Version Changes
Revised. Amendments from Version 1
In the revised Figure 1 we show the cis- and trans-Golgi in cells infected with wt HSV-1 or the Us3 deletion mutant. In the revised Figure 11 we only show the 2 pathways we propose. A new Figure 12 is reproduced from Farnsworth et al., 2007’s Figure 2, showing budding virus interaction with the outer nuclear membrane of a mutant lacking the fusion proteins gB/gH.
Abstract
Background: Herpesvirus capsids are assembled in the nucleus, translocated to the perinuclear space by budding, acquiring tegument and envelope, or released to the cytoplasm via impaired nuclear envelope. One model proposes that envelopment, “de-envelopment” and “re-envelopment” is essential for production of infectious virus. Glycoproteins gB/gH were reported to be essential for de-envelopment, by fusion of the “primary” envelope with the outer nuclear membrane. Yet, a high proportion of enveloped virions generated from genomes with deleted gB/gH were found in the cytoplasm and extracellular space, suggesting the existence of alternative exit routes.
Methods: We investigated the relatedness between the nuclear envelope and membranes of the endoplasmic reticulum and Golgi complex, in cells infected with either herpes simplex virus 1 (HSV-1) or a Us3 deletion mutant thereof, or with bovine herpesvirus 1 (BoHV-1) by transmission and scanning electron microscopy, employing freezing technique protocols.
Results: The Golgi complex is a compact entity in a juxtanuclear position covered by a membrane on the cis face. Golgi membranes merge with membranes of the endoplasmic reticulum forming an entity with the perinuclear space. All compartments contained enveloped virions. After treatment with brefeldin A, HSV-1 virions aggregated in the perinuclear space and endoplasmic reticulum, while infectious progeny virus was still produced.
Conclusions: The data suggest that virions derived by budding at nuclear membranes are intraluminally transported from the perinuclear space via Golgi -endoplasmic reticulum transitions into Golgi cisternae for packaging. Virions derived by budding at nuclear membranes are infective like Us3 deletion mutants, which accumulate in the perinuclear space. Therefore, i) de-envelopment followed by re-envelopment is not essential for production of infective progeny virus, ii) the process taking place at the outer nuclear membrane is budding not fusion, and iii) naked capsids gain access to the cytoplasmic matrix via impaired nuclear envelope as reported earlier.
Keywords: herpes virus, envelopment, egress pathway, endoplasmic reticulum, Golgi complex, intraluminal transport, brefeldin A
Introduction
The Golgi complex plays a crucial role in the secretory pathway. Cargo is transported from the endoplasmic reticulum (ER) to the Golgi complex via vesicles that derive from ER exit sites ( Bonifacino & Glick, 2004). Tubules are involved in anterograde as well as in retrograde transport ( Lippincott-Schwartz et al., 1990; Lippincott-Schwartz et al., 1989). The sole paradigm of vesicular transport between ER and Golgi may evolve to account for the results of new technologies ( Lippincott-Schwartz, 2011). Indeed, cargo may also be transported through an ER-Golgi intermediate compartment (ERGIC) ( Hauri & Schweizer, 1992; Klumperman, 2000; Saraste et al., 2009). Whether the ERGIC is a stable structure, is under debate ( Ben-Tekaya et al., 2005). There are also transitional elements connecting Golgi membranes to ER membranes ( Pavelka & Roth, 2015; Polishchuk & Mironov, 2004; Vivero-Salmerón et al., 2008) possibly enabling direct transportation of cargo from ER cisternae into Golgi cisternae. Once in the Golgi cisternae, cargo is packaged into secretory granules for exocytotic release ( Palade, 1975). Packaging of cargo is accompanied by loss of Golgi membranes. To maintain Golgi structure and function, multiple recycling processes take place ( Orci et al., 1981). Although the structure and function of the Golgi complex has been investigated for decades, many uncertainties remain e.g. Golgi maturation and functionality of the trans Golgi network (TGN) ( Emr et al., 2009).
The Golgi complex plays also a crucial role in herpes virus morphogenesis and intracellular transport ( Roizman et al., 2014). Herpes viruses comprise the capsid, tegument and envelope, with embedded glycoproteins. Capsids are assembled in nuclei of host cells and transported to the Golgi complex concomitantly acquiring the envelope and tegument. Yet, this transport is not fully understood and controversially discussed. For certain, capsids bud at the inner nuclear membrane (INM) acquiring an envelope and tegument. Since the discovery of capsids in the cytoplasm adjacent to the nucleus ( Stackpole, 1969) the virions are believed to be released from the PNS via de-enveloped by fusion of the viral envelope with the outer nuclear membrane (ONM) or with adjacent ER membranes releasing capsid and tegument into the cytoplasmic matrix. These capsids then are re-enveloped by budding at membranes of the TGN ( Mettenleiter et al., 2013) acquiring again an envelope and tegument. Concomitantly, a small concentric transport vacuole is formed enclosing the enveloped virion. This process is referred to as wrapping ( Roizman et al., 2014). Alternatively, capsids may be enveloped at endosomes in cells infected with HSV-1 ( Albecka et al., 2016; Hollinshead et al., 2012) or varicella-zoster virus (VZV) ( Buckingham et al., 2016; Grose et al., 2016). Virions have been repeatedly shown in ER cisternae suggesting that virions are intraluminally transported ( Gilbert et al., 1994; Granzow et al., 1997; ( Harson & Grose, 1995) Radsak et al., 1996; Schwartz & Roizman, 1969; Stannard et al., 1996; Sutter et al., 2012; Whealy et al., 1991; Wild et al., 2002) into ER cisternae, whose membranes are connected to the ONM. Virions may be released from the PNS and ER by formation of vesicles engulfing virions ( Campadelli-Fiume et al., 1991; Epstein, 1962; Harson & Grose, 1995; Torrisi et al., 1992; Torrisi et al., 1999). Alternatively, virions were suggested to be farther transported via ER-Golgi transitions into Golgi cisternae ( Leuzinger et al., 2005; Wild et al., 2015; Wild et al., 2002). Importantly, intraluminal transportation requires mechanisms for preventing the viral envelope from fusion with the membrane the virions are transported along. Thus, virions can either be intraluminally transported or the viral envelope fuses with the ONM or ER membranes. There is no doubt that virions can be intraluminally transported and to aggregate in the PNS-ER compartment, e.g. in the absence of the Us3 kinase, raising the question how capsids gain access to the cytoplasmic matrix.
The question remains how naked capsids gain access to the cytoplasmic matrix. It has been clearly shown that nuclear pores dilate leading to impairment of the nuclear envelope, through which capsids are released from the nuclear periphery into the cytoplasmic matrix ( Leuzinger et al., 2005; Wild et al., 2005; Wild et al., 2009) or disrupted nuclear membranes ( Borchers & Oezel, 1993; Klupp et al., 2011). Notably, pore impairment is the initial step in breakdown of the nuclear envelope ( Terasaki et al., 2001) that takes place when HSV-1 infection proceeds ( Maric et al., 2014). The capsids in the cytoplasmic matrix are transported to any site of the Golgi complex ( Wild et al., 2002) and are enveloped either by wrapping (see above) or by budding into Golgi cisternae and/or vacuoles, which may enlarge to engulf multiple virions ( Homman-Loudiyi et al., 2003; Leuzinger et al., 2005; Stannard et al., 1996; Sutter et al., 2012; Wild et al., 2015; Wild et al., 2002). Capsids bud, though less frequently, also at the ONM and RER membranes ( Leuzinger et al., 2005; Wild et al., 2005), and are intraluminally transported to a yet unknown destination.
Although the phenotypes of the capsid transport across the ONM exhibit all characteristics of budding ( Bonifacino & Glick, 2004; Harrison, 2015; Jahn et al., 2003; Kanaseki et al., 1997; Lee, 2010; Mayer, 2002) the de-envelopment theory is still favored. Fusion of the viral envelope with the ONM requires the glycoproteins gB and gH ( Farnsworth et al., 2007). Nonetheless, various stages of capsid transportation across the ONM and ER membranes in the absence of the glycoproteins gB/gH have been shown (see Figure 2 in Farnsworth et al., 2007). Furthermore, a substantial proportion of virions lacking gB/gH were reported to be in the cytoplasm and extracellular space. These two facts strongly suggest another pathway of virus particles out of the PNS. The Us3 kinase is involved in nucleus to cytoplasm capsid translocation ( Poon et al., 2006) via phosphorylation of viral proteins including gB ( Wisner et al., 2009), UL31 and UL34 ( Mou et al., 2009; Ryckman & Roller, 2004). In the absence of the Us3 kinase, 98% of all produced virions accumulate in the PNS-ER compartment ( Wild et al., 2015). These virions are infective ( Reynolds et al., 2002; Wisner et al., 2009). Us3 is also involved in downregulation of phospholipid biosynthesis ( Wild et al., 2012) induced by HSV-1 ( Sutter et al., 2012), and in blocking of apoptosis ( Wang et al., 2011).
Transportation of virions out of the PNS into ER raises the question of their destination. As stated above, the viral envelope cannot fuse because transportation of a membrane bound particle along membranes needs protection from fusion. A possible destination of intraluminal transported virions could be further transportation into Golgi cisternae via ER-to-Golgi transitions as suggested by ( Leuzinger et al., 2005). ER-to-Golgi transitions have been shown in many cells since the early 1970s ( Claude, 1970). Therefore, we investigated cells infected with BoHV-1, a member of the subfamily varicella virus, or with HSV-1, a member of the subfamily simplex virus, or a Us3 deletion mutant thereof, because the Golgi complex enlarges in the absence of Us3 ( Wild et al., 2012) by cryo-field emission scanning electron microscopy (Cryo-FESEM) and transmission electron microscopy (TEM), employing protocols for improved spatial and temporal resolution ( Ellinger et al., 2010; Mueller, 1992). We show that the Golgi complex is a tightly packed organelle, that ER-to-Golgi transitions are present, and that virions aggregate within the ER after exposure cells to brefeldin A (BFA), which disintegrates the Golgi complex within minutes ( Hess et al., 2000), suggesting an intraluminal transportation route. Moreover, we observe that intraluminal virions are densely coated with a proteinaceous layer that arises during budding at nuclear membranes. Therefore, we propose that the significance of the dense coat is protecting the viral envelope from fusion with the membranes along which virions are transported, in a similar manner as clathrin protects coated vesicles from fusion.
