be converted to T-scores (M = 50; SD = 10), with higher scores representing better communicative participation. The CPIB short form has been validated in HNC survivors.3,14
Other Patient-Reported Variables
Four variables previously identified as significant and unique covariates of communicative participation in HNC survivors were included in this study.6 Two variables related to medical history consisted of time since diagnosis (years) and laryngectomy status (total laryngectomy: yes vs. no). Self-rated speech severity was measured using a single item: patients rated their speech on a 100 mm visual analog scale, with endpoints labeled “easily understandable” (0) to “extremely difficult to understand” (100). Cognitive symptoms were measured using the Neuro-QOL Cognition short form.15 This validated PRO measure consists of 8 items rated on a five point Likert-type scale. Participants rate how often and how much difficulty they have with tasks associated with concentration, thinking, reading, and problem-solving. Summary scores are converted to T-scores (M = 50; SD = 10), with higher scores indicating higher perceived function.
Three psychosocial variables were investigated to determine their unique contribution to communicative participation. Perceived social support was measured using the Multidimensional Scale of Perceived Social Support (MSPSS).16 The MSPSS is a validated scale16,17 that consists of 12 items asking participants about their perceptions of availability of social support from family, friends, and a significant other. Items are rated on a seven point Likert-type scale, ranging from very strong disagreement to very strong agreement. Items include statements such as “There is a special person who is around when I am in need”. Summary scores range from 12 (lowest) to 84 (highest level of social support).
Resilience was measured using the Connor Davidson Resilience Scale, validated short form (CD-RISC).18,19 Participants rate a series of 10 statements about how they might react in the face of adversity (e.g., I can deal with whatever comes my way) using a five point Likert-type scale ranging from not true to true nearly all the time. The total score is the summary score (total possible = 40), with higher scores being better.
Perceived depression was measured using the depression subscale of the Hospital Anxiety and Depression Scale (HADS-D).20 Participants respond to each of 7 items, indicating how often they feel a certain way using a 4 point Likert-type scale (e.g., I can laugh and see the funny side of things). Summary scores for the subscales range from 0 (low levels of depression) to 21 (high levels of depression). A cut-off point of 8 is sensitive for depression.21
Data Analysis
Data were entered into SPSS Version 18.0. The CPIB short form T-scores were used to measure communicative participation. Standard scores were derived for the psychosocial scales (MSPSS, CD-RISC 10, HADS-D) and the NeuroQOL Cognition short form. Time since diagnosis was retained as a continuous variable (years), and laryngectomy status was effect coded (yes = +1; no = −1). Where data from individual PRO measures were incomplete, they were not included in the analysis. Prior to conducting the regression, a correlation analysis was performed to determine relationship borders among the variables.
Multiple regression with sequential predictor entry was performed to determine the unique contribution of a block of psychosocial variables to communicative participation significantly above and beyond other known covariates. This type of analysis was selected because it was appropriate for the sample size and number of variables investigated in this study, and because it reduces the potential for overfit of the model.22 The contribution of a block of known covariates was investigated first, directly replicating findings from a previous study.6 Psychosocial factors were entered second, and the significance and unique contribution of these factors were determined above and beyond variables entered in the first block. To ensure that linear regression model assumptions were tenable, normality, linearity and homoscedasticity of residuals were examined for each model.
Self-rated speech severity, cognitive symptoms, laryngectomy status, and years post-diagnosis were together entered as block 1. The main effects of the three psychosocial variables and interactions were entered together as block 2. Interactions were identified a priori as constructs that were theoretically related23 and were verified with simple correlation analysis (r > .4) conducted prior to the multiple regression.22 The final model was:
Communicative Participation = b0 + b1*Speech severity + b2*Cognitive function + b3*Laryngectomy status + b4*Time since diagnosis + b5*Social support + b6*Depression + b7*Resilience + b8*Depression x Social support + b9*Resilience x Depression + b10*Cognitive function x Depression
Blocks of variables (b1−b4; b5−b10) with significant regression coefficients at the P < .05 level were retained in the model and reported as a change in R2. Partial correlations and effect sizes (sr2) demonstrated the unique contribution of each variable to communicative participation, all others being held constant. Standard errors were reported as measures of precision.
