Abstract
Purpose:
The present study was designed to investigate our hypothesis that NADPH oxidase plays a role in radiation-induced pro-oxidative and pro-inflammatory environments in brain.
Materials and Methods:
C57BL/6 mice received either fractionated whole brain irradiation or sham-irradiation. The mRNA expression levels of pro-inflammatory mediators, such as TNF-α and MCP-1, were determined by quantitative real-time RT-PCR. The protein expression levels of TNF-α, MCP-1, NOX-2 and Iba1 were detected by immunofluorescence staining. The levels of ROS were visualized by in situ DHE fluorescence staining.
Results:
A significant up-regulation of mRNA and protein expression levels of TNF-α and MCP-1 was observed in irradiated mouse brains. Additionally, immunofluorescence staining of Iba1 showed a marked increase of microglial activation in mouse brain after irradiation. Moreover, in situ DHE fluorescence staining revealed that fractionated whole brain irradiation significantly increased production of ROS. Furthermore, a significant increase in immunoreactivity of NOX-2 was detected in mouse brain after irradiation. On the other hand, an enhanced ROS generation in mouse brain after irradiation was markedly attenuated in the presence of NOX inhibitors or NOX-2 neutralizing antibody.
Conclusions:
These results suggest that NOX-2 may play a role in fractionated whole brain irradiation-induced pro-oxidative and pro-inflammatory pathways in mouse brain.
Keywords: Fractionated whole brain irradiation, ROS, NOX-2, Inflammation
Introduction
According to the Central Brain Tumor Registry of the United States (CBTRUS), an estimated 77,670 new cases of primary malignant and non-malignant brain and central nervous system (CNS) tumors are expected to be diagnosed in the United States in 2016 (Ostrom et al. 2015). Radiation therapy has been commonly used as a standard treatment modality for patients with brain tumors. However, the use of radiotherapy for brain tumors has been restricted by the risk of radiation-induced injury to normal brain tissue, which may subsequently lead to both anatomic and functional deficits (Denham & Hauer-Jensen 2002; Stone et al. 2003; Béhin & Delattre 2004; Moulder & Cohen 2007; Greene-Schloesser et al. 2013). Radiation-induced brain injury has traditionally been classified as acute, early delayed (subacute), and late delayed responses depending on its time of onset (Tofilon & Fike 2000; Kim et al. 2008; Ramanan et al. 2010). In general, most of the symptoms and signs of acute and early delayed injuries are reversible whereas late delayed injury is considered irreversible and progressive. In addition, there is a growing awareness that late delayed injury is largely responsible for cognitive impairment, focal deficits, seizures, and increased cranial pressure even in cases with no detectable anatomic abnormalities.
Previous studies have shown that whole brain irradiation may cause a significant deterioration of learning and memory in human as well as in rodent (Akiyama et al. 2001; Brown et al. 2007; Welzel et al. 2008; Chang et al. 2009; Douw et al. 2009; Barlind et al. 2010; Liu et al. 2010; Zhou et al. 2011; Warrington et al. 2012; Warrington et al. 2013). In addition to cognitive impairment, whole brain radiation causes other brain injures including growth hormone deficiency and motor dysfunction (Darzy et al. 2005; Manda et al. 2007; Madaschi et al. 2011; Quik et al. 2012). Although there have been significant progresses in understanding pathogenesis of radiation-mediated brain injury, limited information about the etiology of radiation-induced damage to normal brain tissue is currently available.
Recent evidence has identified that oxidative stress and inflammation are important pathways leading to radiation-induced brain injury (Hong et al. 1995; Chiang et al. 1997; Olschowka et al. 1997; Denham & Hauer-Jensen 2002; Kyrkanides et al. 2002; Gaber et al. 2003; Moore et al. 2005; Baluna et al. 2006; Lee et al. 2010; Lee et al. 2012b). For example, irradiation augmented CNS inflammation through up-regulation of a variety of pro-inflammatory mediators including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, monocyte chemoattractant protein-1 (MCP-1), inducible nitric oxide synthase (iNOS), intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin, which may contribute to the radiation-induced functional impairments in the brain (Hong et al. 1995; Olschowka et al. 1997; Kyrkanides et al. 2002; Gaber et al. 2003; Moore et al. 2005; Baluna et al. 2006; Lee et al. 2010). In addition, oxidative stress may be, at least in part, a significant contributor to disastrous neurotoxic consequences in radiation-induced brain injury (Limoli et al. 2004; Collins Underwood et al. 2008; Manda et al. 2009; Baluchamy et al. 2010; Veeraraghavan et al. 2011). Furthermore, among the enzymatic pro-oxidative systems, the family of NADPH oxidase (NOX), a multi-subunit enzyme complex consisting of NOX-1 to −5, has been predominantly perceived as an important source of ROS causing serious damage to a variety of biomolecules in the brain (Infanger et al. 2006; Bedard & Krause 2007; Collins-Underwood et al. 2008; Qin & Crews 2012).
In the present study, we examined the potential role of NOX-2 in radiation-induced pro-oxidative and pro-inflammatory pathways in mouse brain. To our knowledge, our results provide the first evidence to demonstrate that NOX-2 plays a pivotal role in acute effects on radiation-induced oxidative stress and inflammation in mouse brain.
Materials and Methods
Animals
Ten-week-old C57BL/6 male mice were purchased from Jackson Laboratory (Bar Harbor, ME). Animals were housed under a 12-h light: 12-h dark cycle with food and water provided ad libitum. Animal care was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and this study was approved by the Institutional Animal Care and Use Committee.
Fractionated Whole Brain Irradiation and Tissue Sample Preparation
Following acclimation to the animal facility for a week, fractionated whole brain irradiation was performed in mice as described previously (Lee et al. 2012a). Briefly, 10-week-old mice were anesthetized with ketamine-xylazine (intraperitoneal injection, 100–15 mg/kg) and received a clinical fractionated dose of whole brain irradiation using a 137Cs γ irradiator (Gammacell® 40; Best Threratronics, Ottawa, Ontario, Canada) with a Cerrobend shield to collimate the beam only to whole mouse brain and minimize its exposure to other parts of body. In addition, the radiation dose received by the head of mice was verified using radiochromic film dosimetry, as previously described (Ashpole et al., 2014). Six dosimetry film positions were used to measure representations of the anterior and posterior surfaces as well as the centers of the head and body of the mice. Total cumulative dose of 40 Gy was given as 8 fractions of 5 Gy each, twice per week for 4 weeks; 60 Gy for acute biologically effective dose (BED10) and 106.7 Gy for late complications (BED3). Mice in the control group were only anesthetized. The mice were maintained for 4, 8, and 24 h after the last fractionated dose of whole brain irradiation (n=5 in each group). Mouse brain was rapidly removed after perfusion, hemisected at the midline, and then immediately frozen in liquid nitrogen. The left hemispheres were cryopreserved in 30% sucrose solution for 24 h and processed in Tissue-Tek® optimal cutting temperature (O.C.T.) embedding medium (Sakura Finetek USA, Inc., Torrance, CA) for in situ ROS detection and immunofluorescence staining while the right hemispheres were homogenized and prepared for RT-PCR analysis.
