Abstract
Purpose:
To study the long-term safety profile and visual outcomes of primary intraocular lens (IOL) implantation in infants <6 months of age.
Methods:
This was a retrospective observational study conducted at a tertiary eye care center in South India. Infants under 6 months meeting the selection criteria who underwent cataract surgery (lens aspiration, primary posterior capsulorhexis, and anterior vitrectomy) with primary IOL implantation between January 2008 and December 2011 and minimum 3-year follow-up were included. Patient demographics, serial refractions, visual acuity, complications, and associated amblyopia/strabismus were reviewed. Visual acuity, myopic shift, and complications were the outcome measures.
Results:
Sixty-nine eyes of 38 infants (31 bilateral; mean age: 4.6 months) were reviewed. Mean follow-up was 51 months (range: 36–84). Median logMAR best-corrected visual acuity at the final visit was 0.74 (interquartile range [IQR]: 0.50–0.98) in eyes with bilateral cataracts and 0.87 (IQR: 0.60–1.14) in eyes with unilateral cataracts with an average myopic shift of 6.7 diopters over 4.2 years. Most common postoperative complication was visual axis opacification (VAO) (13 eyes, 18%), necessitating membranectomy followed by pigmentary IOL deposits (11 eyes, 15%), and IOL decentration and glaucoma in four eyes each (5.6%). Mixed linear effect model found no significant association of age, gender, laterality, and postoperative complications with final visual acuity (P ≥ 0.05). Eyes with unilateral cataracts had a greater myopic shift than bilateral cases (P = 0.03).
Conclusion:
Primary IOL implantation in infants <6 months is reasonably safe in appropriately selected infants. VAO was the most common postoperative complication, and a large myopic shift was observed.
Keywords: Complications, glaucoma, infants, primary Intraocular lens implantation, visual axis opacification
Although pediatric cataract surgery has become standardized and safer with modern-day instrumentation and surgical techniques, implantation of intraocular lens (IOL) in the infants remains controversial and challenging. Most surgeons recommend deferring primary IOL implantation in infants below 3 months in unilateral cataracts and <7 months in bilateral cases.[1] On the contrary, the Infant Aphakia Treatment Study (IATS) showed that at 4.5-year follow-up, visual outcomes were similar in pseudophakic and aphakic infants treated with contact lenses (CLs).[2] In addition, pseudophakic children had a greater risk of visual axis opacification (VAO) and additional intraocular surgery. The IATS recommended limiting early primary IOL implantation for selected infants with special concerns of cost and handling of CLs.[2] In contrast, Ram et al. reported that meticulous cataract surgery with IOL implantation in children <2 years was safe with good refractive outcomes.[3] Few other reports show good outcomes of IOL implantation in younger infants over the short follow-up period.[4,5]
Pediatric cataract surgery in a developing country poses additional challenges in terms of affordability, access to eye care, awareness, and follow-up.[6,7] Gogate et al. reported that about 79% of children did not follow-up after primary cataract surgery.[8] On active follow-up, 92% of these children needed further intervention. Therefore, pediatric ophthalmologists in developing countries prefer to implant primary IOL in infants and younger children if considered safe. Currently, there are only few reports of long-term follow-up among these children, especially young infants.
The objective of the current study was to evaluate the safety profile, visual outcomes, and complications of primary IOL implantation in infants <6 months of age in a tertiary care center in Southern India.
Methods
This retrospective study was conducted at a tertiary eye care center in South India. We included children with congenital cataracts <6 months of age, seen at our institute, between January 2008 and December 2011 who underwent cataract surgery with primary IOL implantation. Decision to implant IOL was based on minimum corneal horizontal white to white diameter of 10 mm, without associated anterior-segment dysgenesis/increased intraocular pressure (IOP). The study was approved by our Institutional Review Board and adhered to the tenets of the Declaration of Helsinki. We included children with a minimum of 3-year follow-up and excluded children with traumatic or complicated, postuveitis, and postretinoblastoma cataracts and children with shorter follow-ups.