Materials and methods
Cells and viruses
Vero cells and MDBK cells (European Collection of Cell Cultures) were grown in Dulbecco’s modified minimal essential medium (DMEM; Gibco, Bethesda, MD, USA) supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml) and 10% fetal bovine serum (FBS; Gibco). The Us3 deletion mutant R7041(ΔUs3) and the repair mutant R2641 ( Longnecker & Roizman, 1987; Purves et al., 1987) were kindly provided by Bernard Roizman (The Marjorie B. Kovler Viral Oncology Laboratories, University of Chicago, Illinois, USA). Wild-type herpes simplex virus 1 (wt HSV-1) strain F (Ejercito et al., J. Gen. Virol. 2:357–364, 1968), R7041(ΔUs3) and R2641 were propagated in Vero cells, and bovine herpes virus 1 (BoHV-1: Metzler et al., Arch. Virol. 87: 205–217, 1986) in MDBK cells. Virus yields were determined by plaque titration.
Cryo-fixation for transmission electron microscopy
50 μm thick sapphire disks (Bruegger, Minusio, Switzerland) measuring 3 mm in diameter were coated with 8–10 nm carbon, obtained by evaporation under high vacuum conditions to enhance cell growth and to facilitate detachment of cells from the sapphire disks after embedding. Vero and MDBK cells were grown for 2 days on sapphire disks placed in 6 well plates. Cells were inoculated with R7041(ΔUs3), the repair mutant R2641, wt HSV-1 or BoHV-1 at a MOI of 5, incubated at 37°C, and fixed at 8 to 20 hpi by adding 0.25% glutaraldehyde to the medium prior to freezing in a high-pressure freezing unit (HPM010; Science Services, Munich, Germany) and processed as described in detail ( Wild, 2008). In brief, the frozen water was substituted with acetone in a freeze-substitution unit (FS 7500; Boeckeler Instruments, Tucson, AZ, USA) at -88°C with acetone and subsequently fixed with 0.25% glutaraldehyde and 0.5% osmium tetroxide raising the temperature gradually to +2°C to achieve good contrast of membranes ( Wild et al., 2001), and embedded in epon at 4°C followed by polymerization at 60°C for 2.5 days. After removal of sapphire disks by immersion in liquid nitrogen, serial sections of 60 to 90 nm thickness were analyzed in a transmission electron microscope (CM12; FEI, Eindhoven, The Netherlands) equipped with a CCD camera (Ultrascan 1000; Gatan, Pleasanton, CA, USA) at an acceleration voltage of 100 kV.
Cell exposure to brefeldin A
Cells grown on sapphire disks were inoculated with wt HSV-1 at a MOI of 5 and incubated at 37°C. Stock solution (5 mg BFA solved in 0.5 ml methanol) was diluted with medium 1:10. One µl/ml medium (1µg/ml) of this solution was added to cell cultures at 5, 8, 12 and 16 hpi. Cells were high-pressure frozen at indicated times, and prepared for TEM. To quantify virus phenotypes and their location, 10 images of cellular profiles were taken at random from ultrathin sections of monolayers exposed to BFA from 5, 8, 12 or 16 hpi to 20 hpi of 5 independent experiments. Capsids budding at the INM, ONM, ER and Golgi membranes, capsids undergoing wrapping, as wells as virions in the PNS, ER, Golgi cisternae and vacuoles were counted. The means expressed per cellular profile were compared applying a Student’s t-test using GraphPad Prism 3 software.
For determination of infectious progeny virus produced after BFA exposure, cells were grown in 10 ml Falcon flasks, inoculated with wt HSV-1 at a MOI of 5, and exposed to BFA from 5, 8, 12 or 16 h to 20 hpi. Cells were harvested at 5, 8, 12, 16 and 20 hpi for determination of infectious progeny virus by plaque titration. Since the Golgi complex reacts immediately to BFA by disintegration, infectious viruses produced after BFA exposure were considered to have derived by budding at membranes other than those of the Golgi complex.
Cryo-Field Emission Scanning Electron Microscopy (Cryo-FESEM)
Vero cells were grown in 25 cm 2 cell culture flasks for 2 days prior to inoculation with wt HSV-1, R7041(ΔUs3) or R2641 at MOI of 5. Cells were harvested at 9 to 12 hpi by trypsinization followed by centrifugation at 150 x g for 8 min. Pellets were re-suspended in 1 ml fresh medium, collected in Eppendorf tubes and fixed by adding 0.25% glutaraldehyde to the medium. The suspension was kept in the tubes at 4°C until cells were sedimented. After removal of the supernatant, cells were frozen in a high-pressure freezing machine EM HPM100 (Leica Microsystems, Vienna, Austria) as described in detail previously ( Wild et al., 2012; Wild et al., 2009). Cells were fractured at -120°C in a freeze-fracturing device BAF 060 (Leica Microsystems) in a vacuum of 10 -7 mbar. The fractured surfaces were partially freeze-dried (“etched”) at -105°C for 2 min, and coated with 2.5 nm platinum/carbon by electron beam evaporation at an angle of 45°. Some specimens were coated additionally with 4 nm of carbon to reduce electron beam damage during imaging at high magnifications. Specimens were imaged in an Auriga 40 Cross Beam system (Zeiss, Oberkochen, Germany) equipped with a cryo-stage (Leica Microsystems) at -115°C and an acceleration voltage of 5 kV using the inlens secondary electron detector.
Confocal microscopy
Cells were grown for 2 days on 0.17 mm thick cover slips of 12 mm in diameter (Assistent, Sondheim, Germany) and inoculated with R7041(ΔUs3), wt HSV-1 or R2641 at a MOI of 5 and incubated at 37°C. After fixation with 2% formaldehyde for 25 min at room temperature, cells were briefly washed with PBS and stored in PBS at 4°C until further processing. Then, cells were permeabilized with 0.1% Triton-X-100 at room temperature for 7 min and blocked with 3% bovine serum albumin in PBS containing 0.05% Tween 20 (PBST). To identify the Golgi complex, cells were incubated with recombinant monoclonal antibodies against the cis-Golgi protein GM130, abcam EP892Y (Abcam, Cambridge, UK) at a dilution of 1:1000 for 2 h at room temperature followed by incubation with Alexa 647 (Molecular Probes, Eugene, OR, USA) as secondary antibodies, diluted 1:500, for 1 h at room temperature. For identification of the trans-Golgi, TGN46 (NBP1-49643, Novus Biologicals Europe, Abingdon, UK) was used at a dilution of 1:100, followed by Alexa 488 (Molecular Probes), diluted 1:500. To identify infected cells, antibodies against glycoprotein gB (Novus Biological, Littleton, CO, USA) diluted 1:250, and Alexa 594 (Molecular Probes) diluted 1:500, as secondary antibodies were used. After staining nuclei with 4',6-Diamidino-2-phenylindol (DAPI; Roche, Mannheim, Germany), cells were embedded in glycergel mounting media (Dako North America, Carpinteria, CA, USA) and 25 mg/ml 1,4-diazabicyclo [2.2.2] octane (DABCO; Fluka, Buchs, Switzerland). Specimens were analyzed using a confocal laser scanning microscope (SP2; Leica, Wetzlar, Germany). Images were deconvolved employing the deconvolution algorithm of the program suite Huygens Essential (SVI, Hilversum, The Netherlands).
Results
The Golgi complex is a tightly packed entity situated close to the nucleus
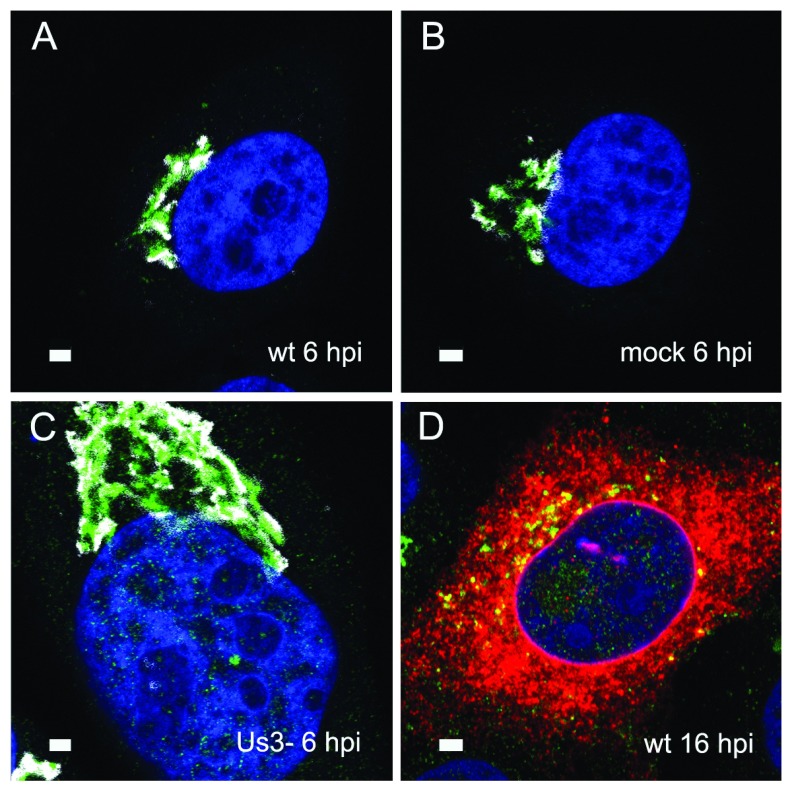
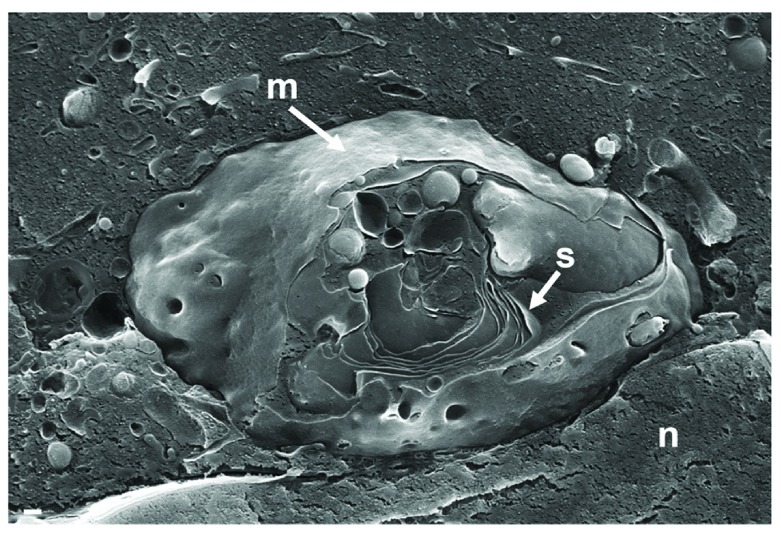
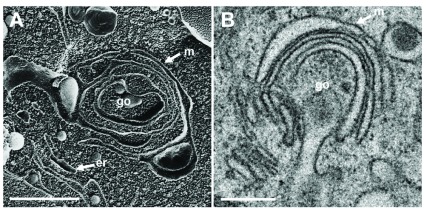
To localize the Golgi complex, we first imaged the Golgi complex by confocal microscopy after labeling the cis-face with anti GM 130 antibodies, and the trans-face with anti TGN46 antibodies in Vero cells. The Golgi complex was always found close to the nucleus in HSV-1, R7041(ΔUs3), or mock infected cell ( Figure 1). At 6 hpi with wt HSV-1, the trans-Golgi was of similar size and shape as in mock infected cells whereas the cis-Golgi had disappeared by 16 hpi, and the trans-Golgi became fragmented. In contrast, the Golgi complex had enormously enlarged after infection with the Us3 deletion mutant R7041(ΔUs3), which is in line with the enlargement of the surface area of Golgi membranes as revealed by electron microscopic morphometry ( Wild et al., 2012), and reflects the significance of Us3 in downregulation of phospholipid synthesis induced by HSV-1 ( Sutter et al., 2012). Next, high resolution cryo-electron microscopy of freeze fractured cells demonstrated the bell-shaped form of the Golgi complex, and its localization close to the nucleus ( Figure 2). This image also shows that the Golgi complex is a complex tightly packed entity with a diameter of approximately 6 µm. The Golgi complex is separated from the cytoplasmic matrix by an intact membrane covering the whole visible surface of the cis-face. The large dimension makes clear that the detected ultrastructural details depend on a large scale on how and where the Golgi complex is hit in a given section plane for studying by TEM. Both freeze-fracture planes and thin sections of central regions show that the membrane of the outermost cisterna covers the cis-side ( Figure 3).
