Abstract
Combinations of chemical and genetic approaches were used to study the function of divalent metal ions in cleavage of RNA by the ribozyme RNase P RNA. We show that different divalent metal ions have differential effects on cleavage site recognition and rescue of cleavage activity by mixing divalent metal ions that do not promote cleavage by themselves. We conclude that efficient and correct cleavage is the result of cooperativity between divalent metal ions bound at different sites in the RNase P RNA-substrate complex. Complementation of a mutant RNase P RNA phenotype as a result of divalent metal ion replacement is demonstrated also. This finding together with other data indicate that one of the metal ions involved in this cooperativity is positioned near the cleavage site. The possibility that the Mg2+/Ca2+ ratio might regulate the activity of biocatalysts that depend on RNA for activity is discussed.
Keywords: RNase P‖divalent metal ions‖tRNA precursors‖tRNA processing
Divalent metal ions are important for the function of many proteins and RNA. The role of the divalent metal ions can be structural and/or catalytic, and in RNA-catalyzed reactions the existence of several categories of divalent metal ion binding sites has been suggested (1–5). The reaction catalyzed by the ribozyme RNase P RNA, the catalytic subunit of the endoribonuclease RNase P (6), requires divalent metal ions; among the ones studied thus far, Mg2+ promotes cleavage most efficiently. The roles of Mg2+ in the RNase P RNA-catalyzed reaction are to induce proper folding of the RNA, to facilitate the interaction with its RNA substrate, and to promote efficient and correct cleavage (7). Cleavage by RNase P RNA has been reported to proceed in the presence of Mn2+ and Ca2+, although at reduced efficiencies (2, 8–15). However, cleavage in the presence of Mn2+ and Ca2+ induces miscleavage (11, 12, 15). These findings make RNase P RNA a suitable model system to study the way by which different divalent metal ions influence RNA-mediated cleavage and thereby expand our knowledge about the role of different categories of divalent metal ion binding sites in the RNase P RNA-catalyzed reaction and in RNA in general.
Here we present data demonstrating RNase P RNA cleavage in the presence of divalent metal ions that do not promote cleavage by themselves, indicating metal ion cooperativity. Our data further suggest that one of the metal ions involved in this cooperativity is positioned in the vicinity of the interaction between the 3′ end of the substrate and RNase P RNA, the “RCCA-RNase P RNA” interaction (interacting residues underlined, ref. 16).
Materials and Methods
The substrates, pATSerCG and pATSerUA, were purchased from Xeragon AG, Switzerland, or generated by run-off transcription using T7 DNA-dependent RNA polymerase (17). Internally labeled pATSerCG, 5′ end-labeled pATSerUA, and the M1 RNA variants were generated as described elsewhere (15, 18).
Cleavage reactions were performed at 37°C as described in detail elsewhere (15) in our standard reaction buffer: 50 mM Tris-HCl (pH 7.2), 5% (wt/vol) polyethylene glycol 6000, 100 mM NH4Cl, and 40 mM total concentration of different divalent metal ions as indicated. The chloride salt of the various Me2+ was used except for Ba2+, for which we used barium acetate. Note that the MnCl2 was stored at −20°C before use.
In the experiments in which the frequency of miscleavage was determined the final concentrations of M1 RNA and substrates were ≈0.25 and ≤0.05 μM, respectively. The frequency of miscleavage at −1 was calculated from the relative amounts of 5′-cleavage products generated from cleavage at the different positions, +1 and −1, using a PhosphorImager (for further details see ref. 15).