Real-time Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Quantitative real-time RT-PCR using TaqMan probes and primers (Applied Biosystems, Foster City, CA) were used for gene expression analyses as described previously (Lee et al. 2010). Amplification of individual genes was performed with Applied Biosystems 7300 real-time PCR system using TaqMan Universal PCR Master Mix and a standard thermal cycler protocol. TaqMan Gene Expression Assay Reagents for mouse TNF-α, MCP-1, and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) were used for specific probes and primers of PCR amplifications. The threshold cycle (CT) was determined, and relative quantification was calculated by the comparative CT method as described previously (Lee et al. 2010).
Immunofluorescence Staining
Brain sections (20 μm) were fixed in 4% (v/v) paraformaldehyde for 15 min at room temperature, rinsed with PBS, and incubated in 0.5% (v/v) Triton X-100 for 15 min. After being washed with PBS, nonspecific binding sites were blocked with 3% (w/v) bovine serum albumin (BSA) in PBS for 1 h at room temperature and incubated with the primary antibody, such as rabbit anti-MCP-1 (1:50, Santa Cruz Biotechnology Inc., Santa Cruz, CA), rabbit anti-TNF-α (1:200, Abcam, Cambridge, MA), rabbit anti-Iba1 (1:200, Wako Chemicals USA Inc., Richmond, VA) or mouse anti-NOX-2 (1:100, BD Biosciences, San Jose, CA), diluted in 1% (w/v) BSA overnight at 4°C. Sections were washed with PBS and incubated with secondary antibody, such as donkey anti-mouse IgG conjugated with Alexa Fluor 488, donkey anti-rabbit IgG conjugated with Alexa Fluor 488 or goat anti-rabbit IgG conjugated with Alexa Fluor 555, 1:400 diluted in PBS in the dark for 1 h. After being washed with PBS, the sections were mounted in Vectashield® mounting medium (Vector Labs., Inc., Burlingame, CA) and examined using a Zeiss AXIO Imager A1m fluorescence microscope. Images were acquired with an AxioCam MRc5 Digital Imaging System (Carl Zeiss MicroImaging, Inc., Thornwood, NY) by a blind observer to the experimental conditions. We mainly focused on the cerebral cortex above corpus callosum of brain to observe any molecular and cellular changes in the irradiated animals. Fluorescence intensity of acquired digital images was quantified by ImageJ software (NIH, Bethesda, MD).
In Situ Detection of ROS
In situ levels of superoxide anion, the main species of ROS, were measured by dihydroethidium (DHE) fluorescence staining. Briefly, brain sections were washed with PBS and incubated with 5 μM DHE solution in a light-protected humidified chamber at 37°C for 30 min. For NOX inhibition ex vivo, tissue sections collected from irradiated brains were incubated ex vivo with 5 μM DHE solution containing either NOX inhibitors, such as apocynin (APO; 1 mM) and diphenylene iodonium (DPI; 20 μM), or NOX-2 neutralizing antibody (1:50 dilution) in PBS at 37°C for 30 min. After incubation, the slides were rinsed with PBS and images of the cortical regions were acquired with a Zeiss AXIO Imager A1m fluorescence microscope by a blind tester. Fluorescence intensity of acquired digital images was quantified by ImageJ software.
Quantitative Analysis of Immunofluorescence and In Situ DHE Staining
For quantitative analysis of fluorescence image data, three images per section were taken and total seven sections per individual animal were analyzed for immunofluorescence and in situ DHE staining. The quantitative immunofluorescent signals from all images were subjected to threshold processing to reduce background signals and measured using the ImageJ Integrated Density analysis.
Statistical Analysis
Statistical analysis of data was completed using SigmaPlot version 11 software (SPSS, Chicago, IL). One-way analysis of variance (ANOVA) was performed to compare mean responses among groups. For each end point, the group means were compared using the Bonferroni least significant difference procedure. A statistical probability (p) value of <0.05 was considered significant.
Results
Fractionated Whole Brain Irradiation Up-regulates mRNA and Protein Expressions of TNF-α and MCP-1 in Mouse Brain
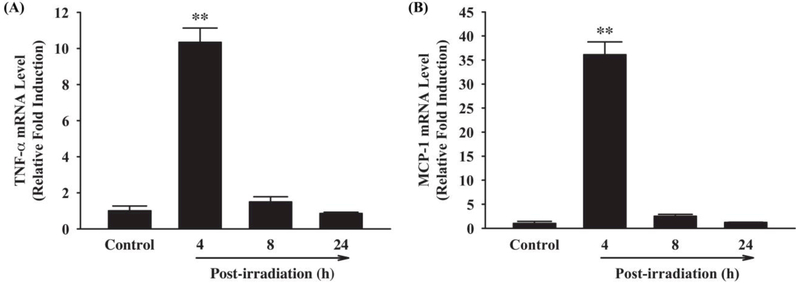
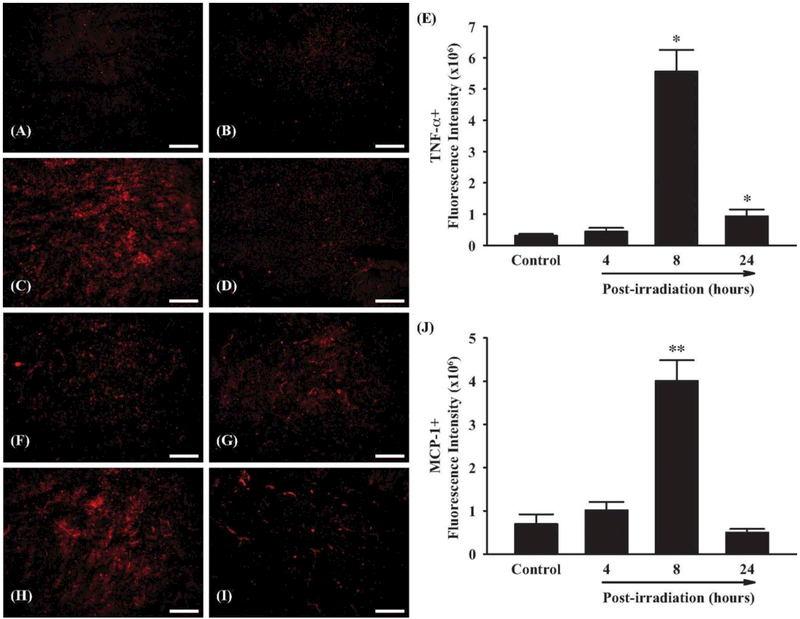
To investigate whether fractionated whole brain irradiation affects pro-inflammatory mediators in mouse brain, mRNA expression levels of TNF-α and MCP-1 were examined by quantitative real-time RT-PCR. As shown in Figure 1, a significant up-regulation of TNF-α and MCP-1 mRNA expressions was observed at 4 h post-irradiation (10.34-fold induction of TNF-α and 36.10-fold induction of MCP-1, respectively, compared to the sham-irradiated control mice). Expression of GAPDH (a housekeeping gene), however, was not affected by irradiation (data not shown). In addition, protein expression levels of TNF-α and MCP-1 in mouse brain were visualized by immunofluorescence staining. As illustrated in Figure 2, markedly increased immunoreactivities of TNF-α and MCP-1 were detected in mouse brain at 8 h after irradiation, whereas very little reactivity was observed in sham-irradiated control mouse brain (Figure 2A-D and F-I). Quantitative analysis of fluorescence intensity demonstrated that fractionated whole brain irradiation significantly induced protein expressions of TNF-α and MCP-1 in mouse brain at 8 h post-irradiation by 18.43-fold increase in TNF-α and 5.76-fold increase in MCP-1, respectively (Figure 2E and J). These results suggest that fractionated whole brain irradiation produces pro-inflammatory environment in mouse brain.