Figure 1. Immunolabelling of the Golgi complex.
Confocal microscopy of the trans-face (green) and the cis-face (white) of Vero cells, immunolabeled with anti TGN46 antibodies (green) and anti GM130 antibodies (white) at 6 h after inoculation with HSV-1 ( A), R7041(ΔUs3) ( C) after mock infection ( B), as well a at 16 hpi with wt HSV-1 ( D) together with immunolabeling of the viral glycoprotein gB (red). The Golgi complex was always in a juxtanuclear position and enormously enlarged after R7041(ΔUs3). Note the enlargement of the nucleus after infection with R7041(ΔUs3). Bars 1 µm.
Figure 2. Cryo-FESEM of a Golgi complex in close vicinity to the nucleus (n) in a Vero cell, at 10 hpi with wt HSV-1.
The entire visible surface is covered by an intact membrane (m) except at the part it is broken away giving view to Golgi stacks (s). Bars: 200 nm.
Figure 3. Golgi fields not involved in virus maturation and transport.
The Golgi complex, as revealed in freeze-fracture planes ( A) and in thin sections ( B), is entirely covered at the cis-face by the membrane (m) of the outermost cisterna. Bars: 100 nm.
ER-to-Golgi transitions
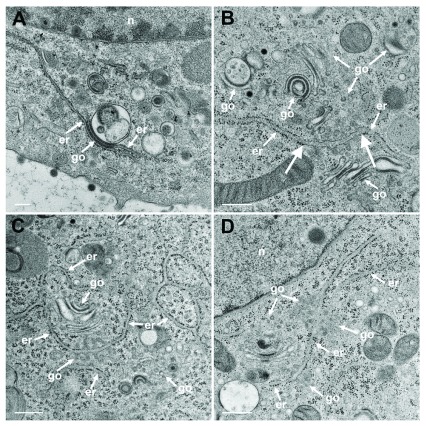
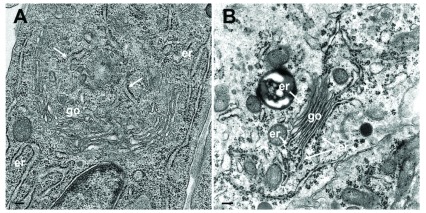
The Golgi complex undergoes dramatic changes during HSV-1 infection finally resulting in fragmentation and dispersion ( Campadelli et al., 1993). However, the Golgi complex is not, or only minimal, involved in envelopment of R7041(ΔUs3) capsids ( Wild et al., 2015). To identify ER-to-Golgi transitions, we thus imaged the Golgi complex in serial sections through wt HSV-1 or R7041(ΔUs3) or BoHV-1 infected cells by transmission electron microscopy after rapidly freezing and freeze-substitution applying a protocol especially suitable to visualize membranes ( Wild et al., 2001). A series of images show that ER membranes continue into Golgi membranes. The ER runs from the perinuclear region into the membranes of the outermost Golgi stack ( Figure 4A). The very same membranes continue again into ER membranes so that this Golgi cisterna is interconnected between ER lamellae. The membranes of the adjacent stack also turn into ER membranes. ER membranes also pass somewhere into central regions of Golgi fields where they are devoid of ribosomes ( Figure 4BC). ER membranes may even connect two Golgi fields ( Figure 4D). The ER forms, as its name implies, a network ( Figure 4C) that connects to the PNS ( Figure 5A).
Figure 4. TEM of R7041(ΔUs3) infected Vero cells at 12 hpi.
( A) The ER (er) runs from the nuclear (n) periphery towards a Golgi field (go), continuing into the membrane of the outermost stack and further into the cytoplasmic matrix. The membranes of the second stack continue also into ER membranes. ( B) An ER cisterna runs through multiple small Golgi fields, whereby the ER membranes turn into Golgi membranes (thick arrows). ( C) ER membranes forming a network continue into Golgi membranes. ( D) ER membranes run through two Golgi fields, turning each time into Golgi membranes. Bars: 500 nm.
Figure 5. The effect of BFA.
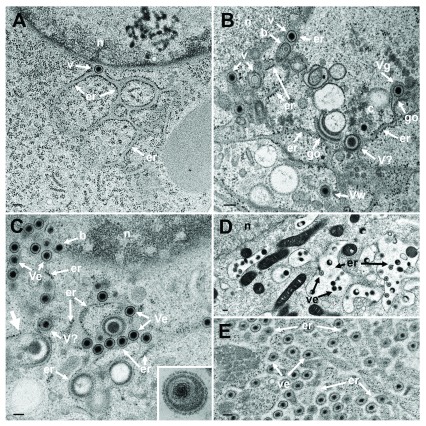
TEM of Vero cells at 9 hpi with R7041(ΔUs3) ( A), at 16 hpi ( B) and at 20 hpi ( C) with wt HSV-1, and at 15 or 17 hpi with wt HSV-1 and BFA exposure ( D and E). ( A) The ER (er) runs from the nucleus (n) towards the cell periphery forming an entity with the PNS that contains a virion (v). ( B) The ER contains virions. One capsid is in the stage of budding (b) into the ER. The ER continues into Golgi (go) membranes at two sites. One Golgi cisterna contains a virion (Vg), one virion has been derived by wrapping (Vw). Close to Golgi stacks, there is probably a virion (V?) of abnormal size. ( C) One capsid buds (b) at the nuclear (n) periphery. The ER is dilated and filled with virions (Ve) and dense material: An ER membrane turns into a Golgi membrane (thick arrow). ( D) After exposure to BFA from 8 to 15 hpi with wt HSV-1, the ER was dilated and contained some virions. ( E) The ER was almost filled with virions after exposure to BFA from 8 to 17 hpi with wt HSV-1. Note that virions in the PNS and ER are covered by a dense coat hiding spikes whereas spikes are clearly apparent on virions in the extracellular space ( C inset). Bars: 200 nm.
Virions are within ER cisternae
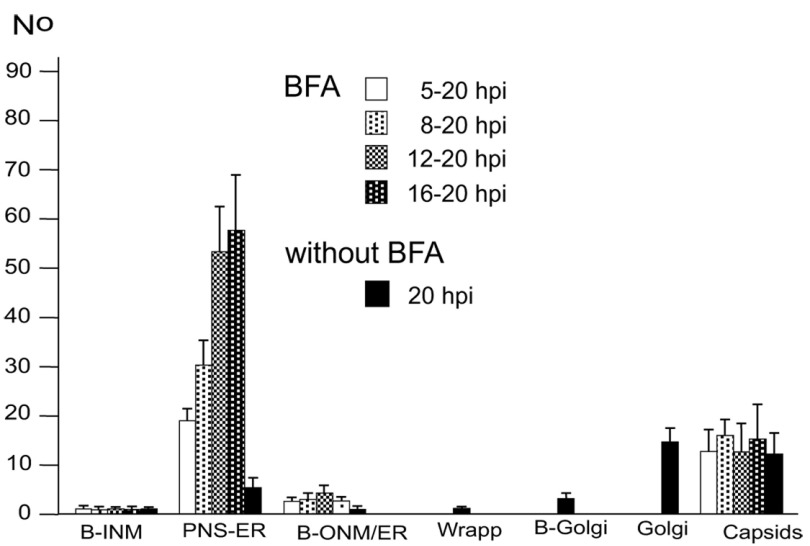
Virions within the ER have been repeatedly shown, the first report dating back to the late 1960ties ( Schwartz & Roizman, 1969). Virions were in the PNS or anywhere in ER cisternae after HSV-1 infection ( Figure 5BC) as well as in Golgi cisternae of which membranes transit into ER membranes ( Figure 5B). Virions accumulate in the PNS-ER compartment late in infection ( Leuzinger et al., 2005; Wild et al., 2015), in the absence of Us3 ( Reynolds et al., 2002; Wisner et al., 2009) or gB/gH ( Farnsworth et al., 2007) or after disintegration of the Golgi complex by BFA ( Chatterjee & Sarkar, 1992; Cheung et al., 1991; Jensen & Norrild, 2002; Whealy et al., 1991). To investigate the effect of BFA on virus release out of the PNS we exposed cells to BFA at 5, 8, 12 and 16 hpi with wt HSV-1, and harvested cells at 20 hpi for quantitative electron microscopic analysis and determination of infectious progeny virus. Electron microscopy revealed dilation of the PNS and ER containing many virions and amorphous material at 15 hpi ( Figure 5D). At 17 hpi, the ER was congested with virions ( Figure 5E). Quantitative analysis of phenotype distribution revealed that virions accumulate in the PNS-ER compartment after BFA administration in a time dependent manner. The number of intraluminal virions was 4 times higher when BFA was added at 8 hpi but 12 times higher than it was added at 16 hpi compared to that in untreated cells ( Figure 6). The number of virus particles interacting with the ONM and ER membranes in BFA exposed cells was about twice as high as in controls whereas the number of capsids in the cytoplasmic matrix did not differ significantly. We thus conclude i) that the interactions at the ONM and ER membranes are budding capsids contributing to accumulation of virions in the PNS and ER, ii) that more capsids bud at the ONM and ER membranes because no Golgi membranes are available after Golgi disintegration induced by BFA, iii) that inhibition of virion release out of the PNS-ER compartment is due to a blockage of the intraluminal transportation pathway after Golgi disintegration, and iv) that the Golgi complex delivered components to budding sites prior to its disintegration by BFA.