The kcat/Km values were calculated based on kcat and Km values, which were determined as reported previously (15). For the calculations we used the 5′-cleavage fragments instead of the 5′-matured products, because we wanted to determine the kinetic constants for cleavage at both positions +1 and −1. The initial rate of cleavage (Vi) measured for each substrate concentration was plotted against the substrate concentration resulting in hyperbolic curves. The kcat and Km values were calculated subsequently by linear regression from Eadie–Hofstee plots. The final concentrations of substrate varied between 0.015 and 59.4 μM, depending on M1 RNA/substrate combination and reaction conditions, whereas the final concentration of M1 RNA was 0.041 μM (or 0.12 μM in some of the cases in which kcat/Km values were >0.005 min−1 μM−1) irrespective of variant. No difference in kcat/Km comparing cleavage of synthetic pATSerCG (this study) and T7 RNA polymerase transcribed pATSerCG (15) was detected.
Results and Discussion
Cleavage in the Presence of Various Divalent Metal Ion Combinations.
To study the effect of various metal ions on cleavage by Escherichia coli RNase P RNA, M1 RNA, different divalent metal ions were added together with Mg2+. We used the precursor pATSerCG as substrate (Fig. 1a), which is a model substrate that has been described elsewhere (15). Noteworthy is also that product release is not a rate-limiting step in cleavage of another substrate of this type (19). Cleavage in the presence of transition metal ions resulted in reduced (see below, Table 1, and data not shown) cleavage rates, concomitant with an increased frequency of miscleavage (Fig. 2a). Addition of Ca2+, Sr2+ or Ba2+ also inhibited the reaction (Fig. 2b and Table 1); but at 5–30 mM of either of these ions, a higher frequency of cleavage at the correct position was observed compared with cleavage in the Mg2+ alone reaction (Fig. 3a). Here we used a mutant M1 RNA, M1C254 RNA (Fig. 1b, Mut 2), because it miscleaves at −1 with an increased frequency compared with wild type (15). However, the wild type showed the same tendency, in particular when Sr2+ was added (Fig. 2). Moreover, the addition of Sr2+ or Ca2+ suppressed the Mn2+-induced miscleavage (Fig. 3b), similar to a previous study in which we studied cleavage in the presence of Mg2+ and Mn2+ (ref. 15). These data show that different divalent metal ions have differential effects on cleavage site recognition while the rate of cleavage in general decreases irrespective of which metal ion was added together with Mg2+ (Table 1; see also below).
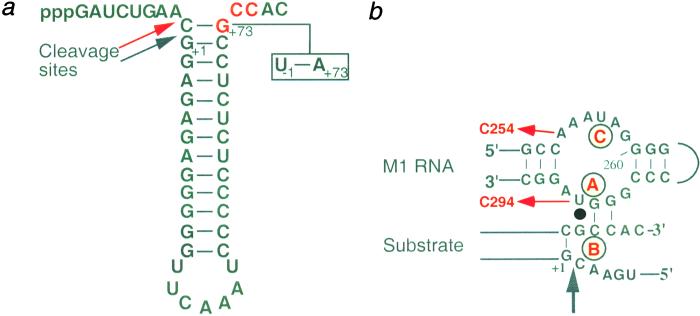
Figure 1.
(a) The secondary structure of the RNase P pATSerCG(UA) model substrate. Arrows indicate the RNase P RNA cleavage sites, where the red arrow represents cleavage at the incorrect position −1. The G+73C+74C+75 motif (marked in red) at the 3′ end is involved in base pairing with RNase P RNA (the RCCA-RNase P RNA interaction) in the RNase P RNA substrate complex (b). The C−1-G+73 and U−1-A+73 (boxed base pair) refer to the substrates pATSerCG and pATSerUA, respectively. (b) Illustration of the RCCA-RNase P RNA interaction (16). The letters A–C refer to divalent metal ion binding sites that have been identified in the P15 loop (18) and in the substrate (21, 24, 27). The red arrows indicate substitutions resulting in Mut1, U to C at 294, and Mut2, A to C at 254. The black arrow marks the consensus RNase P cleavage site.
Table 1.