Preoperative evaluation, ocular/systemic workup, preoperative biometry at the time of the surgery, and the standard pediatric phacoemulsification, anterior vitrectomy, primary posterior capsulotomy, and posterior chamber IOL implantation were performed as described before.[9] The IOL power was under-corrected according to the guidelines published by Enyedi et al.[10] The Sanders-Retzlaff-Kraff II formula was used for IOL power calculation. The operated eyes were implanted with single- or three-piece hydrophobic (Alcon – Acrysof SA60AT, Acrysof MA60AC) or hydrophilic acrylic foldable lenses (RYCF lens, Care group, Gujarat, India; John Fowler, John Fowler Ophthalmics Pvt., Ltd., Bangalore, Karnataka, India; Akreos, Bausch and Lomb, Rochester, USA).
Postoperatively, all patients were prescribed topical tobramycin 0.3% four times a day for a week, atropine sulfate 1%: or Homatropine bromide 2% twice a day for 2 weeks, and prednisolone acetate 1% 8–12 times a day, which was gradually tapered off over 6 weeks. After examination on the 1st postoperative day, an examination under anesthesia was performed at 1–2-week postoperatively at which time glasses were prescribed. All children were followed up at 1 month, thereafter 3 monthly up to 1 year, and then 6 monthly. At each visit, a complete ocular workup was performed which included retinoscopy followed by handheld standard slit-lamp examination to note the IOL centration, ocular inflammation, VAO, if any, and IOP, measured with a Perkins Tonometer (Clement Clarke International, Harlow, UK) or I-care Tonometer (ICare® TAO1i, Finland).
Visual status was assessed with Teller Acuity Cards, Lea symbols, Kay picture charts, or Snellen charts as was most suitable in each case and converted to logMAR scale. Amblyopia treatment in the form of part-time occlusion was advised wherever necessary, and the same was recorded from the patient charts. Data were collected regarding the age at surgery, preoperative visual acuity, ocular alignment, presence of nystagmus, axial length (AL), keratometry, IOL power, and its type and location of implantation using the data. Postoperatively, visual outcome and factors affecting it, refractive status along with myopic shift, complications if any, and need for a second surgery were analyzed.
The child was considered to have glaucoma if the IOP was >22 mmHg with the presence of increased optic nerve cupping and/or myopic refractive shift. IOL was recorded as decentered if the edge of the optic was seen in the pupillary area at undilated or dilated state.
Statistical analysis
Statistical analysis was performed using the R 3.1.2 software (R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.r-project.org). Descriptive statistics were used to summarize the variables. We determined the rate of complications and myopic shift for the whole cohort. Univariate and multivariate analysis were used to study the effect of factors affecting visual outcomes, myopic shift, and incidence of complications. Linear mixed effect model was used to account for the effect of including data from both eyes of the patients. A P < 0.05 was considered statistically significant.
Results
From January 2008 to December 2011, 130 infants underwent cataract surgery with primary IOL implantation at our institute. Of these, 69 eyes of 38 infants under 6 months of age (20 males and 18 females) met the study criteria. Table 1 shows the baseline characteristics of the patients. Median follow-up period was 48 (32–84) months.
Table 1.
Baseline characteristics of the children in the study
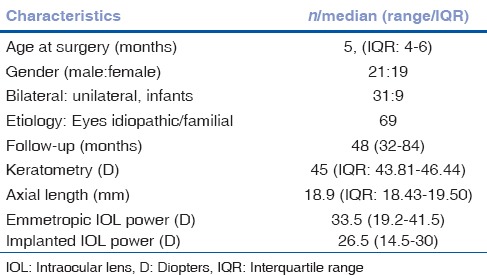
All cataracts were familial/idiopathic in etiology. Thirty-one infants underwent bilateral surgery, while seven had unilateral surgery. Median follow-up duration was 48 months (range: 36–84 months). The IOL was implanted in the capsular bag in 65 (94.4%) eyes, in the sulcus in 3 (4.2%) eyes, and in the bag-sulcus placement in 1 (1.4%) eye.