Figure 1.
Effect of fractionated whole brain irradiation on mRNA expression of TNF-α and MCP-1 in mouse brain. Compared with sham-irradiated controls, fractionated whole brain irradiation significantly up-regulated mRNA expression levels of TNF-α (A) and MCP-1 (B) in mouse brain. Data represent means ± SEM for each group (n=4). **p<0.001 compared to control.
Figure 2.
Effect of fractionated whole brain irradiation on protein expression of TNF-α and MCP-1 in mouse brain. Immunoreactivities of TNF-α (A-D) and MCP-1 (F-I) were visualized in mouse brain. Fractionated whole brain irradiation significantly up-regulated protein expression levels of TNF-α and MCP-1 in mouse brain (E and J). (A and F) Sham-irradiation (Control); (B and G) 4 h post-irradiation; (C and H) 8 h post-irradiation; (D and I) 24 h post-irradiation; (E and J) Quantitative analysis of fluorescence intensity. Data represent means ± SEM for each group (n=4). *p<0.05; **p<0.001 compared to control. Scale bar: 200 μm.
Fractionated Whole Brain Irradiation Increases Microglial Activation in Mouse Brain
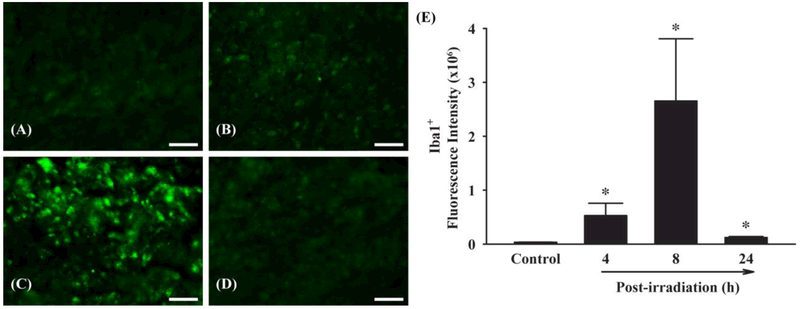
To determine whether fractionated whole brain irradiation affects microglia, the changes in protein expression levels of Iba1, a microglial activation marker, in mouse brain were visualized by immunofluorescence staining (Figure 3). A strong Iba1-positive immunoreactivity was detected in mouse brain at 4 h post-irradiation (16.96-fold increase) and reached to a maximum at 8 h post-irradiation (85.02-fold increase). These data demonstrate that fractionated whole brain irradiation increases activation of microglia in mouse brain.
Figure 3.
Effect of fractionated whole brain irradiation on microglia in mouse brain. Immunoreactivity of Iba1 was visualized in mouse brain. Fractionated whole brain irradiation significantly increased microglial activation in mouse brain (E). (A) Sham-irradiation (Control); (B) 4 h post-irradiation; (C) 8 h post-irradiation; (D) 24 h post-irradiation; (E) Quantitative analysis of fluorescence intensity. Data represent means ± SEM for each group (n=4). *p<0.05 compared to control. Scale bar: 50 μm.
Fractionated Whole Brain Irradiation Increases ROS Generation in Mouse Brain
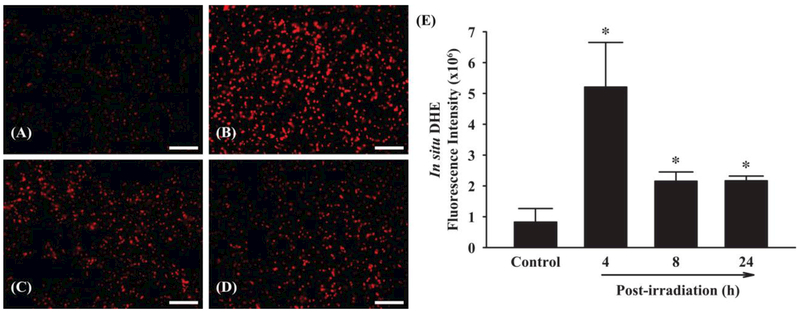
To examine whether fractionated whole brain irradiation affects ROS generation, in situ DHE fluorescence staining for superoxide anion was performed. As depicted in Figure 4, significantly elevated levels of superoxide anion were observed at 4, 8 and 24 h post-irradiation compared with sham-irradiated control mouse brain. Quantitative analysis exhibited that fractionated whole brain irradiation significantly increased ROS generation by 6.32-fold (4 h post-irradiation), 2.61-fold (8 h post-irradiation) and 2.63-fold (24 h post-irradiation), respectively. These results suggest that fractionated whole brain irradiation produces pro-oxidative environment in mouse brain.
Figure 4.
Effect of fractionated whole brain irradiation on reactive oxygen species (ROS) generation in mouse brain. The localization of red fluorescence demonstrates ROS generation in mouse brain (A-D). Fractionated whole brain irradiation significantly increased superoxide anion formation in mouse brain (E). (A) Sham-irradiation (Control); (B) 4 h post-irradiation; (C) 8 h post-irradiation; (D) 24 h post-irradiation; (E) Quantitative analysis of fluorescence intensity. Data represent means ± SEM for each group (n=4). *p<0.05 compared to control. Scale bar: 100 μm.