Summary of the kinetic constants
| M1 RNA | Substrate | Me2+ clevage site |
kcat, min−1
|
Km, μM
|
kcat/Km, μM−1 × min−1
|
|||
|---|---|---|---|---|---|---|---|---|
| +1 | −1 | +1 | −1 | +1 | −1 | |||
| Wild-type | pATSerCG | Mg2+(40) | 4.2 ± 2.4 | 0.13 ± 0.043 | 4.3 ± 2.4 | 4.3 ± 2.4 | 0.97 | 0.027* |
| Mn2+(40) | 0.016 ± 0.012 | 0.017 ± 0.016 | 0.19 ± 0.055 | 0.25 ± 0.10 | 0.084 | 0.068 | ||
| Mg2+/Mn2+(25/15)† | 0.2 | 0.15 | 0.89 | 0.87 | 0.22 | 0.17 | ||
| Mg2+/Sr2+(15/25) | 2.2 ± 0.9 | n | 11 ± 4.2 | n | 0.20 | n | ||
| Mn2+/Sr2+(15/25) | 0.95 ± 0.29 | 0.30 ± 0.11 | 0.30 ± 0.11 | 0.23 ± 0.085 | 3.2 | 1.3 | ||
| Zn2+/Sr2+(15/25) | 0.067 ± 0.044 | 0.074 ± 0.058 | 17 ± 3.6 | 16 ± 8.6 | 0.0039 | 0.0046 | ||
| Ca2+(40) | 0.003 ± 0.0024 | 0.0005 ± 0.0004 | 2.1 ± 1.4 | 2.1 ± 1.4 | 0.0014 | 0.00024* | ||
| Ca2+/Sr2+(15/25) | 0.0013 ± 0.00018 | ND | 0.98 ± 0.55 | ND | 0.0013 | ND | ||
| C294 | pATSerCG | Mg2+(40) | 4.9 ± 1.8 | n | 4.9 ± 2.3 | n | 1.0 | n |
| Mn2+(40) | 0.0004 ± 0.0002 | ND | 1.7 ± 0.4 | ND | 0.00024 | ND | ||
| Mg2+/Mn2+(25/15) | 0.17 ± 0.04 | 0.014 ± 0.005 | 0.31 ± 0.04 | 0.27 ± 0.07 | 0.55 | 0.052 | ||
| Mn2+/Sr2+(15/25) | 0.32 ± 0.09 | 0.013 ± 0.004 | 0.27 ± 0.13 | 0.26 ± 0.14 | 1.2 | 0.05 | ||
| Wild-type | pATSerUA | Mg2+(40) | 9.5 ± 2.4 | n | 2.9 ± 1.2 | n | 3.3 | n |
| Mn2+(40) | 0.0092 ± 0.0019 | n | 0.26 ± 0.22 | n | 0.035 | n | ||
| C254 | pATSerUA | Mg2+(40) | 4.4 ± 2.5 | n | 20 ± 9.6 | n | 0.23 | n |
| Mn2+(40) | 0.12 ± 0.10 | n | 0.72 ± 0.091 | n | 0.17 | n | ||
The kinetic constants kcat and Km and cleavage efficiencies expressed as kcat/Km in the presence of different divalent metal ion combinations, various M1 RNA derivatives, and different substrates are shown. Numbers in parentheses correspond to the final concentrations in mM of indicated divalent metal ions. The numbers (± experimental errors) are averages of at least three independent experiments. ND, not determined; n, no cleavage at −1 detected.
To calculate kcat/Km for cleavage at −1 we first determined the frequency of cleavage at the −1 position and used this number and the initial rates of cleavage at +1 to calculate kcat. Note that approximately the same Km was obtained irrespective of cleavage site (see also ref. 15). The frequencies of cleavage at −1 were: at 40 mM Mg2+, 2.7 ± 0.15%; at 40 mM Ca2+, 16.3 ± 2.0%.
Numbers are taken from ref. 15.