Median best-corrected visual acuity (BCVA) was 0.74 logMAR, Snellen equivalent: 20/100 (IQR: 0.50–0.98) for children with bilateral cataracts and 0.87 logMAR, Snellen equivalent: 20/150 (IQR: 0.60–1.14) for children with unilateral cataracts. At the end of 3 years, 26 eyes (37.7%) had a BCVA of 20/80 (0.6 logMAR) or better, of which only 2 (2.8%) had an acuity of 20/40 or better. Forty-three eyes (62.3%) had acuity worse than 20/80 including seven eyes where visual acuity could not be recorded with a chart. Fig. 1 shows the distribution of final visual acuity among the children with unilateral and bilateral cataracts with follow-up of ≥ 3 years.
Figure 1.
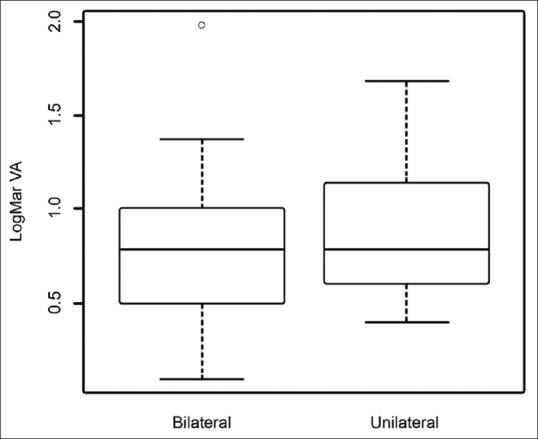
Visual outcomes of primary intraocular lens implantation in infants <6 months. Box and whisker plot showing final visual acuity outcomes following primary intraocular lens implantation in bilateral versus unilateral cataract surgery groups. Final visual acuity was slightly better in eyes with bilateral cataracts, but difference was not statistically significant
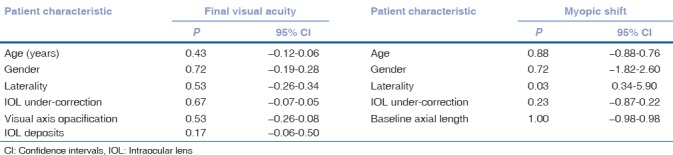
Univariate analysis using a linear mixed effect model found no statistically significant influence of age at surgery, gender, laterality of cataract, planned under correction of IOL power, and development of postoperative complications on final visual acuity (P ≥ 0.05 for all observations) as shown in Table 2.
Table 2.
Univariate analysis with linear mixed model showing the effect of various factors affecting visual acuity and myopic shift at 3 years
Myopic shift
Fig. 2 shows the serial postoperative refraction at each follow-up. There was a steady decline in the postoperative hypermetropia, and at 3-year follow-up (mean patient age: 41.4 months), median spherical equivalent was −0.25 D (IQR: −2.0 to +2.1; range: −12.5 to 10.50 D).
Figure 2.
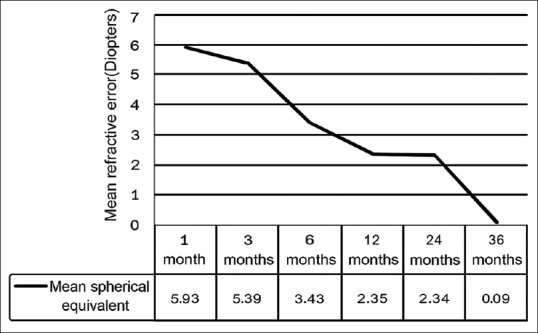
Visual outcomes of primary intraocular lens implantation in infants <6 months. Line diagram showing the serial refraction and myopic shift. Overall, an average myopic shift of 6.02 D was observed at the end of 3 years
Median axial elongation in the eyes with cataracts was 1.73 mm in bilateral cataracts and 2.80 mm in unilateral cataracts at 3-year follow-up. The average myopic shift was 2.00 D/year reaching a median shift of 6.02 D after 3 years.