RESULTS
Participants
Of 102 questionnaires provided to potential participants, 88 were completed and returned, representing an 86% response rate. The respondents’ mean age was 66.0 years (SD = 9.0), and the mean time since cancer diagnosis was 12.2 years (SD = 9.5). The majority of participants were male (58, [65.9%]), consistent with HNC prevalence data (see Table 1).1
TABLE 1.
Demographic Characteristics of Participants (N=88)
| Variable | No. (%) | Mean (SD) | Range | |
|---|---|---|---|---|
| Age | 66.0 (9.0) | 24 – 86 | ||
| Time since cancer diagnosis (in years) | 12.2 (9.5) | 2 – 55 | ||
| Sex | ||||
| Male | 58 (65.9) | |||
| Female | 30 (34.1) | |||
| Race / Ethnicity | ||||
| White / Caucasian | 85 (96.6) | |||
| Asian | 1 (1.1) | |||
| American Indian / Alaskan Native | 1 (1.1) | |||
| Hispanic / Latino | 1 (1.1) | |||
| Education | ||||
| High school or less | 11 (12.5) | |||
| Some vocational or college | 22 (25.0) | |||
| Vocational or college graduate | 37 (42.0) | |||
| Post-graduate (masters; PhD) | 18 (20.5) | |||
| Employment status | ||||
| Employed | 23 (26.1) | |||
| Not working or retired | 59 (67.1) | |||
| Not reported | 6 (6.8) | |||
| Living Situation | ||||
| Alone | 16 (18.2) | |||
| Family | 70 (79.5) | |||
| Not reported | 2 (2.3) | |||
| History of hearing loss | ||||
| Yes | 48 (54.5) | |||
| No | 39 (44.3) | |||
| Not reported | 1 (1.1) | |||
| Cancer Site | ||||
| Larynx | 39 (44.3) | |||
| Oropharynx / hypopharynx | 18 (20.5) | |||
| Oral cavity | 14 (15.9) | |||
| Multiple sites | 14 (15.9) | |||
| Not reported | 3 (3.4) | |||
| Cancer Treatment | ||||
| Surgery alone | 12 (13.6) | |||
| Surgery and radio(chemo)therapy | 67 (76.1) | |||
| Radio(chemo)therapy | 9 (10.2) | |||
| Total laryngectomy | ||||
| Yes | 47 (53.4) | |||
| No | 41 (46.6) | |||
| Primary communication method | ||||
| Natural speech | 35 (39.8) | |||
| Tracheoesophageal speech | 26 (29.5) | |||
| Esophageal speech | 6 (6.8) | |||
| Electrolaryngeal speech | 14 (15.9) | |||
| Other or not reported | 7 (8.0) | |||
Summary Scores
The mean T score for communicative participation in this sample was 49.94 (SD = 12.19; range = 24.20 – 71.00), suggesting a broad range of experiences. These scores are consistent with the HNC survivors on which the CPIB was normed.14 Overall, participants reported mild to moderate speech severity (M = 34.08; SD = 31.06) and average self-perceived cognitive function (M = 50.57; SD = 7.85). They also reported good overall perceived social support (M = 5.54; SD = 1.28) and resilience (M = 32.39; SD = 5.69), comparable to a similarly-aged healthy general American population.24 On average, they reported a level of perceived depression within the normal range (M = 3.94; SD = 3.44). However, 12 (13.6%) participants reported scores of 8 or above on the HADS-D, consistent with perceived depression.21 Additional summary data are provided in Table 2.
TABLE 2.