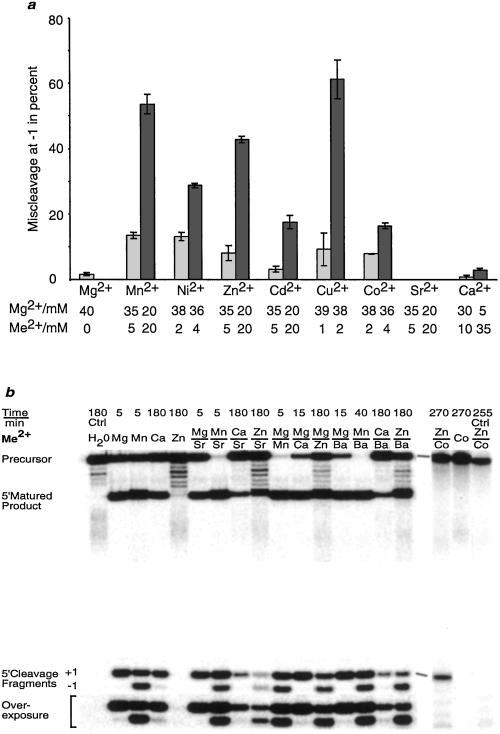
Figure 2.
Cleavage by wild-type M1 RNA in the presence of different divalent metal ion combinations. (a) Diagram showing miscleavage of pATSerCG at the −1 position by wild-type M1 RNA in the presence of different combinations of divalent metal ions as indicated. No miscleavage at −1 was detected when cleavage was performed in the presence of the given Mg2+/Sr2+ combinations. The given values are averages of several independent experiments, and experimental errors are indicated in the figure. (b) Cleavage of [α-32P]UTP internally labeled pATSerCG (except the last three lanes to the right in which we used [γ-32P]ATP 5′ end-labeled pATSerUA, explaining why the 5′-matured cleavage product is not seen) as a function of different divalent metal ion combinations as indicated. In the experiments where two Me2+ were mixed the final concentration of each Me2+ was 20 mM, resulting in a total final divalent metal ion concentration of 40 mM. Time represents the time of incubation of M1 RNA in the presence of pATSerCG (or pATSerUA). The controls were: Ctrl H2O, incubation of pATSerCG in the presence of only H2O for 180 min; Ctrl Zn/Co, incubation of pATSerUA in the presence of 20 mM Zn2+ 20 mM Co(NH3)63+, and no M1 RNA; Co, Co(NH3)63+. Note that pATSerUA was cleaved only at the +1 position, whereas pATSerCG was cleaved both at the correct position +1 and at −1 (see also ref. 15).
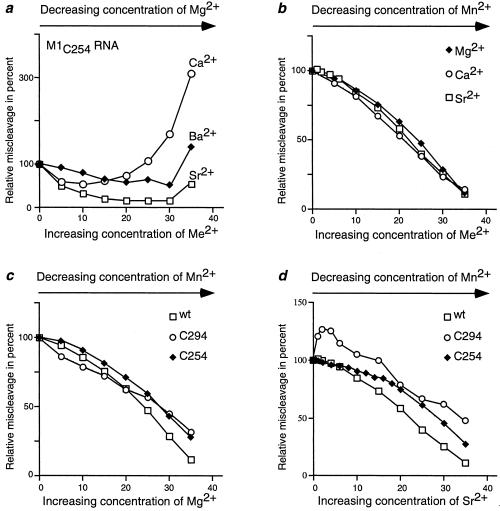
Figure 3.