At 5-year follow-up, the median spherical equivalent was −1.31 D (data available for 10 infants) with a total myopic shift of 8.43 D. Median postoperative keratometry at the end of 3 years was 44.31 D (40.0–48.1 D), while the AL was 20.68 (18.0–27.4) mm.
Univariate analysis using mixed linear effect model found this myopic shift to be significantly higher in unilateral cataracts compared to bilateral cataracts (P = 0.03), while other parameters such as age, gender, IOL under-correction, and preoperative AL did not have a significant association as shown in Table 2.
Postoperative complications
Fig. 3 shows the distribution of various postoperative complications observed in our series.
Figure 3.
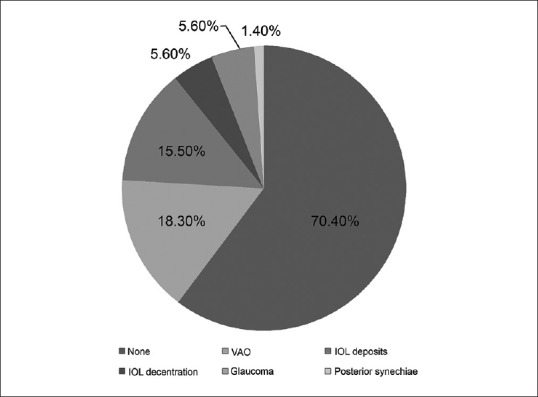
Visual outcomes of primary intraocular lens implantation in infants <6 months. Pie chart showing proportion of various postoperative complications (if any) at the end of 3 years. Most common complication was visual axis opacification followed by intraocular lens decentration and glaucoma
Visual axis opacification
The most common complication was VAO. Thirteen eyes (18.8%) developed VAO necessitating membranectomy at a median follow-up of 6 months. Development of VAO was not significantly associated with age at surgery (odds ratio: 0.79, P = 0.49, confidence interval [CI]: 0.41–1.55), gender (odds ratio: 0.50, P = 0.40, CI: 0.09–2.60), and laterality of cataract (odds ratio: 1.31, P = 0.82, CI: 0.13–13.75) as studied by generalized linear mixed model. There was no recurrence of VAO.
Intraocular lens deposits
In the current study, 11/69 eyes (15.9%) with hydrophilic IOLs developed deposits. This was visually significant only in three eyes necessitating an IOL exchange for a hydrophobic lens. Age at surgery (P = 0.90), gender (P = 0.96), and laterality of cataract (P = 1.02) did not influence the development of IOL deposits.
Intraocular lens decentration
IOL decentration was noticed in 4/69 eyes (5.6%). One eye was a true dislocation into the vitreous cavity, 3 years after surgery, which was explanted following a pars plana vitrectomy, and a three-piece hydrophobic IOL was implanted in the sulcus.
Increased postoperative Inflammation:
Posterior synechiae were noted in 1 eye (1.4%) and increased anterior-chamber inflammation with fibrin in the pupillary region was observed in 1 eye (1.4%) in the early postoperative period. This resolved with frequent use of topical steroids and none of the patients required oral steroids.
Glaucoma
Two eyes (2.9%) developed glaucoma, of which one were managed with topical antiglaucoma medications and one eye underwent Ahmed glaucoma valve implantation 8 months postoperatively. Two eyes (2.8%) developed a transient rise in IOP which resolved with topical antiglaucoma medication over 3 weeks.