Summary of results as a function of sex, cancer site, and laryngectomy status
|
|
CPIB short form |
Speech severity |
Neuro-QoL cognition short form |
HADS-D | MSPSS | CD-RISC 10 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | M (SD) | n | M (SD) | n | M (SD) | n | M (SD) | n | M (SD) | n | M (SD) | ||
| Sex | |||||||||||||
| Female | 29 | 51.41 (11.85) |
30 | 28.77 (28.43) |
30 | 49.36 (8.46) |
30 | 4.57 (4.39) |
30 | 5.50 (1.40) |
30 | 30.10 (6.82) |
|
| Male | 56 | 49.18 (12.39) |
57 | 36.88 (32.24) |
58 | 51.90 (7.51) |
58 | 3.62 (2.82) |
56 | 5.56 (1.22) |
58 | 33.57 (4.69) |
|
| Unknown or not reported |
3 | 1 | 0 | 0 | 2 | 0 | |||||||
| Cancer Location | |||||||||||||
| Larynx | 38 | 50.02 (9.14) |
39 | 33.85 (28.73) |
39 | 52.19 (6.08) |
39 | 3.67 (3.18) |
38 | 5.49 (1.28) |
39 | 32.26 (5.34) |
|
| Oropharynx / hypopharynx |
17 | 48.54 (16.68) |
17 | 35.29 (33.30) |
18 | 48.39 (9.19) |
18 | 3.83 (3.09) |
18 | 5.39 (1.61) |
18 | 33.50 (5.40) |
|
| Oral cavity | 14 | 53.21 (11.31) |
14 | 27.71 (27.06) |
14 | 47.98 (9.57) |
14 | 5.57 (4.70) |
14 | 5.58 (1.30) |
14 | 30.64 (7.40) |
|
| Multiple sites | 13 | 49.20 (12.41) |
13 | 35.86 (38.28) |
14 | 50.44 (8.02) |
14 | 3.00 (2.75) |
13 | 5.81 (0.44) |
14 | 33.14 (4.82) |
|
| Unknown or not reported |
6 | 4 | 3 | 3 | 5 | 3 | |||||||
| Laryngectomy Status | |||||||||||||
| Total Laryngectomy |
44 | 48.38 (9.33) |
47 | 36.81 (31.25) |
47 | 51.23 (6.51) |
47 | 3.83 (3.21) |
45 | 5.54 (1.24) |
47 | 31.74 (5.60) |
|
| Not Laryngectomy |
41 | 51.61 (14.58) |
40 | 30.88 (30.92) |
41 | 49.80 (9.18) |
41 | 4.07 (3.73) |
41 | 5.54 (1.33) |
41 | 33.12 (5.76) |
|
| Unknown or not reported |
3 | 1 | 0 | 0 | 2 | 0 | |||||||
| Averages for Total Sample (complete data) | |||||||||||||
| 85 | 49.94 (12.19) |
87 | 34.08 (31.06) |
88 | 50.57 (7.85) |
88 | 3.94 (3.44) |
86 | 5.54 (1.28) |
88 | 32.39 (5.69) |
||
Note. CPIB short form = Communicative Participation Item bank short form (T score); Speech severity rated on 100mm visual analog scale (0 = easily understandable; 100 = extremely difficult to understand); HADS-D = depression subscale of the Hospital Anxiety and Depression Scale summary score; MSPSS = Multidimensional Scale of Perceived Social Support summary score; CD-RISC 10 = Connor Davidson Resilience 10 item short form summary score.
Variables Related to Communicative Participation
Correlations.
Table 3 presents the zero-order correlations among all of the investigated variables. As hypothesized, better communicative participation was associated with less severely perceived speech problems, less perceived depression, better cognitive function, and better social support.
Table 3.