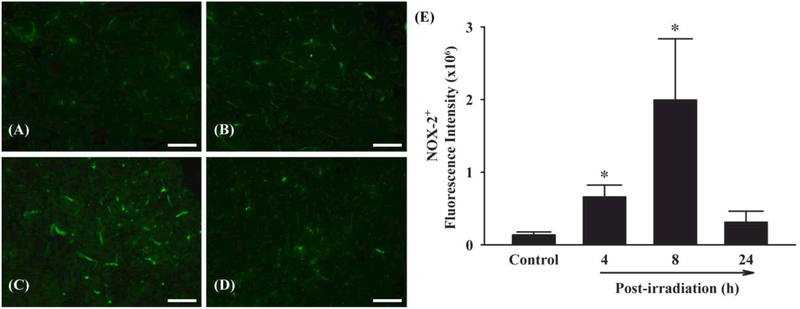
Fractionated Whole Brain Irradiation Up-regulates Protein Expression of NOX-2 in Mouse Brain
To elucidate the potential mechanisms of ROS generation, effects of fractionated whole brain irradiation on protein expression level of NOX-2, a ROS-generating enzyme, in mouse brain were examined. Marked increases in NOX-2-positive immunoreactivity were detected in mouse brain at 4 and 8 h post-irradiation (Figure 5A-D). Quantitative analysis showed that fractionated whole brain irradiation significantly up-regulated NOX-2 protein expression by 4.85-fold (4 h post-irradiation) and 14.68-fold (8 h post-irradiation) compared with sham-irradiated control mouse brain (Figure 5E). These data demonstrate that fractionated whole brain irradiation upregulates NOX-2 expression in mouse brain.
Figure 5.
Effect of fractionated whole brain irradiation on protein expression of NADPH oxidase-2 (NOX-2) in mouse brain. Immunoreactivity of NOX-2 (A-D) was visualized in mouse brain. Fractionated whole brain irradiation significantly up-regulated protein expression level of NOX-2 in mouse brain (E). (A) Sham-irradiation (Control); (B) 4 h post-irradiation; (C) 8 h postirradiation; (D) 24 h post-irradiation; (E) Quantitative analysis of fluorescence intensity. Data represent means ± SEM for each group (n=4). *p<0.05 compared to control. Scale bar: 100 μm.
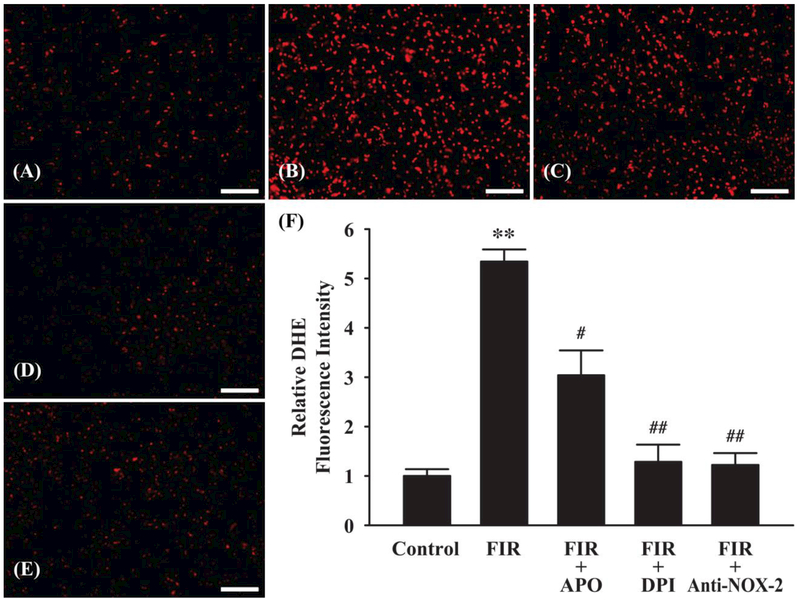
NOX Inhibitors and NOX-2 Neutralizing Antibody Attenuate ROS Generation in Irradiated Mouse Brain
To further delineate the role of NOX-2, effects of NOX inhibitors, such as apocynin (APO) and diphenylene iodonium (DPI), and NOX-2 neutralizing antibody on ROS generation in irradiated mouse brain were examined. As demonstrated in Figure 6A-E, both NOX inhibitors (APO and DPI) and NOX-2 neutralizing antibody (Anti-NOX-2) markedly decreased ROS generation compared with irradiated mouse brain (fractionated whole brain irradiation: FIR). Quantitative analysis demonstrated that fractionated whole brain irradiation-induced ROS generation in mouse brain was significantly attenuated by APO (1.76-fold), DPI (4.16-fold), and anti-NOX-2 (4.37-fold), respectively (Figure 6F). These results suggest that NOX-2 may play a role in ROS generating pathways induced by fractionated whole brain irradiation.
Figure 6.
Effect of NADPH oxidase (NOX) inhibitors and NOX-2 neutralizing antibody on radiation-induced ROS generation in mouse brain. An increase in ROS generation in mouse brain 4 h after fractionated whole brain irradiation (FIR) was markedly and significantly attenuated in the presence of NOX inhibitors (FIR + APO or FIR + DPI) or NOX-2 neutralizing antibody (FIR + anti-NOX-2). (A) Sham-irradiation (Control); (B) 4 h after fractioned whole brain irradiation (FIR); (C) FIR + 1 mM apocynin (APO); (D) FIR + 20 μM diphenylene iodonium (DPI); (E) FIR + anti-NOX-2 antibody; (F) Quantitative analysis of fluorescence intensity. Data represents mean ± SEM for each group (n=4). **p<0.001 compared to control, #p<0.05; ##p<0.001 compared to irradiated brain (FIR). Scale bar: 100 μm.
Discussion
The previous clinical findings associated with radiation exposure to brain tumor patients indicate that various primary cognitive regions are affected and may be even developed to cognitive-communicative deficits, which can significantly affect their quality of life (DeAngelis et al. 1989; Silver et al. 1992; Crossen et al. 1994; Roman & Sperduto 1995; Johannesen et al. 2003; Li et al. 2008; Welzel et al. 2008; Chang et al. 2009; Douw et al. 2009). Progressive impairments in learning and memory, such as decreased verbal memory, spatial memory, attention and novel problem solving ability, were observed in 40~50% of brain tumor patients as long-term consequences of radiation therapy. In addition, pre-clinical studies have shown the pathological consequence of radiation-induced hippocampal damage in animal models with severe neurobehavioral deficits (Lamproglou et al. 1995; Yoneoka et al. 1999; Akiyama et al. 2001; Raber et al. 2004; Rola et al. 2004; Brown et al. 2005; Shi et al. 2006; Barlind et al. 2010; Liu et al. 2010; Zhou et al. 2011; Warrington et al. 2012). At present, there are no successful therapies or effective prevention strategies for radiation-induced brain injury.