Alignment
At initial evaluation, strabismus was noted in seven infants (17.5%; 5 esotropia, 2 exotropia) with nystagmus in 17 infants (42.5%). Following cataract surgery, four children (10%) also underwent squint surgery to correct associated strabismus. At the final visit, however, 16 children (40%) had heterotropia, of which six had esotropia, seven had exotropia (including both intermittent and constant exotropia), while three children had a dissociated vertical deviation.
An overall complication rate of 29.6% was noted with a second surgical intervention required in 17 eyes (20 surgeries).
Discussion
Although there is an increasing trend toward primary IOL implantation in infants, there are limited studies reporting long-term postoperative outcomes in younger infants. The current study found encouraging visual results with 38.7% of eyes in the bilateral surgery group and 22.2% in the unilateral group achieving a BCVA of ≥20/80 (0.6 logMAR) or better. This is similar to the IATS that reported 38.6% of infants treated with CLs and 36.4% of infants with IOL had a BCVA of ≥20/80 at 4.5 years of age (median BCVA 0.90 logMAR in both groups) and 11% of the pseudophakic eyes had a BCVA of 20/40 or better.[2] Lambert et al. reported a mean grating visual acuity of 0.70 ± 0.32 logMAR in children with infants with unilateral cataract <6 months of age.[11] These were slightly better than our results with median logMAR BCVA of 0.78 in all patients, 0.74 in bilateral and 0.87 unilateral cataracts.
This might be attributed to the inclusion of children (4 bilateral and 1 unilateral) with severe corticovisual impairment (with hypoxic-ischemic encephalopathy sequelae on magnetic resonance imaging), cases with poor compliance to regular follow-up, and refractory amblyopia despite very good surgical outcomes. If these are excluded, median BCVA in the study population improved to 0.69 logMAR.
Final BCVA was not significantly different in the unilateral or bilateral cataract groups in this study. This might be attributable to smaller sample size in the unilateral group. Further, age, gender, IOL power, and two most common amblyogenic postoperative complications, i.e., VAO and IOL deposits, did not influence the final visual outcome. This might be attributable to the timely intervention as deemed necessary by a surgeon.
In the current study, the average myopic shift at 3 years was noted to be 6.02 D (i.e., about 2 D/year). Median standard error declined from +5.75 D in the 1st postoperative week to −0.25 D at 3 years. Most of this myopic shift occurred in the 1st year after surgery (1.32 D at 6 months, 1.13 by 1 year), with a slower progression at 2 years (0.03 D). Another large shift was noted at 3 years (2.44 D). Of the ten children who followed-up till 5 years, median myopic shift was found to be by 8.43 D. The axial elongation in the eyes with cataract was more in the unilateral cataract group at 3-year follow-up, contributing to a significantly greater myopic shift in those cases. This is similar to the prior reports by Hussein and Markhan, who reported more AL elongation in unilateral (4.83 ± 1.44 mm) compared to bilateral cataract (4.28 ± 1.55 mm), though this was statistically insignificant (P = 0.26).[12] Fan et al. also reported that in infants (mean age 6.7 months) undergoing primary IOL implantation, mean AL elongation was 2.71 mm in operated eyes versus 3.17 mm in fellow eyes at 3 years.[13] The reported mean myopic shift was 7.11 D and was greater in infants operated before 6 months.[13]
The IATS reported that the baseline AL in cataractous eyes was shorter than the fellow eyes.[14] The rate of AL elongation was lesser in the aphakic group compared to the pseudophakic group (0.17 mm/month vs. 0.24 mm/month, respectively) and was unaffected by age at surgery. The overall myopic shift at the end of 5 years was 8.3 D.[2,15] Furthermore, they reported an overall mean absolute postoperative predictive error of 1.8 ± 1.3 D in those receiving primary IOL with a larger predictive error in eyes with ALs shorter than 18 mm and those with IOL power ≥30 D.[15] This high myopic shift is mainly attributable to continued axial elongation and gradual decrease in the lens power.