Zero Order Correlations
| Variable | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. |
| Outcomes | ||||||||||
| 1.CPIB short form |
-- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
|
Block 1 Predictors |
||||||||||
| 2.SRSS+ | -.64*** | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 3.Time since diagnosis |
.004 | .13 | -- | -- | -- | -- | -- | -- | -- | -- |
|
4.Laryngectomy status+ |
-.13 | .13 | .13 | -- | -- | -- | -- | -- | -- | -- |
| 5.Neuro-QOL cognition short form |
.25** | -.090 | .15 | .13 | -- | -- | -- | -- | -- | -- |
|
Block 2 Predictors |
||||||||||
| 6.HADS-D+ | -.37*** | .16 | -.12 | -.038 | -.57*** | -- | -- | -- | -- | -- |
| 7.MSPSS | .22* | -.090 | .12 | .014 | .27** | -.46*** | -- | -- | -- | -- |
| 8.CD-RISC 10 | .14 | -.078 | .010 | -.051 | .52*** | -.67*** | .23* | -- | -- | -- |
| 9.HADS-D+ x MSPSS |
-.028 | -.037 | .015 | .040 | .11 | -.36*** | .35*** | .17 | -- | -- |
| 10.HADS-D+ x CD-RISC 10 |
-.021 | .046 | .097 | .050 | .31** | -.54*** | .094 | .45*** | .37*** | -- |
| 11.HADS-D+ x Neuro-QOL |
.15 | -.097 | -.15 | -.054 | -.17 | .43*** | .084 | -.43*** | -.21* | -.77*** |
Note. N=83 (complete data).
P < .05
P <.01
P <.001.
Pearson correlations were used for the interval (continuous) level data, while point biserial correlations were used for nominal (categorical) data. + Bold type face = higher score on PRO measure indicates worse outcome (e.g., worse speech, higher depression). Where no bolding exists, higher scores indicate better outcome. For laryngectomy status, a negative score is associated with total laryngectomy. CPIB short form = Communicative Participation Item Bank short form; SRSS = self-reported speech severity, HADS-D = depression subscale of the Hospital Anxiety and Depression scale; MSPSS = Multidimensional Scale of Perceived Social Support, CD-RISC 10 = Connor Davidson Resilience Scale 10 item short form.
Regression Model.
Results of the multiple linear regression with sequential entry are presented in Table 4. Self-rated speech severity, perceived cognitive function, time since HNC diagnosis, and laryngectomy status together accounted for 46.1% of the significant variance in communicative participation in block 1. Together, perceived social support, depression, resilience, and interactions significantly accounted for 12.1% of the additional unique variance in block 2. The final model accounted for 58.2% of the variance in communicative participation. Increased adjusted R2 values in conjunction with increased R2 values increase the validity of these results, reducing the likelihood they are due to overfit of the model or confounding effects between variables.25
Table 4.
Multiple Linear Regression with Sequential Predictor Entry for Communicative Participation.
| Block 1 |
Block 2 |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2change | R2total | R2adj | b | sr2 | R2change | R2total | R2adj | b | sr2 | |||||||
| Model Fit | 0.461 | *** | 0.460 | *** | 0.434 | 0.121 | ** | 0.582 | ** | 0.524 | ||||||
| Coeff | ||||||||||||||||
| Intercept | 57.448 | *** | 54.569 | *** | ||||||||||||
| SRSS+ | -0.246 | *** | 0.277 | -2.180 | *** | 0.276 | ||||||||||
| Time since diagnosis |
0.082 | 0.010 | 0.074 | 0.002 | ||||||||||||
|
Laryng status+ |
-0.987 | 0.003 | -0.952 | 0.005 | ||||||||||||
| Neuro- QOL cognition short form |
2.442 | * | 0.036 | 0.582 | 0.001 | |||||||||||
|
HADS- D+ |
-6.015 | *** | 0.082 | |||||||||||||
| MSPSS | -0.177 | 0.000 | ||||||||||||||
| CD- RISC 10 |
-1.847 | 0.010 | ||||||||||||||
| HADS-D x MSPSS |
-2.046 | 0.019 | ||||||||||||||
| HADS-D x CD- RISC 10 |
0.338 | 0.001 | ||||||||||||||
| HADS-D x Neuro- QOL |
2.713 | * | 0.023 | |||||||||||||
Note. N=83 (complete data).