Figure 6. Means and standard deviations of the phenotype of HSV-1 infected Vero cells.
BFA was added to monolayers at 5, 8, 12 or 16 hpi (MOI of 5) and incubated until 20 hpi. For control, inoculated cells were incubated for 20 h without addition of BFA. Cells were rapidly frozen at 20 hpi and processed for electron microscopy. The phenotypes of envelopment were counted in 10 cellular profiles of 5 independent experiments: Capsids budding at the INM (B-INM), at the ONM and ER membranes (B-ONM/ER) and at the Golgi complex (B-Golgi); virions in the PNS-ER compartment (PNS-ER); virions derived by wrapping (Wrapp); virions in Golgi cisternae or large vacuoles (Golgi); capsids in the cytoplasmic matrix (capsids).
Virions in the PNS-ER compartment are infective
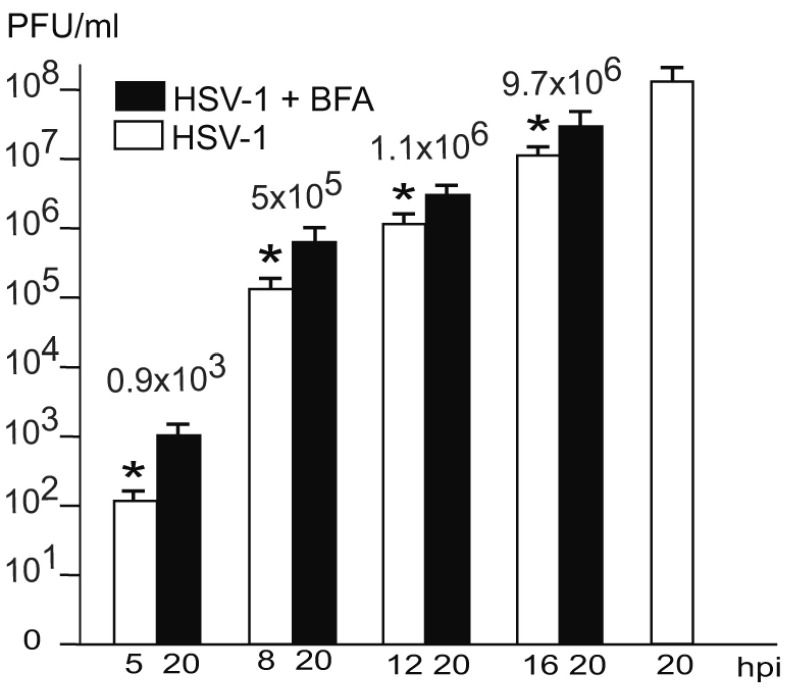
Us3 is not essential ( Poon et al., 2006; Reynolds et al., 2002; Ryckman & Roller, 2004; Wisner et al., 2009). Us3 deletion mutants accumulating in the PNS are infective ( Wild et al., 2015). Hence, it is reasonable to assume that wt HSV-1 virions in the PNS-ER compartment are also infective. To prove this idea, we determined infectious progeny virus by plaque titration at the time point of BFA administration and at 20 hpi. The Golgi complex completely disintegrates within less than 5 minutes after exposure of cells to BFA ( Hess et al., 2000). Therefore, capsids cannot be enveloped by Golgi membranes anymore, or at least only for 5 minutes. As shown in Figure 6, there were no capsid interactions with cell membranes, such as Golgi membranes or endosomes whereas the PNS-ER compartment was full of virions. Nonetheless, infectious progeny virus was produced in a time depended manner. The later BFA was added the more infectious viruses were produced by 20 hpi ( Figure 7). Since virions accumulated invariably in the PNS-ER compartment in BFA exposed cells, and capsids were found to bud at the INM, ONM and ER membranes, we conclude that virions derived by budding at nuclear membranes and ER membranes are infective. The transport of capsids across the ONM generally believed to be fusion – de-envelopment – is budding as discussed below because of the phenotype and because it takes place also in the absence of the fusion proteins gB/gH.
Figure 7. Virus yields at the time of BFA administration or controls (white) and at 20 hpi (black).
The difference between virus yields (indicated with numbers) at the time of BFA addition and harvesting at 20 hpi is considered to be due to virus production after Golgi disintegration. These infectious virions correspond to the virions accumulating in the PNS-ER compartment. n = 4, p<0.01.
PNS, ER and Golgi complex form an entity
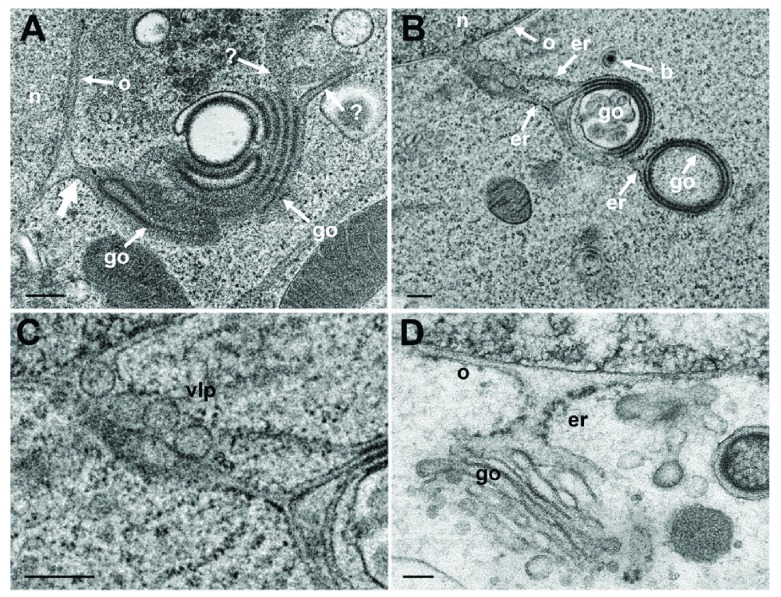
The nuclear envelope is part of the ER. The outer nuclear membrane (ONM) is stubbed with ribosomes. The ONM continues into ER cisternae, which, in turn, merge with Golgi cisternae. Golgi cisternae were also found to connect to the PNS via short ER-Golgi intermediates in R7041(ΔUs3) infected Vero cells ( Figure 8ABC) and BoHV-1 infected MDBK cells ( Figure 8D). ER-Golgi intermediates contained virus like particles ( Figure 8B). The continuum between PNS and Golgi cisternae is considered likely to serve as a direct, short and efficient pathway to transport virions form the site of budding to Golgi cisternae for packaging.
Figure 8. TEM of Vero cells at 12 hpi with R7041(ΔUs3) and of BoHV-1 infected MDBK cells, showing Golgi fields close to the nucleus (n).
( A) Golgi (go) membranes continue (thick arrow) into the ONM (o) as well as towards the cytoplasm indicated by (?) because the destination is unknown. ( B) Golgi membranes continue via ER membranes (er) into the ONM. The ER contains 4 virus-like particles. ( C) Details of panel B. ( D) PNS, ER and Golgi complex form an entity in a BoHV-1 infected MDBK cell ( D: This figure has been reproduced with permission of P. Wild et al., Micron 33, 2002, Elsevier). Bars 200 nm.
Virions are within Golgi cisternae and/or vacuoles
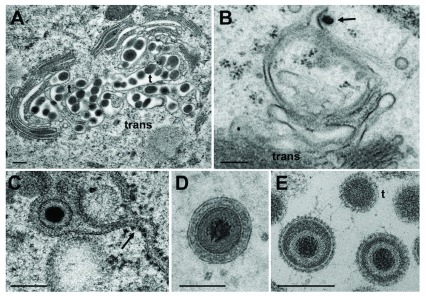
Capsids are postulated to be enveloped at the trans Golgi network ( Mettenleiter et al., 2006) by a process designated wrapping. However, capsids can bud at any location of the Golgi complex ( Figure 9B) and vacuoles as have been shown for HSV-1 ( Leuzinger et al., 2005; Stannard et al., 1996), BoHV-1 ( Wild et al., 2002) and pseudorabies virus ( Klupp et al., 2008), and even at endosomes ( Albecka et al., 2016; Hollinshead et al., 2012). The result of wrapping is a small concentric vacuole ( Figure 9D) containing a single virion as shown elsewhere in detail ( Leuzinger et al., 2005; Wild et al., 2005). Golgi cisternae and vacuoles can contain one to numerous virions in a given section plane ( Figure 9A). The cavities at the trans-face of the Golgi complex in Figure 9A are more likely to represent cisternae rather than vacuoles because of their shape and location. The virions had gained access either by budding or by intraluminal transportation via ER-Golgi intermediates, as might be the case also in Figure 9C. Note that the viral envelope including spikes are covered by a dense layer in narrow Golgi cisternae ( Figure 9C) whereas spikes are visible on virions in wide Golgi cisternae or large vacuoles ( Figure 5 inset) and in the extracellular space ( Figure 9E) as shown previously ( Leuzinger et al., 2005; Wild et al., 2005). From the facts that virions are within ER and Golgi cisternae, and that the ONM continues via ER membranes into Golgi membranes forming an entity, we postulate that virions can be intraluminally transported from the PNS via ER into Golgi cisternae.
Figure 9. Virions in Golgi cisternae versus budding of capsids.
( A) Golgi cisternae engulfing BoHV-1 virions at 20 hpi. Many of them are tangentially (t) sectioned. ( B) Budding BoHV-1 capsid at a Golgi membrane of the cis-face (arrow). ( C) HSV-1 virion in a Golgi cisterna that connects to the ER (arrow). Note the dense content within the ER and Golgi cisterna indicating little loss of material during processing. ( D) Concentric vacuole derived by wrapping containing a single BoHV-1 virion. The space between viral envelope and vacuolar membrane is always filled in well preserved cells. ( E) Virions in a large vacuole or cisterna exhibiting clearly spikes even in tangentially (t) sectioned virions. Bars: 200 nm.