Relative miscleavage of pATSerCG at the −1 position under various conditions. (a and b) Miscleavage at the −1 position as a function of increasing [Me2+] as indicated. (a) 100 = 8.0 ± 2.3% for cleavage at −1 by Mut2 (M1C254 RNA) in the presence of 40 mM Mg2+. (b) 100 = 43 ± 3.8% miscleavage at −1 by wild-type M1 RNA in the presence of 40 mM Mn2+. (c and d) Miscleavage at the −1 position by various M1 RNA derivatives as a function of increasing concentrations of Mg2+ (c) and Sr2+ (d) where 100 refers to cleavage in the presence of only Mn2+. For wild-type M1 RNA 100 = 43 ± 3.8%, for Mut1 (M1C294 RNA) 100 = 5.7 ± 1.3%, and for Mut2 (M1C254 RNA) 100 = 68 ± 5%. Each point is an average of several independent experiments and the total concentration of divalent metal ions was 40 mM and varied as indicated.
These results encouraged us to investigate whether cleavage can be promoted in the presence of other metal ion combinations. As shown in Fig. 2b, the combinations Zn2+/Sr2+, Zn2+/Ba2+, and Zn2+/Co(NH3)63+ supported cleavage although at reduced rates compared with reactions in Mg2+ alone. Co(NH3)63+ is considered to be an analogue of the fully solvated Mg2+ ion (20), which binds stronger to M1 RNA compared with Mg2+ (21). Here we used this analogue to investigate whether cleavage is influenced by its presence. Cleavage efficiencies, expressed as kcat/Km values for the Zn2+/Sr2+ and other combinations, are given in Table 1. These findings are unexpected given that Sr2+, Ba2+, Zn2+, and Co(NH3)63+ alone do not support cleavage under these conditions [Fig. 2b and no M1 RNA cleavage products in the presence of Sr2+ or Ba2+ alone were detected after 17 or ≈3.7 h of incubation, respectively (data not shown)]. Similar results were observed by using tRNA precursors as substrates (data not shown). Strikingly, the combinations Mn2+/Sr2+ and Mn2+/Ba2+ resulted in an increased efficiency of cleavage compared with cleavage in the presence of Mg2+ (or Mn2+) alone (Fig. 2b, Table 1), whereas the combination Ca2+/Sr2+ promoted cleavage as efficiently as Ca2+ alone. Thus the efficiency of cleavage by M1 RNA depends on metal ion combination in a differential manner. Moreover, we argue that in these mixed metal ion experiments it is likely that the transition metal ions Mn2+ and Zn2+ are involved in generating the nucleophile, whereas Sr2+, Ba2+, and Co(NH3)63+ play supportive structural roles, e.g. stabilizing the M1 RNA substrate (RS) interaction. The data suggest that these categories of divalent metal ions operate in concert and that efficient and correct cleavage is the result of cooperativity between divalent metal ions bound at different binding sites in the RS complex, most likely in the vicinity of the cleavage site. Our findings that the structure of M1 RNA is very similar under conditions where activity was detected as judged from Pb2+ cleavage data are in agreement with this suggestion (refs. 15 and 21; unpublished data).
The RCCA-RNase P RNA Interaction and Metal Ion Cooperativity.
From the above it is clear that certain divalent metal ions complement each other with respect both to cleavage rates and cleavage site recognition in the reaction catalyzed by M1 RNA. Base substitutions in the P15 loop, a domain of M1 RNA that interacts with the RCCA motif at the 3′ end of the substrate, the RCCA-RNase P RNA interaction (interacting residues underlined; Fig. 1b), result in reduced activity and changes in divalent metal ion binding in M1 RNA (16, 22). Thus, we decided to combine the chemical approach discussed above with a genetic approach to study whether the divalent metal ion(s) bound in P15 (Fig. 1b) are involved in the cooperativity between divalent metal ions bound at different metal binding sites in the RS complex. In addition, we wanted to investigate whether a divalent metal ion replacement would result in complementation of a mutant phenotype. The two mutant M1 RNAs used carried changes in P15, one a uridine (U) to cytidine (C) replacement at 294 (Mut1), and the other harbored an adenosine (A) to C change at 254 (Mut2; Fig. 1b).