P < 0.05
P < 0.01
P < 0.001.
Block 1 F-change test df = 4, 78; Block 2 df = 6, 72. + Bold type face = higher score on PRO measure indicates worse outcome (e.g., worse speech, higher depression). Where no bolding exists, higher scores indicate better outcome. For laryngectomy status, a negative score is associated with total laryngectomy. Coeff = coefficients, Laryng = laryngectomy status, SRSS = self-reported speech severity, HADS-D = depression subscale of the Hospital Anxiety and Depression scale; MSPSS = Multidimensional Scale of Perceived Social Support, CD-RISC 10 = Connor Davidson Resilience Scale 10 item short form.
To determine the unique contribution of individual variables to communicative participation with all others being held constant, partial correlations and their effect sizes (sr2) were examined (Table 4). In the final model, self-rated speech severity, perceived depression, and the interaction between depression and cognition uniquely predicted 27.7%, 8.2%, and 2.3% of the variance in communicative participation respectively, holding all else constant. To understand the interaction, predicted values were plotted for each group (high/low cognitive function) vs. levels of perceived depression (see Figure 1). The effect of depression on communicative participation was greater for patients with low levels of perceived cognitive function. In individuals with low cognitive function (1 SD below mean), only those with low levels of depression (1 SD below mean) experienced a benefit in communicative participation, whereas perceived depression did not have as great an effect on communicative participation in those with higher levels of cognitive function (1 SD above mean).
Figure 1.
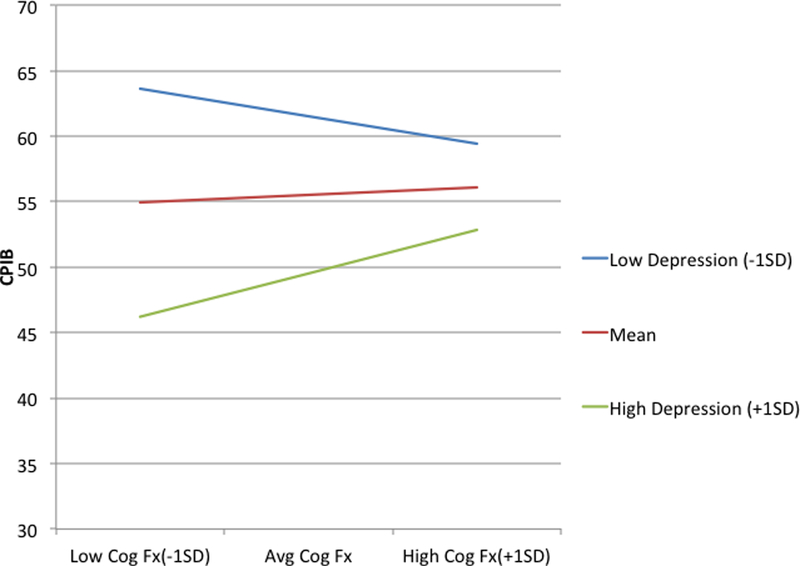
The relationship between low (−1SD) vs. high (+1SD) depression and perceived cognitive function (Cog fx) on CPIB short form T scores
DISCUSSION
Results from this study suggest that communicative participation in HNC survivors is associated with multiple variables. Consistent with a previous investigation,6 self-rated speech severity, perceived cognitive function, time since HNC diagnosis, and laryngectomy status together predicted 46.1% of the significant variance in communicative participation. In particular, self-rated speech severity was a strong, unique predictor, accounting for 27.7% of variance in the model. This result is unsurprising; other studies have shown that everyday communication is primarily affected by the severity of self-rated speech difficulties.7,26 This is particularly important among HNC survivors, whose voice and speech function often fails to return to baseline even well after treatment has ended.26, 27
While self-rated speech severity is the strongest predictor of communicative participation, other variables are associated with communication outcomes. In this study, perceived social support, depression, resilience, and interactions together accounted for an additional 12.1% of the significant unique variance in communicative participation among this group of HNC survivors. This result highlights the important and novel role of psychosocial factors, particularly since 13 other variables (e.g., disease site, self-reported measures of pain) were not found to make any significant contribution to communication outcomes previously.6
Among the psychosocial variables included in this study, perceived depression emerged as the strongest variable, accounting for 8.2% of the unique variance in communicative participation, all other variables being held constant. Those with higher levels of depression reported worse communicative participation, consistent with other patient populations.7 This result is especially salient because 12 (13.6%) participants reported scores consistent with perceived depression.21 These findings are remarkable among participants who were on average 12.2 years post-HNC diagnosis. They also are consistent with a recent study identifying 19.5% of HNC patients with depression, either in the two years prior to their cancer diagnosis or in the year following diagnosis.28 Clearly, depression remains a critical target for interventions because it affects so many HNC outcomes.11