Recent studies have demonstrated that pro-oxidative and pro-inflammatory environments have been, at least in part, implicated in the radiation-induced brain injury (Hong et al. 1995; Chiang et al. 1997; Olschowka et al. 1997; Denham & Hauer-Jensen 2002; Gaber et al. 2003; Baluna et al. 2006; Collins-Underwood et al. 2008; Lee et al. 2010; Conner et al. 2011). However, details of specific molecular and cellular mechanisms responsible for oxidative stress and inflammation in radiation-induced brain injury remain unclear. Prognosis of radiationinduced brain injury by evaluating time-course of ROS induction in addition to analyses of gene and protein expressions of pro-inflammatory mediators may contribute to understanding selective biochemical pathways leading to neurodegeneration in fractionated whole brain irradiated brain. In the present study, we demonstrated that NADPH oxidase may contribute to molecular and cellular responses in radiation-induced oxidative stress and inflammation following fractionated whole brain irradiation.
The present study first observed enhanced expression of pro-inflammatory mediators, such as cytokine and chemokine, in the brain following fractionated whole brain irradiation. A significant increase of TNF-α, a pro-inflammatory cytokine and critical regulator of immune activation, and MCP-1, a member of CC chemokine family playing a critical role in monocyte chemotaxis and transmigration (Lee et al. 2003), was observed following fractionated whole brain irradiation. In a previous study (Lee et al. 2010), mRNA and protein expression levels of TNF-α, IL-1β, and MCP-1 were significantly up-regulated in hippocampal and cortical regions isolated from rat brain irradiated with a single, large dose (10 Gy). In addition, there are several reports demonstrating up-regulation of a variety of other pro-inflammatory markers, including IL-6, macrophage inflammatory protein 2 (MIP-2), ICAM-1, iNOS, and cyclooxygenase-2 (COX-2), may be associated with the molecular responses of the brain to a single, large dose (10, 25 or 35 Gy) of irradiation (Hong et al. 1995; Olschowka et al. 1997; Kyrkanides et al. 2002; Moore et al. 2005; Schnegg et al. 2012). Therefore, these studies provide sufficient evidence demonstrating the potential contribution of pro-inflammatory environment to radiation-induced brain injury. Our results further revealed that the peak mRNA and its concomitant protein expressions of TNF-α and MCP-1 occurred at 4 h and 8 h post-irradiation, respectively (Figure 1 and Figure 2). These time-course analyses suggest that mRNA and protein expression levels of pro-inflammatory mediators at different time points may be critically associated with pathological prognosis of radiation-induced brain injury. Moreover, a clinically relevant fractionated radiation regimen in the current study may provide more reliable information of the events occurring after a clinical whole brain irradiation compared with a single, large dose irradiation. According to the linear-quadratic model with an assumption of α/β ratio of 3 Gy for late responding effects in the brain (Fowler 1989), the biologically effective dose (BED3) of fractionation regimen (a total of 40 Gy; 8 fractions of 5 Gy in 4 weeks resulting in 106.7 Gy) in this study corresponds to a typical regimen in the clinic, such as 30 fractions of 2 Gy (a total of 60 Gy) in 6 weeks resulting in 100.2 Gy. Therefore, biological effect under current fractionated whole brain irradiation regimen may be more relevant to the clinical situation than those after a single, large dose of irradiation in the aforementioned previous studies. Yet, assessing cumulative damages associated with fractionated dose irradiation still requires careful investigation on how each fraction contributes to quickly accumulating inflammatory responses and further exhibiting substantially exacerbated cellular and molecular responses by a dynamic series of cellular injury, ongoing repair, and other pertaining pathophysiologic processes in the brain. In addition, the age at which subjects are exposed to radiation needs to be accounted for understanding these cumulative damages by fractionated whole brain irradiation due to the complexity of radiation-induced cognitive deficits depending on ages (Forbes et al. 2013; Blomstrand M et al. 2014).
Although the potential contribution of complex and dynamic multiple cell types in the brain to the overexpression of pro-inflammatory mediators after irradiation remains unclear, microglia, the primary immune effector cells in the CNS, are considered as a key causative player in this process (Block et al. 2007). In the present study, the results indicated that a significant increase in microglial activation was detected in irradiated brain (Figure 3). Previous in vitro studies suggested that whole brain irradiation-induced pro-inflammatory environments in the brain may be mediated through activation of microglia that resulted in a significant up-regulation of mRNA and protein expressions of pro-inflammatory mediators including TNF-α, IL-1β, IL-6, COX-2, and MCP-1 (Chiang & McBride 1991; Kyrkanides et al. 2002; Hwang et al. 2006; Lee et al. 2010). In addition, in vivo studies support that an overexpression of pro-inflammatory mediators (Chiang et al. 1997; Kyrkanides et al. 2002; Lee et al. 2010) and an increase in the number of activated microglia (Monje et al. 2002; Conner et al. 2011; Schnegg et al. 2013) were detected in the brain following irradiation. Our data also indicate that fractionated whole brain irradiation significantly increased the number of activated microglia at 4 h post-irradiation and the activation reached maximum at 8 h post-irradiation, suggesting that microglial activation is responsible for acute inflammatory responses, such as up-regulation of TNF-α and MCP-1 expressions.
Oxidative stress has been identified as one of the mechanistic pathways leading to radiation-induced brain injury (Limoli et al. 2004; Manda et al. 2007; Zhao et al. 2007; Collins-Underwood et al. 2008; Kim et al. 2008; Baluchamy et al. 2010; Veeraraghavan et al. 2011). In the present study, ROS generation following fractionated whole brain irradiation was observed by in situ DHE fluorescence staining in mouse brain. Interestingly, a significant increase of ROS generation was reached peak at 4 h post-irradiation and maintained highly up to 24 h post-irradiation (Figure 4). In a previous in vitro study (Limoli et al. 2004), levels of ROS were elevated in a dose-dependent manner from 6 h after exposure of X-rays in neural precursor cells. Another in vitro study (Collins-Underwood et al. 2008) demonstrated that irradiation on brain endothelial cells caused a significant and dose-dependent increase in ROS generation at 1 h postirradiation. Therefore, these findings from other groups support that irradiation cause an acute response of redox system in the brain. More importantly, the novel findings in this study are time-dependent ROS generation and concomitant inflammatory response. It is still unclear whether oxidative response precedes inflammatory event or vice versa; however, due to an early imbalance in the redox system observed at 4 h post-irradiation compared with the significant protein induction of pro-inflammatory mediators (TNF-α and MCP-1) at 8 h post-irradiation, it is possible that the radiation-mediated increase in oxidative stress may be responsible for these pro-inflammatory events. Particularly, previous studies explored that neuronal damage or reduced neurogenesis associated with a series of inflammation and oxidative stress following irradiation may contribute to radiation-induced late effects by dynamic interactions among multiple cell types of the brain (Raber et al. 2004; Rola et al. 2004; Fishman et al. 2009; Oh et al. 2013). However, a putative connection between acute pro-oxidant/pro-inflammatory responses and chronic pathologies following irradiation in the brain is vital to better understand chronic effects of radiation-induced brain injury.