The current study had an overall complication rate of 29.6% with 17 eyes (23.9%) requiring a second surgical intervention (20 surgeries). Supplement Table 1 (702KB, tif) compares the distribution of refractive outcomes and complications in the current study with the major studies in the reported literature.
Comparison of the incidence of complications in the current study to major studies in the literature showing a significantly less incidence of major complications (visual axis opacification and glaucoma)
Like prior major studies, VAO was the most common complication in our series involving 13 eyes (18.8%) at 6 months postoperatively. Trivedi et al. reported that 37.9% of eyes developed VAO (with hydrophobic acrylic IOL) requiring secondary surgical intervention at a median follow-up of 4.8 months.[16] Average age at surgery for eyes with VAO was 3.8 months compared with 5.4 months, for those whose visual axis remained clear. In the current study, 18.3% of the eyes developed VAO. Majority of these children developed VAO within 6 months of surgery requiring membranectomy, except one eye who underwent membranectomy at 3 years. None had a recurrence. Lundvall and Zetterström reported VAO in 67% of infant eyes that had cataract extraction with IOL implantation even though a posterior capsulorhexis and an anterior vitrectomy were performed.[4] Sukhija et al., in their study on IOL implantation under 2 years of age, reported reopacification of visual axis in 13.3% of eyes and suggested that VAO might be determined by the site of IOL implantation and adequacy of anterior vitrectomy.[17]
Another factor affecting the clarity of visual axis is the formation of deposits on the IOL surface. In the current study, 11 eyes (15.9%) with hydrophilic IOLs had pigment deposits. However, these were visually significant in only three eyes requiring an IOL exchange for a hydrophobic IOL. Similar complication and resurgery rates have been noted with hydrophilic IOL implantation as compared to hydrophobic IOLs.[18] In one of the previous publications from our institute, we observed increased incidence of significant IOL deposits when hydrophilic IOLs were implanted in infants.[19] This was possibly due to the IOL material and its processing which attracts calcium and phosphorus ions in the irrigating solutions and viscoelastics to form deposits over the IOL. These eyes underwent IOL explantation to prevent sight-threatening amblyopia. Although, at our institution, we have used these for a short time, many years ago, when the use of these IOLs was dependent on the surgeon's choice, in view of increased risk of deposits, we recommend avoiding the use of hydrophilic IOLs in young children. As of now in our routine practice, we no longer use the hydrophilic IOLs in children and recommend avoiding the use of hydrophilic IOLs in young children.
Development of postoperative glaucoma in pseudophakic children remains a concern for all pediatric ophthalmologists. The IATS[20] reported incidence of glaucoma to be 16% in the aphakic eyes and 19% in the pseudophakic eyes at 5-year follow-up and suggested younger age at the time of surgery and smaller corneal diameter to be significant risk factors for development of glaucoma. Other studies[5,21] have reported that IOL implantation may exert a protective effect against development of glaucoma in these eyes and reported a lower incidence. In the current study, two eyes (2.9%) developed glaucoma; one was following vitreoretinal surgery for a dislocated IOL, while another one was without predisposing factors or secondary intervention and developed glaucoma secondary to cataract surgery. In addition, two eyes developed a transient rise in IOP which resolved with topical antiglaucoma medication over 3 weeks.
Preoperatively, seven infants (17.5%) had manifest strabismus; five had esotropia, while two had exotropia. This was associated with nystagmus in 17 infants. Of these, four children required squint surgery following the cataract extraction to correct the ocular alignment. However, at the final visit, 16 children (40%) had heterotropia, of which six had esotropia, seven had exotropia (including both intermittent and constant exotropia), while three children had a dissociated vertical deviation. Bothun et al. have reported manifest strabismus in 38 pseudophakic infants (66.7%) at the end of 1 year.[22] They also found a lesser tendency for strabismus when early cataract removal had been done stressing the need for timely intervention.