Perceived depression also affects other areas of functioning, such as resilience. While resilience did not significantly account for unique variance in communicative participation in this study, perceived depression and resilience shared 45% of their variance (r = −.67; r2 = 0.45). Because those who are depressed may also be less likely to adapt to HNC treatment and recover, this may be a strong confounding factor in determining relationships with outcomes such as communicative participation. These relationships deserve future study in longitudinal investigations, particularly as patients experience differing levels of stress and adversity during treatment and recovery. This is important because resilience is thought to be amenable to change as part of psychosocial interventions.29
Perceived depression also emerged as a factor that was moderately associated with perceived cognitive function (r = −.57; r2 = 0.32); together, these variables interacted to uniquely predict 2.3% of the variance in communicative participation. The interaction revealed that individuals with low perceived depression reported better communicative participation. Those who had low perceived cognitive function and high depression were differentially affected in their communicative participation. At higher levels of perceived depression, we hypothesize that the more aware individuals are about their reduced cognitive function, the more they might become further isolated. In contrast, those who are less affected by problems with thinking may be better able to successfully meet their communication needs, despite some difficulties with emotional health. The link between perceived cognitive function and depression has been reported in other cancer populations,30 although the directionality of this relationship is unknown. This study also highlights the increasing importance of considering perceived cognitive function among HNC survivors.
While increased perceived social support overall was positively associated with increased communicative participation (r = .22; r2 = .048), it did not emerge as a unique predictor in this study. Previous investigations have shown equivocal results about the effects of perceived social support on various PRO measures after HNC.9,10 Yet, qualitative studies consistently reveal that supportive connections help HNC patients cope.5,8
Results from this study should be considered in light of the sample of participants. Many participants were recruited through online support groups, and all were paid. It is not known whether this approach may have negatively or positively affected outcomes. For example, individuals who seek out support groups may require more support and have worse psychosocial outcomes. In contrast, it may be that individuals involved in support groups have higher than expected outcomes due the reinforcement they receive through the group. All outcomes in this study must be interpreted with these potential biases in mind.
Results from this study also need to be considered with regard to the method of data analysis and its reporting. While the R2 metric is considered the gold standard measure of the effect size of regression, its interpretation may be limited if the model has too many predictor variables, if the variables are not carefully selected, or if the order of entry of the variables into the model is not considered.25 While these factors were considered in designing this study, it is unknown whether they affected our results.