ER-to-Golgi transitions in uninfected cells
ER-to-Golgi transitions are not only established in infected cells, but also in cultured epithelial cells ( Figure 10A) or in cells in organs, e.g. parathyroid gland, which was prepared by perfusion fixation ( Wild et al., 1985) according to conventional protocols ( Figure 10B). These observations suggest that ER to Golgi transitions may also serve as a direct transportation route e.g. for proteins to be finally released by exocytosis.
Figure 10.
( A) TEM image of a cultured epithelial cell in which the Golgi complex is embedded in the ER. The ER membranes (arrows) run into the Golgi complex, close to a structure that probably represents a tangential section of the Golgi organizing center. ( B) TEM image of a parathyroid cell prepared according to conventional protocols showing Golgi membranes (go) continuing into ER membranes (er). Bars: 200 nm.
Copyright: © 2018 Wild P et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Copyright: © 2018 Wild P et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Discussion
The Golgi complex is among the first organelles that rapidly disintegrate during processing for electron microscopy after improper fixation and processing ( Han et al., 2013; Wild et al., 2001). To minimize disintegration, we employed a technique that leads to improved retention of cellular material ( Cope & Williams, 1969; Weibull et al., 1984), and to improved spatial and temporal resolution ( Mueller, 1992). This is especially important for analytical studies of cells in which the Golgi complex is involved in highly dynamic processes such as packaging of proteins into granules ( Ellinger et al., 2010; Orci et al., 1981; Wild et al., 1982) in the secretory pathway, or envelopment of capsids and vacuole formation ( Figure 11) for delivery of hundreds of virions to the cell periphery ( Leuzinger et al., 2005; Wild et al., 2015; Wild et al., 2002). Because of the difficulties in preservation of the Golgi ultrastructure ( Ellinger et al., 2010; Han et al., 2013; Wild et al., 2001), and because of its complexity, three-dimensional structure is poorly understood. Cryo-FESEM revealed the Golgi complex to be a complex tightly packed structure. The membrane of the outermost cisternae may completely cover the cis-face. TEM also revealed that the Golgi complex is embedded in the ER system, with multiple membrane connections forming a Golgi-ER entity.
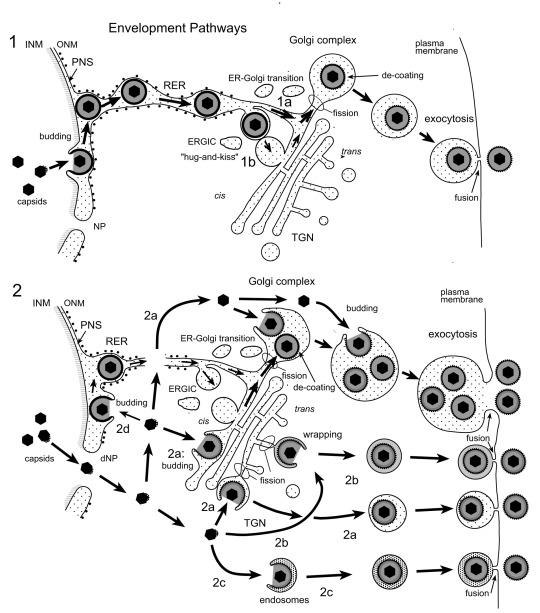
Figure 11. Schematic representation of proposed herpes virus envelopment pathways.
(1) Capsids bud at the INM into the PNS acquiring tegument and an envelope covered with a dense coat. These perinuclear virions are transported into the RER and further via Golgi transitions (1a) or the ERGIC (“hug-and-kiss”, 1b) into Golgi cisternae where they are packaged into transport vacuoles, which are detached from Golgi membranes by fission. The dense coat is shed off while vacuoles are transported to the cell periphery for exocytotic release of uncoated virions into the extracellular space. (2) Capsids gain direct access to the cytoplasmic matrix via dilated nuclear pores (dNP), and are transported to any site of the Golgi complex. They either bud into Golgi cisternae and vacuoles, respectively (2a) or are enveloped by a process designated wrapping (2b) that involves budding and concomitant formation of a small transport vacuole engulfing a single virion. Capsids can also be enveloped by endosomal membranes (2c). Occasionally, capsids may bud at the OM or RER (2d), and the resulting virions are intraluminally transported as in pathway 1. Finally, vacuoles derived by fission from Golgi membranes or from membranes of vacuoles or endosomes transport virions to the cell periphery and release them into the extracellular space via exocytosis. The dense coat, which derived during the budding process at the INM and ONM and probably protects the viral envelope from fusion with membranes the virions are transported along, is shed of (de-coating) in transport vacuoles at latest when virions are released into the extracellular space. During budding at Golgi cisternae and vacuoles, a dense rim of tegument is closely attached to the inner layer of the viral envelope. However, no dense coat is formed so that spikes (glycoproteins) are readily seen in high resolution micrographs.
The Golgi complex fragments and disperses about 16 hpi with HSV-1 ( Campadelli et al., 1993) adding additional difficulties for understanding Golgi function in herpes virus envelopment. Simultaneous immune labelling revealed that the cis-Golgi had disappeared by 16 hpi with wt HSV-1 whereas the trans-Golgi had fragmented. In contrast, the cis- and trans- Golgi had enlarged already by 6 hpi with the Us3 deletion mutant R7041(ΔUs3), which is related to the enhanced phospholipid synthesis in the absence of Us3 ( Wild et al., 2012). The significance of Us3 on the Golgi complex is under study. To address the significance of the Golgi complex in virus envelopment and virus transportation, we thus investigated infected cells between 8 hpi (the approximate time of onset of envelopment) and 16 hpi. Our data clearly show that the Golgi complex is localized in a juxtanuclear position appearing as a compact entity by cryo-FESEM. Golgi membranes continue into ER membranes which in turn connect to the ONM forming a continuum between Golgi cisternae and PNS. Thus, the presence of virions within the PNS, ER cisternae and Golgi cisternae strongly suggests that the ER-to-Golgi transition is used as a direct intraluminal pathway to deliver virions from the PNS into Golgi cisternae ( Figure 11, pathway 1). This idea is supported by the fact that about 80 HSV-1 virions per mean cell volume were within ER cisternae at 12 and 16 hpi but close to 300 by 24 hpi ( Wild et al., 2015) suggesting that virus transportation out of the ER is inhibited after Golgi fragmentation ( Campadelli et al., 1993). Virus transportation out of the ER is also drastically inhibited after BFA exposure. The ER dilates and secretory protein transport from the ER to the Golgi complex is impeded after BFA treatment ( Fujiwara et al., 1988; Misumi et al., 1986). Hence, the integrity of the Golgi complex is crucial for export of both secretory proteins and HSV-1 out of the ER suggesting that HSV-1 release from the ER to the Golgi complex follows a similar pathway as secretory proteins either by vesicle formation involving cop II ( Klumperman, 2000) or equivalent, or via ER-Golgi transitions.
According to the currently stressed herpes virus egress theory ( Mettenleiter et al., 2013), formation of infectious herpes viruses follows a complicated uneconomic pathway involving primary envelopment by budding of capsids at the INM, de-envelopment of capsids by fusion of the viral envelope with the ONM releasing capsids and tegument into the cytoplasmic matrix, and re-envelopment by wrapping at the trans Golgi network. The interaction of the viral envelope with the ONM was described the first time in 1968 ( Darlington & Moss, 1968) and identified as budding of capsids from the cytoplasmic matrix into the PNS. About 30 years later, it was tried to prove that this process is fusion ( Kopp et al., 2002; Naldinho-Souto et al., 2006; Skepper et al., 2001). Fact is that the phenotypes of the process taking place at the ONM are identical with those at the INM ( Darlington & Moss, 1968; Leuzinger et al., 2005; Wild et al., 2005; Wild et al., 2012) and that they show all characteristics of budding. Budding requires proteins that are able to induce positive and negative curvatures. Budding at the INM is driven by UL31/UL34 ( Bigalke & Heldwein, 2016; Bigalke & Heldwein, 2017; Bigalke et al., 2014; Hagen et al., 2015). UL31 and UL34 are also present at the ONM even in cells infected with Us3 deletion mutants ( Reynolds et al., 2002) those envelopes are unable to fuse with the ONM ( Reynolds et al., 2002; Wisner et al., 2009). Therefore, the presence of UL31/UL34 at the ONM cannot be the result of membrane transportation from the INM to the ONM via budding and subsequent fusion as often used as arguments for the presence of viral proteins at the ONM ( Skepper et al., 2001). Furthermore, UL34 was shown to localize at the ER ( Yamauchi et al., 2001) and that UL31 is required for its dislocation to the INM.
Us3 deletion mutants arising by budding at the INM cannot be de-enveloped at the ONM and, hence, accumulate in the PNS. Nonetheless, Us3 deletion mutants are infective ( Reynolds et al., 2002; Ryckman & Roller, 2004; Wild et al., 2015) implying that all essential proteins must have been transported to the nuclear periphery so that they can become part of virions deriving by budding at the INM. The same is considered likely to be true for wt HSV-1 virions because they differ from Us3 deletion mutants only in the ability to be released out of the PNS. Indeed, by constructing a triple-fluorescent recombinant it was shown that the capsids fusion protein VP26 localized in the nucleus, the tegument fusion protein VP16 and the envelope glycoprotein H localized at the nuclear rim early in infection ( de Oliveira et al., 2008). In VZV, it was shown that biosynthesis of glycoprotein starts within 2 h after infection ( Yao et al., 1993). Production of infectious progeny virus after exposure cells to BFA indicate that they have derived by budding at nuclear membranes and ER membranes see ( Figure 6), and that e.g. glycosylation of glycoproteins had to take place prior to disintegration of the Golgi complex. The glycoprotein gH was detected in the cytoplasm as well as at the nuclear periphery at 4 hpi, ( de Oliveira et al., 2008), i.e. much earlier prior to the onset of budding. Therefore, we assume that infectious virions can be produced after administration of BFA at 5 hpi by budding at nuclear membranes though at a limited number. The later cells were exposed to BFA the more infectious virions were produced suggesting that the later the Golgi complex disintegrated the more proteins and lipids could have been produced. Considering fragmentation of the Golgi complex by about 16 hpi with wt HSV-1 ( Campadelli et al., 1993) the question arises where are the large number of virions produced after Golgi fragmentation?