Overall, the current study reported a slightly lower rate of the postoperative complications. As per our protocols, postoperatively, topical steroids were given in a tapering dose starting 8–12 times a day along with 1% atropine sulfate eye drops twice daily. Only one eye developed a fibrinous reaction in the early postoperative period, for which the frequency of steroids was increased, and none of our patients required oral steroids.
Two of our patients required a second surgery for IOL decentration, of which one was a true dislocation into the vitreous cavity. None of the children in this study developed endophthalmitis or retinal detachment till the last follow-up.
The limitations of our study are its retrospective nature, different protocols for vision testing at different ages, and difficulty in monitoring patching compliance. However, despite its limitations, this study is one of the largest studies to look at the visual outcomes and postoperative complications in infants under 6 months of age.
Conclusion
The current study suggests that primary IOL implantation in the selected children (without anterior-segment dysgenesis or glaucoma, having age appropriate corneal diameter and AL) is safe even in experienced hand in infants <6 months of age. However, further studies with longer follow-up studies of these cohort of patients are necessary.
Financial support and sponsorship
Hyderabad Eye Research Foundation.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kumar P, Lambert SR. Evaluating the evidence for and against the use of IOLs in infants and young children. Expert Rev Med Devices. 2016;13:381–9. doi: 10.1586/17434440.2016.1153967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambert SR, Lynn MJ, Hartmann EE, DuBois L, Drews-Botsch C, et al. Infant Aphakia Treatment Study Group. Comparison of contact lens and intraocular lens correction of monocular aphakia during infancy: A randomized clinical trial of HOTV optotype acuity at age 4.5 years and clinical findings at age 5 years. JAMA Ophthalmol. 2014;132:676–82. doi: 10.1001/jamaophthalmol.2014.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ram J, Brar GS, Kaushik S, Sukhija J, Bandyopadhyay S, Gupta A, et al. Primary intraocular lens implantation in the first two years of life: Safety profile and visual results. Indian J Ophthalmol. 2007;55:185–9. doi: 10.4103/0301-4738.31937. [DOI] [PubMed] [Google Scholar]
- 4.Lundvall A, Zetterström C. Primary intraocular lens implantation in infants: Complications and visual results. J Cataract Refract Surg. 2006;32:1672–7. doi: 10.1016/j.jcrs.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 5.O'Keefe M, Fenton S, Lanigan B. Visual outcomes and complications of posterior chamber intraocular lens implantation in the first year of life. J Cataract Refract Surg. 2001;27:2006–11. doi: 10.1016/s0886-3350(01)00973-7. [DOI] [PubMed] [Google Scholar]
- 6.Khandekar R, Sudhan A, Jain BK, Shrivastav K, Sachan R. Pediatric cataract and surgery outcomes in central India: A hospital based study. Indian J Med Sci. 2007;61:15–22. [PubMed] [Google Scholar]
- 7.Khanna RC, Foster A, Krishnaiah S, Mehta MK, Gogate PM. Visual outcomes of bilateral congenital and developmental cataracts in young children in South India and causes of poor outcome. Indian J Ophthalmol. 2013;61:65–70. doi: 10.4103/0301-4738.107194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gogate P, Patil S, Kulkarni A, Mahadik A, Tamboli R, Mane R, et al. Barriers to follow-up for pediatric cataract surgery in Maharashtra, India: How regular follow-up is important for good outcome. The Miraj Pediatric Cataract Study II. Indian J Ophthalmol. 2014;62:327–32. doi: 10.4103/0301-4738.116465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta A, Kekunnaya R, Ramappa M, Vaddavalli PK. Safety profile of primary intraocular lens implantation in children below 2 years of age. Br J Ophthalmol. 2011;95:477–80. doi: 10.1136/bjo.2010.184606. [DOI] [PubMed] [Google Scholar]