Other limitations to this study also exist. For example, participants were on average 12.2 years post-HNC diagnosis. Future studies should include patients at earlier phases of recovery to determine whether these relationships differ throughout treatment and recovery. In addition, 47 (53.4%) participants indicated that they had undergone total laryngectomy. However, results from this study revealed that laryngectomy status did not significantly account for any unique variance in communicative participation. A previous study6 also showed that while better communicative participation was associated with not having undergone a total laryngectomy, it only accounted for 3.5% of the variance in scores. At least among the current participants, these results suggest that inclusion of a large group of patients with total laryngectomy did not significantly affect the study’s external validity. An additional limitation of this study was the self-reported nature of the data. Future research should investigate the association between communicative participation with measures such as clinician-rated speech intelligibility, vocal fold function using videolaryngostroboscopy, or clinically measured depression. Other variables that predict communicative participation also need to be identified, such as treatment type, cancer location, disease stage, severity of comorbidities, or radiation dosage. The current study was not designed to answer these questions, and is limited by the self-reported nature of the data. A final limitation of this study was its cross-sectional design; caution is warranted in interpreting associations and causality, with a need for future longitudinal (including pre-treatment) investigations to track changes over time.
Despite the stated limitations, our study demonstrates that beyond voice and speech severity, psychosocial factors such as perceived depression are important considerations when assessing communication outcomes in HNC survivors. Clinicians may use PROs such as those used in this study to inform counseling and design future interventions related to communication in everyday settings in this population.
ACKNOWLEDGMENTS
We gratefully acknowledge funding support from NIH/NCI R01CA177635 (PI: Eadie), and the University of Washington SPHSC Vocal Function Lab.
Footnotes
Note: Portions of this paper were presented at the American Head and Neck Society’s 9th International Conference on Head and Neck Cancer in Seattle, WA, in July, 2016.
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures 2016 Atlanta: American Cancer Society; 2016. [Google Scholar]
- 2.Karnell LH, Funk GF, Hoffman HT. Assessing head and neck cancer patient outcome domains. Head & Neck 2000;22(1):6–11. [DOI] [PubMed] [Google Scholar]
- 3.Eadie TL, Lamvik K, Baylor CR, Yorkston KM, Kim J, Amtmann D. Communicative participation and quality of life in head and neck cancer. Ann Otol Rhinol Laryngol 2014;123(4):257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eadie TL, Yorkston KM, Klasner ER, et al. Measuring communicative participation: A review of self-report instruments in speech-language pathology. Am J Speech Lang Pathol 2006;15(4):307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nund RL, Rumbach AF, Debattista BC, et al. Communication changes following non-glottic head and neck cancer management: the perspectives of survivors and carers. Int J Speech Lang Pathol 2015;17(3):263–272. [DOI] [PubMed] [Google Scholar]
- 6.Bolt S, Eadie T, Yorkston K, Baylor C, Amtmann D. Variables associated with communicative participation after head and neck cancer. JAMA-Otolaryngol Head Neck Surg 2016;142(12):1145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baylor C, Yorkston K, Bamer A, et al. Variables associated with communicative participation in people with multiple sclerosis: A regression analysis. Am J Speech-Lang Pathol 2010;19(2):143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fletcher BS, Cohen MZ, Schumacher K, Lydiatt W. A blessing and a curse: Head and neck cancer survivors’ experiences. Cancer Nursing 2012;35(2):126–132. [DOI] [PubMed] [Google Scholar]
- 9.Karnell LH, Christensen AJ, Rosenthal EL, Magnuson JS, Funk GF. Influence of social support on health-related quality of life outcomes in head and neck cancer. Head & Neck 2007;29(2):143–146. [DOI] [PubMed] [Google Scholar]