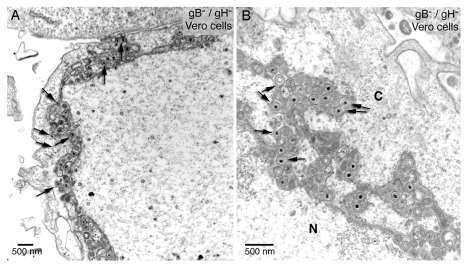
For de-envelopment via fusion of the viral envelope with the ONM fusion proteins would be required. Theglycoproteins B and H (gB/gH), members of the quartet of fusion proteins responsible for cell entry ( Turner et al., 1998), have been claimed to be responsible for fusion of the viral envelope with the ONM ( Farnsworth et al., 2007). Indeed, they clearly showed by electron microscopy striking interactions with the ONM and ER membranes in cells infected with a gB/gH deletion mutant. The crux is, first, that the viral envelope cannot fuse with ONM in the absence of gB/gH, second, numerous virions accumulated in the PNS and adjacent ER cisternae and, third, none the virus membrane interactions exhibit characteristics of fusion. Therefore, the phenotypes shown in Figure 12, a reproduction of Figure 2 of this report ( Farnsworth et al., 2007) with permission of the copyright holder, represent undoubtedly various stages of capsids budding from the cytoplasmic matrix into the PNS and ER cisternae. Furthermore, in Vero cells infected with the gB/gH deletion mutant, three times more virions were found in the cytoplasm compared to wild type infected Vero cells, and about a third in the extracellular space. Similar relations though less pronounced were found in other cell types. Taken together, gB/gH are not involved in release of virions out of the PNS via fusion. The process taking place at the ONM claimed to be fusion is budding absolutely implying another transportation route than that of de-envelopment by fusion of the viral envelope with the ONM.
Figure 12. Virus transport across the ONM in the absence of the fusion proteins gB/gH.
gB/gH null virions accumulate in the PNS-ER compartment. The arrows (inserted by P. Wild) point to interactions of virus particles with the ONM and ER membranes showing the characteristics of budding. The fusion proteins gB/gH are missing. Therefore, these virus membrane interactions represent various stages of budding – not of fusion. Reproduced from Farnsworth et al., 2007, Figure 2), copyright (2007) National Academy of Sciences, U.S.A.
Wrapping demands an enormous amount of membranes that would need to be provided to the TGN, which, to our knowledge, is not clear yet. However, capsids can be wrapped at diverse sides of the Golgi complex or can bud into Golgi cisternae and/or vacuoles via another pathway than wrapping. There are numerous reports showing HSV-1 virions in vacuoles and/or Golgi cisternae ( Homman-Loudiyi et al., 2003; Leuzinger et al., 2005; Stannard et al., 1996; Sutter et al., 2012; Wild et al., 2015; Wild et al., 2002). Budding of capsids at various sites of Golgi membranes as well as virions in Golgi cisternae and vacuoles were also shown in pseudorabies virus infected cells ( Klupp et al., 2008). These virions in all these vacuoles and/or Golgi cisternae did not arise by wrapping because wrapping results in a single virion in a concentric vacuole as shown in Figure 9D and described in detail ( Wild et al., 2005). They may have entered Golgi cisternae by budding into them or by intraluminal transportation via ER-to-Golgi transitions. Transient elements from cis and trans Golgi sides have been shown in various cells ( Pavelka & Roth, 2015). It was also suggested that the cis-Golgi approaches the ER and contacts the ER exit sites in the yeast Saccharomyces cerevisiae to capture cargo for transportation to the Golgi complex ( Kurokawa et al., 2014). This ‘hug-and-kiss’ behavior could be another route to transfer virions, which are intraluminally transported to ER exit sites, into the Golgi cisternae.
In cells prepared for improved resolution, we found no valid arguments for de-envelopment by fusion of the viral envelope with the ONM. There are a number of facts that argue clearly against the de-envelopment theory. First, the morphology of the process taking place at the INM and ONM are identical showing all characteristics for budding ( Darlington & Moss, 1968; Leuzinger et al., 2005; Wild et al., 2005; Wild et al., 2012) but none for fusion ( Haluska et al., 2006; Kanaseki et al., 1997) considering fundamentals of membrane bound transportation ( Leabu, 2006; Peters et al., 2004; White, 1992). Second, virions have been repeatedly shown within ER cisternae ( Gilbert et al., 1994; Granzow et al., 1997; Leuzinger et al., 2005; Radsak et al., 1996; Schwartz & Roizman, 1969; Stannard et al., 1996; Sutter et al., 2012; Wild et al., 2002). To reach this location, either virions need to be transported from the PNS into the ER, or capsids have to bud from the cytoplasmic matrix into the ER. If virions can be intraluminally transported out of the PNS the viral envelope must be protected from fusion with membranes the virions are transported along. If capsids have the ability to bud at ER membranes capsids are very likely to be able to bud at the ONM since the ONM is part of the ER. Third, virions can accumulate to large numbers in the PNS, e.g. in the absence of the protein kinase Us3 ( Poon et al., 2006; Reynolds et al., 2002; Wild et al., 2015). Strangely enough, these virions are infective ( Reynolds et al., 2002; Ryckman & Roller, 2004; Wild et al., 2015) despite the inability of the envelope to fuse with the ONM ( Wisner et al., 2009) clearly contradicting the theory that de- and re-envelopment is essential to become infective ( Klupp et al., 2011). Fourth, the equivalent to budding is the formation of coated pits resulting in coated vesicles ( Owen & Luzio, 2000; Pearse et al., 2000). Coated vesicles derive e.g. from the plasma membrane and are transported towards the Golgi complex where they fuse with Golgi membranes ( Orci et al., 1981). However, coated vesicles must be uncoated to gain the ability for fusion. The coat consists of clathrin that drives formation of coated pits and finally coated vesicles and protects them from fusion. Clathrin was claimed to be involved in envelopment of HSV-6 capsids at Golgi membranes together with viral proteins ( Mori et al., 2008). Budding of HSV-1 capsids at the INM is driven by the nuclear envelopment complex, UL31/UL34, ( Bigalke & Heldwein, 2015; Bigalke et al., 2014; Hagen et al., 2015) located at the nuclear rim ( Mou et al., 2009).
Budding at the INM, ONM and ER membranes starts with deposition of dense substances that finally result in a dense coat at the viral envelop. The dense coat is readily seen on virions in the PNS, ER and, inconsistently, in Golgi cisternae and large vacuoles containing many virions. The dense coat suggests that it protects virions from fusion with membranes the virions is transported along but allows virus transportation from the PNS into ER and Golgi cisternae. However, in large Golgi cisternae and/or vacuoles, many virions are without dense coat. Instead, spikes are visible like at virions in the extracellular space. Budding at Golgi membranes takes place without dense coat formation.
Conclusions
Golgi membranes interconnect with ER membranes in cells infected with HSV-1, a Us3 deletion mutant thereof or with BoHV-1 as well as in uninfected cells. The ER continues into the ONM, that turns into the INM at sites of nuclear pores. Consequently, the PNS, ER and Golgi complex forms an entity that can be only visualized by TEM, either when the membranes of all three compartments are luckily hit in the same plane of a given section, or by 3D-reconstruction after imaging of serial sections, focused ion beam (FIB) microscopy or electron tomography. Fact is that virions are intraluminally transported out of the PNS into the ER. This implies that the viral envelope needs to be protected from fusion with the membranes the virions are transported along. Thus, the significance of the dense coat, which derives during budding of capsids at nuclear membranes, is protecting the viral envelope from fusion in a similar manner as clathrin protects coated vesicles from fusion. The ER-to-Golgi transitions, virions in Golgi cisternae with a similar dense coat as virions in the PNS and ER, and inhibition of virion transportation out of the PNS and ER after disruption of the Golgi complex by BFA, strongly suggest that virions are intraluminally transported from the PNS through the ER into Golgi cisternae. The process at the ONM reported to be fusion is budding that takes place in the absence of the fusion proteins gB/gH. Therefore, we propose, first, that intraluminal transport is the only pathway for virions out of the PNS, and, second, that capsids gain direct access to the cytoplasm from the nucleus via dilated nuclear pores and impaired nuclear envelope, and are than enveloped by membranes of the Golgi complex, of vacuoles or endosomes The function of gB/gH and other proteins, such as the Us3 kinase, need to be reinvestigated and discussed in an unbiased context of herpes virus morphogenesis and egress.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2018 Wild P et al.
Data associated with the article are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication). http://creativecommons.org/publicdomain/zero/1.0/
Dataset 1: Raw images for Figure 1– Figure 5, Figure 8– Figure 10. DOI, 10.5256/f1000research.12252.d179644 ( Wild et al., 2017a)
Dataset 2: Raw values for Figure 6 and Figure 7. DOI, 10.5256/f1000research.12252.d179645 ( Wild et al., 2017b)
Acknowledgments
We thank Bernard Roizman, University of Chicago, for providing the Us3 deletion mutant.