- 10.Enyedi LB, Peterseim MW, Freedman SF, Buckley EG. Refractive changes after pediatric intraocular lens implantation. Am J Ophthalmol. 1998;126:772–81. doi: 10.1016/s0002-9394(98)00247-5. [DOI] [PubMed] [Google Scholar]
- 11.Lambert SR, Lynn M, Drews-Botsch C, Loupe D, Plager DA, Medow NB, et al. A comparison of grating visual acuity, strabismus, and reoperation outcomes among children with aphakia and pseudophakia after unilateral cataract surgery during the first six months of life. J AAPOS. 2001;5:70–5. doi: 10.1067/mpa.2001.111015. [DOI] [PubMed] [Google Scholar]
- 12.Hussin HM, Markham R. Changes in axial length growth after congenital cataract surgery and intraocular lens implantation in children younger than 5 years. J Cataract Refract Surg. 2009;35:1223–8. doi: 10.1016/j.jcrs.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Fan DS, Rao SK, Yu CB, Wong CY, Lam DS. Changes in refraction and ocular dimensions after cataract surgery and primary intraocular lens implantation in infants. J Cataract Refract Surg. 2006;32:1104–8. doi: 10.1016/j.jcrs.2006.01.097. [DOI] [PubMed] [Google Scholar]
- 14.Lambert SR, Lynn MJ, DuBois LG, Cotsonis GA, Hartmann EE, Wilson ME, et al. Axial elongation following cataract surgery during the first year of life in the Infant Aphakia Treatment Study. Invest Ophthalmol Vis Sci. 2012;53:7539–45. doi: 10.1167/iovs.12-10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.VanderVeen DK, Nizam A, Lynn MJ, Bothun ED, McClatchey SK, Weakley DR, et al. Predictability of intraocular lens calculation and early refractive status: The Infant Aphakia Treatment Study. Arch Ophthalmol. 2012;130:293–9. doi: 10.1001/archophthalmol.2011.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trivedi RH, Wilson ME, Jr, Bartholomew LR, Lal G, Peterseim MM. Opacification of the visual axis after cataract surgery and single acrylic intraocular lens implantation in the first year of life. J AAPOS. 2004;8:156–64. doi: 10.1016/j.jaapos.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Sukhija J, Ram J, Kaur S. Complications in the first 5 years following cataract surgery in infants with and without intraocular lens implantation in the Infant Aphakia Treatment Study. Am J Ophthalmol. 2014;158:1360–1. doi: 10.1016/j.ajo.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Kleinmann G, Zaugg B, Apple DJ, Bleik J. Pediatric cataract surgery with hydrophilic acrylic intraocular lens. J AAPOS. 2013;17:367–70. doi: 10.1016/j.jaapos.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Pehere NK, Bojja S, Vemuganti GK, Vaddavalli PK, Samant M, Jalali S, et al. Opacification of intraocular lenses implanted during infancy: A clinicopathologic study of 4 explanted intraocular lenses. Ophthalmology. 2011;118:2128–320. doi: 10.1016/j.ophtha.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Freedman SF, Lynn MJ, Beck AD, Bothun ED, Örge FH, Lambert SR, et al. Glaucoma-related adverse events in the first 5 years after unilateral cataract removal in the Infant Aphakia Treatment Study. JAMA Ophthalmol. 2015;133:907–14. doi: 10.1001/jamaophthalmol.2015.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asrani S, Freedman S, Hasselblad V, Buckley EG, Egbert J, Dahan E, et al. Does primary intraocular lens implantation prevent “aphakic” glaucoma in children? J AAPOS. 2000;4:33–9. doi: 10.1016/s1091-8531(00)90009-0. [DOI] [PubMed] [Google Scholar]
- 22.Bothun ED, Cleveland J, Lynn MJ, Christiansen SP, Vanderveen DK, Neely DE, et al. One-year strabismus outcomes in the Infant Aphakia Treatment Study. Ophthalmology. 2013;120:1227–31. doi: 10.1016/j.ophtha.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of the incidence of complications in the current study to major studies in the literature showing a significantly less incidence of major complications (visual axis opacification and glaucoma)