To elucidate origin of ROS generation following fractionated whole brain irradiation, we examined expression levels of NOX-2. NOX-2, the primary phagocytic oxidase (Bedard & Krause 2007; Jaquet et al. 2009; Pendyala & Natarajan 2010), has been frequently studied and demonstrated its critical role of ROS formation in glial cells and neurons under various neuropathological conditions (Green et al. 2001; Serrano et al. 2003; Zekry et al. 2003; Infanger et al. 2006; Qin et al. 2006; Jackman et al. 2009; Drummond et al. 2011; Surace & Block 2012). In the present study, we demonstrated that NOX-2 expression was markedly elevated at 4 h and 8 h post-irradiation (Figure 5) and its pharmacological inhibitions (DPI, APO, and a neutralizing antibody) dramatically ameliorated the production of ROS (Figure 6), which strongly suggest NOX-2 contributes to radiation-induced oxidative stress in the brain. Previous studies have observed specific markers in the redox system to investigate a primary cause of excessive ROS generation after irradiation (Rola et al. 2007; Collins-Underwood et al. 2008; Fishman et al. 2009; Veeraraghavan et al. 2011). However, the detailed mechanism underlying this process is still unknown and needs to be further investigated.
Conclusions
The present study demonstrated the evidence that NOX-2 may play a potential role in fractionated whole brain irradiation-mediated pro-oxidative and pro-inflammatory pathways in mouse brain. These findings may provide a foundation for defining cellular and molecular basis of radiation-induced brain injury that will lead to new opportunities for preventive and therapeutic interventions for brain tumor patients who receive radiation therapy.
Acknowledgements
The work was supported by the National Institute of Neurological Disorders and Stroke under Grant Number R01NS056218.
Footnotes
Declaration of Interest
The authors report no conflicts of interest.
References
- Akiyama K, Tanaka R, Sato M, Takeda N. 2001. Cognitive dysfunction and histological findings in adult rats one year after whole brain irradiation. Neurol Med Chir. 41:590–598. [DOI] [PubMed] [Google Scholar]
- Ashpole NM, Warrington JP, Mitschelen MC, Yan H, Sosnowska D, Gautam T, Farley JA, Csiszar A, Ungvari Z, Sonntag WE. Systemic influences contribute to prolonged microvascular rarefaction after brain irradiation: a role for endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2014;307(6):H858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluchamy S, Zhang Y, Ravichandran P, Ramesh V, Sodipe A, Hall JC, Jejelowo O, Gridley DS, Wu H, Ramesh GT. 2010. Differential oxidative stress gene expression profile in mouse brain after proton exposure. In Vitro Cell Dev Biol Anim. 46:718–725. [DOI] [PubMed] [Google Scholar]
- Baluna RG, Eng TY, Thomas CR. 2006. Adhesion molecules in radiotherapy. Radiat Res. 166:819–831. [DOI] [PubMed] [Google Scholar]
- Barlind A, Karlsson N, Björk-Eriksson T, Isgaard J, Blomgren K. 2010. Decreased cytogenesis in the granule cell layer of the hippocampus and impaired place learning after irradiation of the young mouse brain evaluated using the IntelliCage platform. Exp Brain Res. 201:781–787. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. 2007. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 87:245–313. [DOI] [PubMed] [Google Scholar]
- Béhin A, Delattre JY. 2004. Complications of radiation therapy on the brain and spinal cord. Semin Neurol. 24:405–417. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. 2007. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 8:57–69. [DOI] [PubMed] [Google Scholar]
- Blomstrand M, Kalm M, Grand´er R, Björk-Eriksson T, Blomgren K. 2014. Different reactions to irradiation in the juvenile and adult hippocampus. Int J Radiat Biol. 90:807–815. [DOI] [PubMed] [Google Scholar]
- Brown WR, Blair RM, Moody DM, Thore CR, Ahmed S, Robbins ME, Wheeler KT. 2007. Capillary loss precedes the cognitive impairment induced by fractionated whole-brain irradiation: a potential rat model of vascular dementia. J Neurol Sci. 257:67–71. [DOI] [PubMed] [Google Scholar]
- Brown WR, Thore CR, Moody DM, Robbins ME, Wheeler KT. 2005. Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res. 164:662–668. [DOI] [PubMed] [Google Scholar]
- Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA. 2009. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 10:1037–1044. [DOI] [PubMed] [Google Scholar]
- Chiang CS, Hong JH, Stalder A, Sun JR, Withers HR, McBride WH. 1997. Delayed molecular responses to brain irradiation. Int J Radiat Biol. 72:45–53. [DOI] [PubMed] [Google Scholar]
- Chiang CS, McBride WH. 1991. Radiation enhances tumor necrosis factor α production by murine brain cells. Brain Res. 566:265–269. [DOI] [PubMed] [Google Scholar]
- Collins-Underwood JR, Zhao W, Sharpe JG, Robbins ME. 2008. NADPH oxidase mediates radiation-induced oxidative stress in rat brain microvascular endothelial cells. Free Radic Biol Med. 45:929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner KR, Forbes ME, Lee WH, Lee YW, Riddle DR. 2011. AT1 receptor antagonism does not influence early radiation-induced changes in microglial activation or neurogenesis in the normal rat brain. Radiat Res. 176:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossen JR, Garwood D, Glatstein E, Neuwelt EA. 1994. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 12: 627–642. [DOI] [PubMed] [Google Scholar]
- Darzy KH, Pezzoli SS, Thorner MO, Shalet SM. 2005. The dynamics of growth hormone (GH) secretion in adult cancer survivors with severe GH deficiency acquired after brain irradiation in childhood for nonpituitary brain tumors: evidence for preserved pulsatility and diurnal variation with increased secretory disorderliness. J Clin Endocrinol Metab. 90: 2794–2803. [DOI] [PubMed] [Google Scholar]
- DeAngelis LM, Delattre JY, Posner JB. 1989. Radiation-induced dementia in patients cured of brain metastases. Neurology. 39:789–796. [DOI] [PubMed] [Google Scholar]
- Denham JW, Hauer-Jensen M. 2002. The radiotherapeutic injury - a complex ‘wound’. Radiother Oncol. 63:129–145. [DOI] [PubMed] [Google Scholar]