In the first set of experiments we observed that suppression of the Mn2+-induced miscleavage of pATSerCG required approximately the same concentration of Mg2+ for Mut1 and wild-type M1 RNA (Fig. 3c). However, when Sr2+ was added, a higher concentration was needed for Mut1 compared with the case in which wild-type was used. In fact, a small but reproducible increase in miscleavage at lower [Sr2+] was observed for Mut1 (Fig. 3d). Compared with cleavage by wild type it also seems that for Mut2 a higher concentration of in particular Sr2+ was required to suppress the Mn2+-induced miscleavage. These data suggest that structural changes in P15 can influence cleavage site recognition differentially in a divalent metal ion-dependent manner.
Next we determined the efficiency of cleavage (kcat/Km) for Mut1, Mut2, and wild-type M1 RNA in the presence of Mg2+ or Mn2+ by measuring the kinetic constants kcat and Km. As shown in Table 1, wild type and Mut1 cleaved pATSerCG with the same efficiency in the Mg2+-alone reaction. This result is expected given that the only change introduced by using Mut1 is a change from a GU- to a GC-base pair in the RS complex (Fig. 1b). Surprisingly, in the Mn2+-alone reaction, the activity for Mut1 was down ≈4,000-fold, whereas wild-type activity was reduced only ≈10-fold (≈100-fold using pATSerUA). The dramatic decrease in activity for Mut1 is due to a reduction in kcat with only a slight decrease in Km. This finding is in contrast to wild type (and Mut2, see below), in which changes in both kcat and Km were observed when Mg2+ was replaced with Mn2+. The efficiency of cleavage of the substrate pATSerUA for the other mutant, Mut2, in the presence of Mg2+ was reduced ≈15 times compared with the activity of the wild type, mainly because of an increase in Km. This is in keeping with our previous data using pATSerCG (15). Replacement of Mg2+ by Mn2+ resulted in a decrease in kcat/Km for the wild type (see above), whereas the cleavage efficiency of the mutant was affected only slightly. Comparing the numbers obtained in the Mn2+-alone reactions for wild type and Mut2 reveals a modest increase in kcat/Km for the mutant, indicating that replacement of divalent metal ions indeed can result in suppression of a mutant phenotype. These findings further stress the importance of the structure of the P15 loop for cleavage activity and divalent metal ion binding in the RS complex.
Elsewhere (18), we have suggested that Mg2+ (referred to “MeA2+”) positioned in the vicinity of U294 in M1 RNA contributes to function by stabilizing the RS complex (A in Fig. 1b). Taken together with the present data we suggest that the Me2+-dependent stabilization of the interaction between the 3′ end of the substrate and M1 RNA, in particular the +73/294 base pair (see also refs. 15 and 23), is essential to sustain efficient cleavage at the correct position. Hence, MeA2+ is suggested to be one of the Me2+ involved in divalent metal ion cooperativity in the RS complex. This is in keeping with our findings when we used the M1 RNA variants carrying substitutions at 294 or 254 (Mut1 and Mut2, respectively; see above). Unpublished studies of cleavage activity in the presence of Mg2+ or Mn2+ as a function of the orientation of the +73/294 base pair in the RS complex as well as previously published data (24, 25) give further support to this suggestion. We propose that the other Me2+ ion(s) is the one(s) that is engaged directly in generating the nucleophile. Based on this interpretation, certain divalent metal ions can fulfill the role as a MeA2+ ion, e.g. Mg2+, Ca2+, Sr2+, and Ba2+, and stabilize the RCCA-RNase P RNA interaction. As demonstrated here, cleavage in the presence of either of these Me2+ result in cleavage mainly at the correct position. By contrast, Mn2+ does not function well as a MeA2+ ion, and consequently substantial miscleavage occurs in its presence. The other transition metal ion, Zn2+, is even less active because it fails to function as a MeA2+ ion and thus does not promote cleavage alone. However, as suggested above, Zn2+ can act as the metal ion(s) generating the nucleophile but requires the presence of a metal ion that can fulfill the role of a MeA2+ ion, e.g. Sr2+, to give cleavage. Moreover, Co(NH3)63+ is considered to be an analog of fully solvated magnesium (20). Hence if Zn2+ cannot fulfill the role of a MeA2+ ion, then the coordination at the MeA2+ ion binding site does not need to be inner sphere coordination, because the combination Zn2+/Co(NH3)63+ was found to give activity.