Funding Statement
This study was supported by the Foundation for Scientific Research at the University of Zürich, Switzerland.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; referees: 1 approved
References
- Albecka A, Laine RF, Janssen AF, et al. : HSV-1 Glycoproteins Are Delivered to Virus Assembly Sites Through Dynamin-Dependent Endocytosis. Traffic. 2016;17(1):21–39. 10.1111/tra.12340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Tekaya H, Miura K, Pepperkok R, et al. : Live imaging of bidirectional traffic from the ERGIC. J Cell Sci. 2005;118(Pt 2):357–367. 10.1242/jcs.01615 [DOI] [PubMed] [Google Scholar]
- Bigalke JM, Heldwein EE: Structural basis of membrane budding by the nuclear egress complex of herpesviruses. EMBO J. 2015;34(23):2921–2936. 10.15252/embj.201592359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigalke JM, Heldwein EE: Nuclear Exodus: Herpesviruses Lead the Way. Annu Rev Virol. 2016;3(1):387–409. 10.1146/annurev-virology-110615-042215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigalke JM, Heldwein EE: Have NEC Coat, Will Travel: Structural Basis of Membrane Budding During Nuclear Egress in Herpesviruses. Adv Virus Res. 2017;97:107–141. 10.1016/bs.aivir.2016.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigalke JM, Heuser T, Nicastro D, et al. : Membrane deformation and scission by the HSV-1 nuclear egress complex. Nat Commun. 2014;5: 4131. 10.1038/ncomms5131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Glick BS: The mechanisms of vesicle budding and fusion. Cell. 2004;116(2):153–166. 10.1016/S0092-8674(03)01079-1 [DOI] [PubMed] [Google Scholar]
- Borchers K, Oezel M: Simian agent 8 (SA8): morphogenesis and ultrastructure. Zentralbl Bakteriol. 1993;279(4):526–536. 10.1016/S0934-8840(11)80425-1 [DOI] [PubMed] [Google Scholar]
- Buckingham EM, Jarosinski KW, Jackson W, et al. : Exocytosis of Varicella-Zoster Virus Virions Involves a Convergence of Endosomal and Autophagy Pathways. J Virol. 2016;90(19):8673–8685. 10.1128/JVI.00915-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli G, Brandimarti R, Di Lazzaro C, et al. : Fragmentation and dispersal of Golgi proteins and redistribution of glycoproteins and glycolipids processed through the Golgi apparatus after infection with herpes simplex virus 1. Proc Natl Acad Sci U S A. 1993;90(7):2798–2802. 10.1073/pnas.90.7.2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G, Farabegoli F, Di Gaeta S, et al. : Origin of unenveloped capsids in the cytoplasm of cells infected with herpes simplex virus 1. J Virol. 1991;65(3):1589–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claude A: Growth and differentiation of cytoplasmic membranes in the course of lipoprotein granule synthesis in the hepatic cell. I. Elaboration of elements of the Golgi complex. J Cell Biol. 1970;47(3):745–766. 10.1083/jcb.47.3.745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Sarkar S: Studies on endoplasmic reticulum--Golgi complex cycling pathway in herpes simplex virus-infected and brefeldin A-treated human fibroblast cells. Virology. 1992;191(1):327–337. 10.1016/0042-6822(92)90195-U [DOI] [PubMed] [Google Scholar]
- Cheung P, Banfield BW, Tufaro F: Brefeldin A arrests the maturation and egress of herpes simplex virus particles during infection. J Virol. 1991;65(4):1893–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope GH, Williams MA: Quantitative studies on the preservation of choline and ethanolamine phosphatides during tissue preparation for electron microscopy. I. Glutaraldehyde, osmium tetroxide, Araldite methods. J Microsc. 1969;90(1):31–46. 10.1111/j.1365-2818.1969.tb00692.x [DOI] [PubMed] [Google Scholar]
- Darlington RW, Moss LH, 3rd: Herpesvirus envelopment. J Virol. 1968;2(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira AP, Glauser DL, Laimbacher AS, et al. : Live visualization of herpes simplex virus type 1 compartment dynamics. J Virol. 2008;82(10):4974–4990. 10.1128/JVI.02431-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger A, Vetterlein M, Weiss C, et al. : High-pressure freezing combined with in vivo-DAB-cytochemistry: a novel approach for studies of endocytic compartments. J Struct Biol. 2010;169(3):286–293. 10.1016/j.jsb.2009.10.011 [DOI] [PubMed] [Google Scholar]
- Emr S, Glick BS, Linstedt AD, et al. : Journeys through the Golgi--taking stock in a new era. J Cell Biol. 2009;187(4):449–453. 10.1083/jcb.200909011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein MA: Observations on the mode of release of herpes virus from infected HeLa cells. J Cell Biol. 1962;12(3):589–597. 10.1083/jcb.12.3.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth A, Wisner TW, Webb M, et al. : Herpes simplex virus glycoproteins gB and gH function in fusion between the virion envelope and the outer nuclear membrane. Proc Natl Acad Sci U S A. 2007;104(24):10187–10192. 10.1073/pnas.0703790104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T, Oda K, Yokota S, et al. : Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988;263(34):18545–18552. [PubMed] [Google Scholar]
- Gilbert R, Ghosh K, Rasile L, et al. : Membrane anchoring domain of herpes simplex virus glycoprotein gB is sufficient for nuclear envelope localization. J Virol. 1994;68(4):2272–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzow H, Weiland F, Jöns A, et al. : Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J Virol. 1997;71(3):2072–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose C, Buckingham EM, Carpenter JE, et al. : Varicella-Zoster Virus Infectious Cycle: ER Stress, Autophagic Flux, and Amphisome-Mediated Trafficking. Pathogens. 2016;5(4): pii: E67. 10.3390/pathogens5040067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen C, Dent KC, Zeev-Ben-Mordehai T, et al. : Structural Basis of Vesicle Formation at the Inner Nuclear Membrane. Cell. 2015;163(7):1692–1701. 10.1016/j.cell.2015.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluska CK, Riske KA, Marchi-Artzner V, et al. : Time scales of membrane fusion revealed by direct imaging of vesicle fusion with high temporal resolution. Proc Natl Acad Sci U S A. 2006;103(43):15841–15846. 10.1073/pnas.0602766103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HM, Bouchet-Marquis C, Huebinger J, et al. : Golgi apparatus analyzed by cryo-electron microscopy. Histochem Cell Biol. 2013;140(4):369–381. 10.1007/s00418-013-1136-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SC: Viral membrane fusion. Virology. 2015;479–480:498–507. 10.1016/j.virol.2015.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harson R, Grose C: Egress of varicella-zoster virus from the melanoma cell: a tropism for the melanocyte. J Virol. 1995;69(8):4994–5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri HP, Schweizer A: The endoplasmic reticulum-Golgi intermediate compartment. Curr Opin Cell Biol. 1992;4(4):600–608. 10.1016/0955-0674(92)90078-Q [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess MW, Müller M, Debbage PL, et al. : Cryopreparation provides new insight into the effects of brefeldin A on the structure of the HepG2 Golgi apparatus. J Struct Biol. 2000;130(1):63–72. 10.1006/jsbi.2000.4230 [DOI] [PubMed] [Google Scholar]
- Hollinshead M, Johns HL, Sayers CL, et al. : Endocytic tubules regulated by Rab GTPases 5 and 11 are used for envelopment of herpes simplex virus. EMBO J. 2012;31(21):4204–4220. 10.1038/emboj.2012.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homman-Loudiyi M, Hultenby K, Britt W, et al. : Envelopment of human cytomegalovirus occurs by budding into Golgi-derived vacuole compartments positive for gB, Rab 3, trans-golgi network 46, and mannosidase II.[erratum appears in J Virol. Arch. 2003 Jul; 77 (14): 8179]. J Virol. 2003;77(5):3191–3203. 10.1128/JVI.77.5.3191-3203.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Lang T, Südhof TC: Membrane fusion. Cell. 2003;112(4):519–533. 10.1016/S0092-8674(03)00112-0 [DOI] [PubMed] [Google Scholar]
- Jensen HL, Norrild B: Temporal morphogenesis of herpes simplex virus type 1-infected and brefeldin A-treated human fibroblasts. Mol Med. 2002;8(4):210–224. [PMC free article] [PubMed] [Google Scholar]
- Kanaseki T, Kawasaki K, Murata M, et al. : Structural features of membrane fusion between influenza virus and liposome as revealed by quick-freezing electron microscopy. J Cell Biol. 1997;137(5):1041–1056. 10.1083/jcb.137.5.1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman J: Transport between ER and Golgi. Curr Opin Cell Biol. 2000;12(4):445–449. 10.1016/S0955-0674(00)00115-0 [DOI] [PubMed] [Google Scholar]
- Klupp B, Altenschmidt J, Granzow H, et al. : Glycoproteins required for entry are not necessary for egress of pseudorabies virus. J Virol. 2008;82(13):6299–6309. 10.1128/JVI.00386-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klupp BG, Granzow H, Mettenleiter TC: Nuclear envelope breakdown can substitute for primary envelopment-mediated nuclear egress of herpesviruses. J Virol. 2011;85(16):8285–8292. 10.1128/JVI.00741-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp M, Klupp BG, Granzow H, et al. : Identification and characterization of the pseudorabies virus tegument proteins UL46 and UL47: role for UL47 in virion morphogenesis in the cytoplasm. J Virol. 2002;76(17):8820–8833. 10.1128/JVI.76.17.8820-8833.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K, Okamoto M, Nakano A: Contact of cis-Golgi with ER exit sites executes cargo capture and delivery from the ER. Nat Commun. 2014;5: 3653. 10.1038/ncomms4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leabu M: Membrane fusion in cells: molecular machinery and mechanisms. J Cell Mol Med. 2006;10(2):423–427. 10.1111/j.1582-4934.2006.tb00409.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KK: Architecture of a nascent viral fusion pore. EMBO J. 2010;29(7):1299–1311. 10.1038/emboj.2010.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuzinger H, Ziegler U, Schraner EM, et al. : Herpes simplex virus 1 envelopment follows two diverse pathways. J Virol. 2005;79(20):13047–13059. 10.1128/JVI.79.20.13047-13059.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J: An evolving paradigm for the secretory pathway? Mol Biol Cell. 2011;22(21):3929–3932. 10.1091/mbc.E11-05-0452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Donaldson JG, Schweizer A, et al. : Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990;60(5):821–836. 10.1016/0092-8674(90)90096-W [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan LC, Bonifacino JS, et al. : Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56(5):801–813. 10.1016/0092-8674(89)90685-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R, Roizman B: Clustering of genes dispensable for growth in culture in the S component of the HSV-1 genome. Science. 1987;236(4801):573–576. 10.1126/science.3033823 [DOI] [PubMed] [Google Scholar]