- Douw L, Klein M, Fagel SS, van den Heuvel J, Taphoorn MJ, Aaronson NK, Postma TJ, Vandertop WP, Mooij JJ, Boerman RH, et al. 2009. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 8:810–818. [DOI] [PubMed] [Google Scholar]
- Drummond GR, Selemidis S, Griendling KK, Sobey CG. 2011. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 10:453–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman K, Baure J, Zou Y, Huang TT, Andres-Mach M, Rola R, Suarez T, Acharya M, Limoli CL, Lamborn KR, Fike JR. 2009. Radiation-induced reductions in neurogenesis are ameliorated in mice deficient in CuZnSOD or MnSOD. Free Radic Biol Med. 47: 1459–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes ME, Paitsel M, Bourland JD, Riddle DR. 2013. Systemic effects of fractionated, wholebrain irradiation in young adult and aging rats. Radiat Res. 180:326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JF. 1989. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 62:679–694. [DOI] [PubMed] [Google Scholar]
- Gaber MW, Sabek OM, Fukatsu K, Wilcox HG, Kiani MF, Merchant TE. 2003. Differences in ICAM-1 and TNF-α expression between large single fraction and fractionated irradiation in mouse brain. Int J Radiat Biol. 79:359–366. [DOI] [PubMed] [Google Scholar]
- Green SP, Cairns B, Rae J, Errett-Baroncini C, Hongo JA, Erickson RW, Curnutte JT. 2001. Induction of gp91-phox, a component of the phagocyte NADPH oxidase, in microglial cells during central nervous system inflammation. J Cereb Blood Flow Metab. 21:374–384. [DOI] [PubMed] [Google Scholar]
- Greene-Schloesser D, Moore E, Robbins ME. 2013. Molecular pathways: radiation-induced cognitive impairment. Clin Cancer Res. 19:2294–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Chiang CS, Campbell IL, Sun JR, Withers HR, McBride WH. 1995. Induction of acute phase gene expression by brain irradiation. Int J Radiat Oncol Biol Phys. 33: 619–626. [DOI] [PubMed] [Google Scholar]
- Hwang SY, Jung JS, Kim TH, Lim SJ, Oh ES, Kim JY, Ji KA, Joe EH, Cho KH, Han IO. 2006. Ionizing radiation induces astrocyte gliosis through microglia activation. Neurobiol Dis. 21:457–467. [DOI] [PubMed] [Google Scholar]
- Infanger DW, Sharma RV, Davisson RL. 2006. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal. 8:1583–1596. [DOI] [PubMed] [Google Scholar]
- Jackman KA, Miller AA, De Silva TM, Crack PJ, Drummond GR, Sobey CG. 2009. Reduction of cerebral infarct volume by apocynin requires pretreatment and is absent in Nox2-deficient mice. Br J Pharmacol. 156:680–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquet V, Scapozza L, Clark RA, Krause KH, Lambeth JD. 2009. Small-molecule NOX inhibitors: ROS-generating NADPH oxidases as therapeutic targets. Antioxid Redox Signal. 11:2535–2552. [DOI] [PubMed] [Google Scholar]
- Johannesen TB, Lien HH, Hole KH, Lote K. 2003. Radiological and clinical assessment of longterm brain tumour survivors after radiotherapy. Radiother Oncol. 69:169–176. [DOI] [PubMed] [Google Scholar]
- Kim JH, Brown SL, Jenrow KA, Ryu S. 2008. Mechanisms of radiation-induced brain toxicity and implications for future clinical trials. J Neurooncol. 87:279–286. [DOI] [PubMed] [Google Scholar]
- Kyrkanides S, Moore AH, Olschowka JA, Daeschner JC, Williams JP, Hansen JT, Kerry O’Banion M. 2002. Cyclooxygenase-2 modulates brain inflammation-related gene expression in central nervous system radiation injury. Brain Res Mol Brain Res. 104:159–169. [DOI] [PubMed] [Google Scholar]
- Lamproglou I, Chen QM, Boisserie G, Mazeron JJ, Poisson M, Baillet F, Le Poncin M, Delattre JY. 1995. Radiation-induced cognitive dysfunction: an experimental model in the old rat. Int J Radiat Oncol Biol Phys. 31:65–70. [DOI] [PubMed] [Google Scholar]
- Lee WH, Sonntag WE, Mitschelen M, Yan H, Lee YW. 2010. Irradiation induces regionally specific alterations in pro-inflammatory environments in rat brain. Int J Radiat Biol. 86:132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WH, Warrington JP, Sonntag WE, Lee YW. 2012a. Irradiation alters MMP-2/TIMP-2 system and collagen type IV degradation in brain. Int J Radiat Oncol Biol Phys. 82:1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YW, Cho HJ, Lee WH, Sonntag WE. 2012b. Whole brain radiation-induced cognitive impairment: pathophysiological mechanisms and therapeutic targets. Biomol Ther. 20:357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YW, Hennig B, Toborek M. 2003. Redox-regulated mechanisms of IL-4-induced MCP-1 expression in human vascular endothelial cells. Am J Physiol Heart Circ Physiol. 284: H185–H192. [DOI] [PubMed] [Google Scholar]
- Li J, Bentzen SM, Li JL, Renschler M, Mehta MP. 2008. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 71:64–70. [DOI] [PubMed] [Google Scholar]
- Limoli CL, Giedzinski E, Rola R, Otsuka S, Palmer TD, Fike JR. 2004. Radiation response of neural precursor cells: linking cellular sensitivity to cell cycle checkpoints, apoptosis and oxidative stress. Radiat Res. 161:17–27. [DOI] [PubMed] [Google Scholar]
- Liu Y, Xiao S, Liu J, Zhou H, Liu Z, Xin Y, Suo WZ. 2010. An experimental study of acute radiation-induced cognitive dysfunction in a young rat model. Am J Neuroradiol. 31:383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaschi S, Fiorino C, Losa M, Lanzi R, Mazza E, Motta M, Perna L, Brioschi E, Scavini M, Reni M. 2011. Time course of hypothalamic-pituitary deficiency in adults receiving cranial radiotherapy for primary extrasellar brain tumors. Radiother Oncol. 99:23–28. [DOI] [PubMed] [Google Scholar]
- Manda K, Ueno M, Anzai K. 2009. Cranial irradiation-induced inhibition of neurogenesis in hippocampal dentate gyrus of adult mice: attenuation by melatonin pretreatment. J Pineal Res. 46:71–78. [DOI] [PubMed] [Google Scholar]
- Manda K, Ueno M, Moritake T, Anzai K. 2007. Radiation-induced cognitive dysfunction and cerebellar oxidative stress in mice: protective effect of α-lipoic acid. Behav. Brain Res. 177:7–14. [DOI] [PubMed] [Google Scholar]
- Monje ML, Mizumatsu S, Fike JR, Palmer TD. 2002. Irradiation induces neural precursor-cell dysfunction. Nat Med. 8:955–962. [DOI] [PubMed] [Google Scholar]
- Moore AH, Olschowka JA, Williams JP, Okunieff P, O’Banion MK. 2005. Regulation of prostaglandin E2 synthesis after brain irradiation. Int J Radiat Oncol Biol Phys. 62: 267–272. [DOI] [PubMed] [Google Scholar]