RNA Catalysis, Various Metal Ions, and Biological Relevance.
The divalent metal ions Mg2+ and Ca2+ are bulk biological ions. Addition of Ca2+ together with Mg2+ resulted in a decrease in cleavage activity when the ratio between Ca2+ and Mg2+ was increased (Fig. 4). These two metal ions show similar affinity for RNA (21). These findings raise the interesting possibility that the activity of biocatalysts that depend on RNA for activity are up- or down-regulated depending on the intracellular concentrations of Mg2+ and Ca2+. In this context it is worth noting for example that the flux of Ca2+ is perturbed in tumor cells (26). As a consequence, this perturbation might influence the activity of various RNA and thereby the growth of a tumor cell.
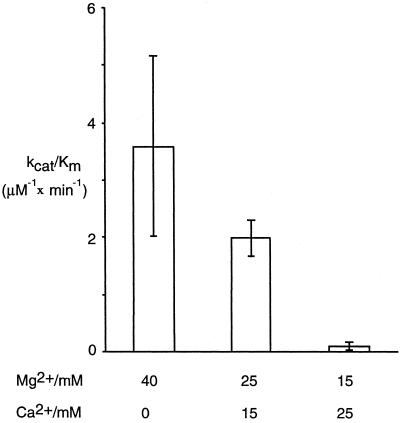
Figure 4.
Cleavage efficiency as a function of different Mg2+/Ca2+ ratios. Diagrams show kcat/Km values for cleavage of patisserie by wild-type M1 RNA at different Mg2+/Ca2+ ratios. The given values are averages of three independent experiments, and the bars indicate experimental errors.
Concluding Remarks.
Our findings demonstrate the power of a chemical genetic approach to extract information about the importance of different categories of divalent metal ion binding sites in the ribozyme M1 RNA cleavage reaction. Moreover, considering both correctness and cleavage efficiency, our data show that cleavage by M1 RNA is optimized in the presence of Mg2+ alone. Our present findings also support and extend our previous model (16) that the RCCA-RNase P RNA interaction results in recoordination of Me2+ such that efficient cleavage occurs at the correct position. Finally, other ribozymes as well as many enzymes require divalent metal ions for function and thus the approach used here can be applied also to these systems to obtain functional information about different divalent metal ion binding sites/metal ions.
Acknowledgments
We thank our colleagues for discussions, Drs. S. Dasgupta, D. Hughes, E. G. H. Wagner, and A. Virtanen for critical reading of the manuscript. This work was supported by grants from the Swedish Natural Science Research Council and the Foundation for Strategic Research (to L.A.K.).