- 10.Howren MB, Christensen AJ, Karnell LH, Van Liew JR, Funk GF. Influence of pretreatment social support on health-related quality of life in head and neck cancer survivors: results from a prospective study. Head & Neck 2013;35(6):779–787 [DOI] [PubMed] [Google Scholar]
- 11.Howren MB, Christensen AJ, Karnell LH, Funk GF. Psychological factors associated with head and neck cancer treatment and survivorship: Evidence and opportunities for behavioral medicine. J Consult Clin Psychol 2013;81(2):299–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asiedu Y Quality of life in head & neck cancer patients: A service evaluation exploring quality of life and psychological resilience as surrogate markers Presentation at the 9th International Head & Neck Cancer Quality of Life Conference, Liverpool, UK; 2014. http://www.headandneckcancer.co.uk/File.ashx?id=13341. Accessed May 14, 2017. [Google Scholar]
- 13.Campbell BH, Marbella A., Layde PM Candidate’s thesis: quality of life and recurrence concer in survivors of head and neck cancer. Laryngscope 2000;110(6); 895–906. [DOI] [PubMed] [Google Scholar]
- 14.Baylor C, Yorkston K, Eadie T, Kim J, Chung H, Amtmann D. The Communicative Participation Item Bank (CPIB): Item bank calibration and development of a disorder-generic short form. J Speech Lang Hear Res 2013;56(4):1190–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cella D, Lai JS, Nowinski CJ et al. (2012). Neuro-QoL: Brief measures of health-related quality of life for clinical research in neurology. Neurol 2012;78(23):1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimet GD, Dahlem NW, Zimet SG, Farley GK. The Multidimensional Scale of Perceived Social Support. J Pers Assess 1988;52(1):30–41. [DOI] [PubMed] [Google Scholar]
- 17.Osman A, Lamis DA, Freedenthal S, Gutierrez PM, McNaughton-Cassill M. The Multidimensional Scale of Perceived Social Support: Analyses of internal reliability, measurement invariance, and correlates across gender. J Pers Assess 2014;96(1):103–112. [DOI] [PubMed] [Google Scholar]
- 18.Connor KM, Davidson JRT. Development of a new resilience scale: The Connor-Davidson Resilience Scale (CD-RISC). Depression Anxiety 2003;18:76–82. [DOI] [PubMed] [Google Scholar]
- 19.Campbell-Sills L, Stein MB. Psychometric analysis and refinement of the Connor-Davidson Resilience Scale (CD-RISC): validation of a 10 item measure of resilience. J Trauma Stress 2007;20:1019–28. [DOI] [PubMed] [Google Scholar]
- 20.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psych scand 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- 21.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital and Depression Scale: An updated literature review. J Psychosom Res 2002;52(2):69–77. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences (3rd ed). Mahwah, New Jersey: Laurence Erlbaum; 2003. [Google Scholar]
- 23.Richardson GE. The metatheory of resilience and resiliency. J Clin Psychol 2002;58:307–321. doi:10.1002/jclp.10020 [DOI] [PubMed] [Google Scholar]
- 24.Jeste DV, Savla GN, Thompson WK, et al. Association between older age and more successful aging: Critical role of resilience and depression. Am J Psychiatry 2013;170(2):188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider A, Hommel G, Blettner M. Linear regression analysis: Part 14 of a series on evaluation of scientific publications. Dtsch Arztebl Int 2010;107(44):776–782. doi: 10.3238/arztebl.2010.0776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eadie TL, Otero D, Cox S, et al. The relationship between communicative participation and postlaryngectomy speech outcomes. Head & Neck 2016;38(1):E1955–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dwivedi RC, Kazi RA, Agrawal N, et al. Evaluation of speech outcomes following treatment of oral and oropharygeal cancers. Cancer Treatment Rev 2009;35:417–424. [DOI] [PubMed] [Google Scholar]
- 28.Rieke K, Boilesen E, Lydiatt W, Schmid KK, Houfek J, Watanabe-Galloway S. Population-based retrospective study to investigate preexisting and new depression diagnosis among head and neck cancer patients. Cancer Epidemiol 2016;43:42–48. [DOI] [PubMed] [Google Scholar]
- 29.Luthar SS, Cicchetti D. The construct of resilience: Implications for interventions and social policies. Development Psychopathol 2000;12:857–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vardy JL, Dhillon HM, Pond GR, et al. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: A prospective, longitudinal, controlled study. J Clin Oncol 2015;33(34):4085–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]