- Moulder JE, Cohen EP. 2007. Future strategies for mitigation and treatment of chronic radiationinduced normal tissue injury. Semin Radiat Oncol. 17:141–148. [DOI] [PubMed] [Google Scholar]
- Oh SB, Park HR, Jang YJ, Choi SY, Son TG, Lee J. 2013. Baicalein attenuates impaired hippocampal neurogenesis and the neurocognitive deficits induced by γ-ray radiation. Br J Pharmacol. 168:421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olschowka JA, Kyrkanides S, Harvey BK, O’Banion MK, Williams JP, Rubin P, Hansen JT. 1997. ICAM-1 induction in the mouse CNS following irradiation. Brain Behav Immun. 11:273–285. [DOI] [PubMed] [Google Scholar]
- Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Solan JS. 2015. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol 17:iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendyala S, Natarajan V. 2010. Redox regulation of Nox proteins. Respir Physiol Neurobiol. 174:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin B, Cartier L, Dubois-Dauphin M, Li B, Serrander L, Krause KH. 2006. A key role for the microglial NADPH oxidase in APP-dependent killing of neurons. Neurobiol Aging. 27:1577–1587. [DOI] [PubMed] [Google Scholar]
- Qin L, Crews FT. 2012. NADPH oxidase and reactive oxygen species contribute to alcoholinduced microglial activation and neurodegeneration. J Neuroinflammation. 9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik EH, Valk GD, Drent ML, Stalpers LJ, Kenemans JL, Koppeschaar HP, van Dam PS. 2012. Reduced growth hormone secretion after cranial irradiation contributes to neurocognitive dysfunction. Growth Horm IGF Res. 22:42–47. [DOI] [PubMed] [Google Scholar]
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. 2004. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 162:39–47. [DOI] [PubMed] [Google Scholar]
- Ramanan S, Zhao W, Riddle DR, Robbins ME. 2010. Role of PPARs in radiation-induced brain injury. PPAR Res. 2010:234975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. 2004. Radiationinduced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 188:316–330. [DOI] [PubMed] [Google Scholar]
- Rola R, Zou Y, Huang TT, Fishman K, Baure J, Rosi S, Milliken H, Limoli CL, Fike JR. 2007. Lack of extracellular superoxide dismutase (EC-SOD) in the microenvironment impacts radiation-induced changes in neurogenesis. Free Radic Biol Med. 42:1133–1145; discussion 1131–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman DD, Sperduto PW. 1995. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys. 31:983–998. [DOI] [PubMed] [Google Scholar]
- Schnegg CI, Greene-Schloesser D, Kooshki M, Payne VS, Hsu FC, Robbins ME. 2013. The PPARδ agonist GW0742 inhibits neuroinflammation, but does not restore neurogenesis or prevent early delayed hippocampal-dependent cognitive impairment after whole-brain irradiation. Free Radic Biol Med. 61:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnegg CI, Kooshki M, Hsu FC, Sui G, Robbins ME. 2012. PPARδ prevents radiation-induced proinflammatory responses in microglia via transrepression of NF-κB and inhibition of the PKCα/MEK1/2/ERK1/2/AP-1 pathway. Free Radic Biol Med. 52:1734–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano F, Kolluri NS, Wientjes FB, Card JP, Klann E. 2003. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 988:193–198. [DOI] [PubMed] [Google Scholar]
- Shi L, Adams MM, Long A, Carter CC, Bennett C, Sonntag WE, Nicolle MM, Robbins M, D’Agostino R, Brunso-Bechtold JK. 2006. Spatial learning and memory deficits after wholebrain irradiation are associated with changes in NMDA receptor subunits in the hippocampus. Radiat Res. 166:892–899. [DOI] [PubMed] [Google Scholar]
- Silber JH, Radcliffe J, Peckham V, Perilongo G, Kishnani P, Fridman M, Goldwein JW, Meadows AT. 1992. Whole-brain irradiation and decline in intelligence: the influence of dose and age on IQ score. J Clin Oncol. 10:1390–1396. [DOI] [PubMed] [Google Scholar]
- Stone HB, Coleman CN, Anscher MS, McBride WH. 2003. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 4:529–536. [DOI] [PubMed] [Google Scholar]
- Surace MJ, Block ML. 2012. Targeting microglia-mediated neurotoxicity: the potential of NOX2 inhibitors. Cell Mol Life Sci. 69:2409–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofilon PJ, Fike JR. 2000. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 153:357–370. [DOI] [PubMed] [Google Scholar]
- Veeraraghavan J, Natarajan M, Herman TS, Aravindan N. 2011. Low-dose γ-radiation-induced oxidative stress response in mouse brain and gut: regulation by NFκB-MnSOD crosssignaling. Mutat Res. 718:44–55. [DOI] [PubMed] [Google Scholar]
- Warrington JP, Ashpole N, Csiszar A, Lee YW, Ungvari Z, Sonntag WE. 2013. Whole brain radiation-induced vascular cognitive impairment: mechanisms and implications. J Vasc Res. 50:445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington JP, Csiszar A, Mitschelen M, Lee YW, Sonntag WE. 2012. Whole brain radiationinduced impairments in learning and memory are time-sensitive and reversible by systemic hypoxia. PLoS One. 7:e30444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welzel G, Fleckenstein K, Schaefer J, Hermann B, Kraus-Tiefenbacher U, Mai SK, Wenz F. 2008. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Phys. 72:1311–1318. [DOI] [PubMed] [Google Scholar]
- Yoneoka Y, Satoh M, Akiyama K, Sano K, Fujii Y, Tanaka R. 1999. An experimental study of radiation-induced cognitive dysfunction in an adult rat model. Br J Radiol. 72: 1196–1201. [DOI] [PubMed] [Google Scholar]
- Zekry D, Epperson TK, Krause KH. 2003. A role for NOX NADPH oxidases in Alzheimer’s disease and other types of dementia? IUBMB Life. 55:307–313. [DOI] [PubMed] [Google Scholar]
- Zhao W, Diz DI, Robbins ME. 2007. Oxidative damage pathways in relation to normal tissue injury. Br J Radiol. 8:S23–S31. [DOI] [PubMed] [Google Scholar]
- Zhou H, Liu Z, Liu J, Wang J, Zhou D, Zhao Z, Xiao S, Tao E, Suo WZ. 2011. Fractionated radiation-induced acute encephalopathy in a young rat model: cognitive dysfunction and histologic findings. Am J Neuroradiol. 32:1795–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]