Abbreviation
- RS
M1 RNA substrate
References
- 1.Gesteland R F, Cech T R, Atkins J F. In: The RNA World. 2nd Ed. Gesteland R F, Cech T R, Atkins J F, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1999. [Google Scholar]
- 2.Guerrier-Takada C, Haydock K, Allen L, Altman S. Biochemistry. 1986;25:1509–1515. doi: 10.1021/bi00355a006. [DOI] [PubMed] [Google Scholar]
- 3.Grosshans C A, Cech T R. Biochemistry. 1989;28:6888–6894. doi: 10.1021/bi00443a017. [DOI] [PubMed] [Google Scholar]
- 4.Feig A L, Scott W G, Uhlenbeck O C. Science. 1998;279:81–84. doi: 10.1126/science.279.5347.81. [DOI] [PubMed] [Google Scholar]
- 5.Streicher B, Westhof E, Schroeder R. EMBO J. 1996;15:2556–2564. [PMC free article] [PubMed] [Google Scholar]
- 6.Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S. Cell. 1983;35:849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- 7.Altman S, Kirsebom L A. In: The RNA World. Gesteland R F, Cech T R, Atkins J F, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1999. pp. 351–380. [Google Scholar]
- 8.Gardiner K J, Marsh T L, Pace N R. J Biol Chem. 1985;260:5415–5419. [PubMed] [Google Scholar]
- 9.Kazakov S, Altman S. Proc Natl Acad Sci USA. 1991;88:9193–9197. doi: 10.1073/pnas.88.20.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith D, Burgin A B, Haas E S, Pace N R. J Biol Chem. 1992;267:2429–2436. [PubMed] [Google Scholar]
- 11.Kufel J, Kirsebom L A. J Mol Biol. 1994;244:511–521. doi: 10.1006/jmbi.1994.1749. [DOI] [PubMed] [Google Scholar]
- 12.Kufel J, Kirsebom L A. Proc Natl Acad Sci USA. 1996;93:6085–6090. doi: 10.1073/pnas.93.12.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warnecke J M, Fürste J P, Hardt W-D, Erdmann V A, Hartmann R K. Proc Natl Acad Sci USA. 1996;93:8924–8928. doi: 10.1073/pnas.93.17.8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warnecke J M, Held R, Busch S, Hartmann R K. J Mol Biol. 1999;290:433–445. doi: 10.1006/jmbi.1999.2890. [DOI] [PubMed] [Google Scholar]
- 15.Brännvall M, Kirsebom L A. J Mol Biol. 1999;292:53–63. doi: 10.1006/jmbi.1999.3048. [DOI] [PubMed] [Google Scholar]
- 16.Kirsebom L A, Svärd S G. EMBO J. 1994;13:4870–4876. doi: 10.1002/j.1460-2075.1994.tb06814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milligan J F, Groebe D R, Whiterell G W, Uhlenbeck O C. Nucleic Acids Res. 1987;29:8783–8798. doi: 10.1093/nar/15.21.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kufel J, Kirsebom L A. RNA (NY) 1998;4:777–788. doi: 10.1017/s1355838298970923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perreault J-P, Altman S. J Mol Biol. 1993;230:750–756. doi: 10.1006/jmbi.1993.1197. [DOI] [PubMed] [Google Scholar]
- 20.Misra V K, Draper D E. Biopolymers. 1998;48:113–135. doi: 10.1002/(SICI)1097-0282(1998)48:2<113::AID-BIP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 21.Brännvall M, Mikkelsen N E, Kirsebom L A. Nucleic Acids Res. 2001;29:1426–1432. doi: 10.1093/nar/29.7.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kufel J, Kirsebom L A. J Mol Biol. 1996;263:685–698. doi: 10.1006/jmbi.1996.0608. [DOI] [PubMed] [Google Scholar]
- 23.Tallsjö A, Kufel J, Kirsebom L A. RNA (NY) 1996;2:299–307. [PMC free article] [PubMed] [Google Scholar]
- 24.Perreault J-P, Altman S. J Mol Biol. 1992;226:399–409. doi: 10.1016/0022-2836(92)90955-j. [DOI] [PubMed] [Google Scholar]
- 25.Oh B-K, Frank D N, Pace N R. Biochemistry. 1998;37:7277–7283. doi: 10.1021/bi973100z. [DOI] [PubMed] [Google Scholar]
- 26.Berridge M J, Bootman M D, Lipp P. Nature (London) 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 27.Zuleeg T, Hartmann R K, Kreutzer R, Limmer S. J Mol Biol. 2001;305:181–189. doi: 10.1006/jmbi.2000.4299. [DOI] [PubMed] [Google Scholar]