- Maric M, Haugo AC, Dauer W, et al. : Nuclear envelope breakdown induced by herpes simplex virus type 1 involves the activity of viral fusion proteins. Virology. 2014;460–461:128–137. 10.1016/j.virol.2014.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A: Membrane fusion in eukaryotic cells. Annu Rev Cell Dev Biol. 2002;18:289–314. 10.1146/annurev.cellbio.18.032202.114809 [DOI] [PubMed] [Google Scholar]
- Mettenleiter TC, Klupp BG, Granzow H: Herpesvirus assembly: a tale of two membranes. Curr Opin Microbiol. 2006;9(4):423–429. 10.1016/j.mib.2006.06.013 [DOI] [PubMed] [Google Scholar]
- Mettenleiter TC, Müller F, Granzow H, et al. : The way out: what we know and do not know about herpesvirus nuclear egress. Cell Microbiol. 2013;15(2):170–8. 10.1111/cmi.12044 [DOI] [PubMed] [Google Scholar]
- Misumi Y, Misumi Y, Miki K, et al. : Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J Biol Chem. 1986;261(24):11398–11403. [PubMed] [Google Scholar]
- Mori Y, Koike M, Moriishi E, et al. : Human herpesvirus-6 induces MVB formation, and virus egress occurs by an exosomal release pathway. Traffic. 2008;9(10):1728–1742. 10.1111/j.1600-0854.2008.00796.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou F, Wills E, Baines JD: Phosphorylation of the U L31 protein of herpes simplex virus 1 by the U S3-encoded kinase regulates localization of the nuclear envelopment complex and egress of nucleocapsids. J Virol. 2009;83(10):5181–5191. 10.1128/JVI.00090-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M: The integrating power of cryofixation-based electron microscopy in biology. Acta Microscopica. 1992;1:37–46. [Google Scholar]
- Naldinho-Souto R, Browne H, Minson T: Herpes simplex virus tegument protein VP16 is a component of primary enveloped virions. J Virol. 2006;80(5):2582–4. 10.1128/JVI.80.5.2582-2584.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Montesano R, Perrelet A: Exocytosis-endocytosis as seen with morphological probes of membrane organization. Methods Cell Biol. 1981;23:283–300. 10.1016/S0091-679X(08)61504-6 [DOI] [PubMed] [Google Scholar]
- Owen DJ, Luzio JP: Structural insights into clathrin-mediated endocytosis. Curr Opin Cell Biol. 2000;12(4):467–474. 10.1016/S0955-0674(00)00118-6 [DOI] [PubMed] [Google Scholar]
- Palade G: Intracellular aspects of the process of protein synthesis. Science. 1975;189(4200):347–358. 10.1126/science.1096303 [DOI] [PubMed] [Google Scholar]
- Pavelka M, Roth J: Functional ultrastructure: atlas of tissue biology and pathology. Springer Vienna.2015;57–77. 10.1007/978-3-7091-1830-6 [DOI] [Google Scholar]
- Pearse BM, Smith CJ, Owen DJ: Clathrin coat construction in endocytosis. Curr Opin Struct Biol. 2000;10(2):220–228. 10.1016/S0959-440X(00)00071-3 [DOI] [PubMed] [Google Scholar]
- Peters C, Baars TL, Bühler S, et al. : Mutual control of membrane fission and fusion proteins. Cell. 2004;119(5):667–678. 10.1016/j.cell.2004.11.023 [DOI] [PubMed] [Google Scholar]
- Polishchuk RS, Mironov AA: Structural aspects of Golgi function. Cell Mol Life Sci. 2004;61(2):146–158. 10.1007/s00018-003-3353-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon AP, Benetti L, Roizman B: U S3 and U S3.5 protein kinases of herpes simplex virus 1 differ with respect to their functions in blocking apoptosis and in virion maturation and egress. J Virol. 2006;80(8):3752–3764. 10.1128/JVI.80.8.3752-3764.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves FC, Longnecker RM, Leader DP, et al. : Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J Virol. 1987;61(9):2896–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radsak K, Eickmann M, Mockenhaupt T, et al. : Retrieval of human cytomegalovirus glycoprotein B from the infected cell surface for virus envelopment. Arch Virol. 1996;141(3–4):557–572. 10.1007/BF01718317 [DOI] [PubMed] [Google Scholar]
- Reynolds AE, Wills EG, Roller RJ, et al. : Ultrastructural localization of the herpes simplex virus type 1 U L31, U L34, and U S3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J Virol. 2002;76(17):8939–8952. 10.1128/JVI.76.17.8939-8952.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B, Knipe DM, Whitley RJ: Herpes simplex viruses. In: Fields Virology K D M, P M, Howley, editor. Wolters Kluver/Lipincott Wiliams & Wilkins, Philadelphia.2014;2:1823–1897. [Google Scholar]
- Ryckman BJ, Roller RJ: Herpes simplex virus type 1 primary envelopment: UL34 protein modification and the US3-UL34 catalytic relationship. J Virol. 2004;78(1):399–412. 10.1128/JVI.78.1.399-412.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J, Dale HA, Bazzocco S, et al. : Emerging new roles of the pre-Golgi intermediate compartment in biosynthetic-secretory trafficking. FEBS Lett. 2009;583(23):3804–3810. 10.1016/j.febslet.2009.10.084 [DOI] [PubMed] [Google Scholar]
- Schwartz J, Roizman B: Concerning the egress of herpes simplex virus from infected cells: electron and light microscope observations. Virology. 1969;38(1):42–49. 10.1016/0042-6822(69)90126-3 [DOI] [PubMed] [Google Scholar]
- Skepper JN, Whiteley A, Browne H, et al. : Herpes simplex virus nucleocapsids mature to progeny virions by an envelopment --> deenvelopment --> reenvelopment pathway. J Virol. 2001;75(12):5697–5702. 10.1128/JVI.75.12.5697-5702.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackpole CW: Herpes-type virus of the frog renal adenocarcinoma. I. Virus development in tumor transplants maintained at low temperature. J Virol. 1969;4(1):75–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stannard LM, Himmelhoch S, Wynchank S: Intra-nuclear localization of two envelope proteins, gB and gD, of herpes simplex virus. Arch Virol. 1996;141(3–4):505–524. 10.1007/BF01718314 [DOI] [PubMed] [Google Scholar]
- Sutter E, de Oliveira AP, Tobler K, et al. : Herpes simplex virus 1 induces de novo phospholipid synthesis. Virology. 2012;429(2):124–135. 10.1016/j.virol.2012.04.004 [DOI] [PubMed] [Google Scholar]
- Terasaki M, Campagnola P, Rolls MM, et al. : A new model for nuclear envelope breakdown. Mol Biol Cell. 2001;12(2):503–510. 10.1091/mbc.12.2.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi MR, Di Lazzaro C, Pavan A, et al. : Herpes simplex virus envelopment and maturation studied by fracture label. J Virol. 1992;66(1):554–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi MR, Gentile M, Cardinali G, et al. : Intracellular transport and maturation pathway of human herpesvirus 6. Virology. 1999;257(2):460–471. 10.1006/viro.1999.9699 [DOI] [PubMed] [Google Scholar]
- Turner A, Bruun B, Minson T, et al. : Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J Virol. 1998;72(1):873–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivero-Salmerón G, Ballesta J, Martínez-Menárguez JA: Heterotypic tubular connections at the endoplasmic reticulum-Golgi complex interface. Histochem Cell Biol. 2008;130(4):709–717. 10.1007/s00418-008-0471-2 [DOI] [PubMed] [Google Scholar]
- Wang X, Patenode C, Roizman B: U S3 protein kinase of HSV-1 cycles between the cytoplasm and nucleus and interacts with programmed cell death protein 4 (PDCD4) to block apoptosis. Proc Natl Acad Sci U S A. 2011;108(35):14632–14636. 10.1073/pnas.1111942108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibull C, Villiger W, Carlemalm E: Extraction of lipids during freeze-substitution of Acholeplasma laidlawii-cells for electron microscopy. J Microsc. 1984;134(Pt 2):213–216. 10.1111/j.1365-2818.1984.tb02513.x [DOI] [PubMed] [Google Scholar]
- Whealy ME, Card JP, Meade RP, et al. : Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J Virol. 1991;65(3):1066–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM: Membrane fusion. Science. 1992;258(5084):917–924. 10.1126/science.1439803 [DOI] [PubMed] [Google Scholar]
- Wild P: Electron microscopy of viruses and virus-cell interactions. Methods Cell Biol. 2008;88:497–524. 10.1016/S0091-679X(08)00425-1 [DOI] [PubMed] [Google Scholar]
- Wild P, Bitterli D, Becker M: Quantitative changes of membranes in rat parathyroid cells related to variations of serum calcium. Lab Invest. 1982;47(4):370–374. [PubMed] [Google Scholar]
- Wild P, de Oliveira AP, Sonda S, et al. : The herpes simplex virus 1 U S3 regulates phospholipid synthesis. Virology. 2012;432(2):353–360. 10.1016/j.virol.2012.06.020 [DOI] [PubMed] [Google Scholar]
- Wild P, Engels M, Senn C, et al. : Impairment of nuclear pores in bovine herpesvirus 1-infected MDBK cells. J Virol. 2005;79(2):1071–1083. 10.1128/JVI.79.2.1071-1083.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild P, Gloor S, Vetsch E: Quantitative aspects of membrane behavior in rat parathyroid cells after depression or elevation of serum calcium. Lab Invest. 1985;52(5):490–496. [PubMed] [Google Scholar]
- Wild P, Käch A, Lucas MS: High resolution scanning electron microscopy of the nuclear surface in herpes simplex virus 1 infected cells. In: Scanning electron microscopy for the life sciences Schatten H, editor. Cambridge University Press, New York, USA.2012. 10.1017/CBO9781139018173.009 [DOI] [Google Scholar]
- Wild P, Kaech A, Schraner E, et al. : Dataset 1 in: Endoplasmic reticulum-to-Golgi transitions upon herpes virus infection. F1000Research. 2017a. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild P, Kaech A, Schraner E, et al. : Dataset 2 in: Endoplasmic reticulum-to-Golgi transitions upon herpes virus infection. F1000Research. 2017b. Data Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild P, Leisinger S, de Oliveira AP, et al. : Herpes simplex virus 1 Us3 deletion mutant is infective despite impaired capsid translocation to the cytoplasm. Viruses. 2015;7(1):52–71. 10.3390/v7010052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild P, Schraner EM, Adler H, et al. : Enhanced resolution of membranes in cultured cells by cryoimmobilization and freeze-substitution. Microsc Res Tech. 2001;53(4):313–321. 10.1002/jemt.1098 [DOI] [PubMed] [Google Scholar]
- Wild P, Schraner EM, Cantieni D, et al. : The significance of the Golgi complex in envelopment of bovine herpesvirus 1 (BHV-1) as revealed by cryobased electron microscopy. Micron. 2002;33(4):327–337. 10.1016/S0968-4328(01)00037-3 [DOI] [PubMed] [Google Scholar]
- Wild P, Senn C, Manera CL, et al. : Exploring the nuclear envelope of herpes simplex virus 1-infected cells by high-resolution microscopy. J Virol. 2009;83(1):408–419. 10.1128/JVI.01568-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner TW, Wright CC, Kato A, et al. : Herpesvirus gB-induced fusion between the virion envelope and outer nuclear membrane during virus egress is regulated by the viral US3 kinase. J Virol. 2009;83(7):3115–3126. 10.1128/JVI.01462-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi Y, Shiba C, Goshima F, et al. : Herpes simplex virus type 2 UL34 protein requires UL31 protein for its relocation to the internal nuclear membrane in transfected cells. J Gen Virol. 2001;82(Pt 6):1423–1428. 10.1099/0022-1317-82-6-1423 [DOI] [PubMed] [Google Scholar]
- Yao Z, Jackson W, Forghani B, et al. : Varicella-zoster virus glycoprotein gpI/gpIV receptor: expression, complex formation, and antigenicity within the vaccinia virus-T7 RNA polymerase transfection system. J Virol. 1993;67(1):305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]