Abstract
Objective To test social cognitive predictors of medication adherence in racial/ethnic minority youth living with HIV using a conceptual model. Methods Youth were participants in two descriptive studies by the Adolescent Trials Network for HIV/AIDS Interventions. Minority youth ages 16–24 years who were prescribed antiretroviral medication were included ( N = 956). Data were collected through chart extraction and/or laboratory testing and by Audio Computer-Assisted Self-Interview. Results 39% of youth reported suboptimal adherence. Path analysis was used to explore predictors of medication adherence. Higher self-efficacy predicted higher readiness and adherence. Greater social support predicted higher self-efficacy. Psychological symptoms and substance use were associated with several predictors and lower adherence. Conclusions The model provided a plausible framework for understanding adherence in this population. Culturally competent, but individually tailored, interventions focused on increasing self-efficacy to take medication and reducing risk behaviors (e.g., substance use) may be helpful for racial or ethnic minority youth with HIV.
Keywords: adolescents, chronic illness, disparities, health behavior, HIV/Aids, psychosocial functioning, race/ethnicity
In the United States, it is estimated that one-third of all new HIV diagnoses are among people <25 years of age ( CDC, 2011 ). The rates of new and existing infections continue to be disproportionately higher in racial and ethnic minorities, particularly among Black and Latino adolescents and young adults ( CDC, 2008 ). Advances in medical treatment, particularly antiretroviral medications (ART), have resulted in rapid declines in HIV-associated morbidity and mortality, allowing for HIV-infected adolescents and young adults (hereafter called “youth”) to manage their HIV infection as a chronic disease ( CASCADE, 2003 ). There is strong evidence that adherence to ART is a primary determinant of virological suppression, disease progression, and mortality ( Benator et al., 2015 ; Eaton, Saag, & Mugavero, 2014 ; Stricker et al., 2014 ).
Racial and/or ethnic minority youth are at particular risk of poor adherence to ART and, thus, of having detectable viral load ( MacDonell, Naar-King, Huszti, & Belzer, 2013 ; Simoni et al., 2012 ). The goal of taking ART is to reduce viral load count so that there are so few copies of the virus that it is considered “below detection.” Having a viral load count below detection is associated with better health outcomes for people with HIV, including slowing or stopping the advance of the disease. “Suboptimal adherence,” which we define as taking ART as prescribed <90% of the time, has public health implications as well as personal risks. High viral load increases the likelihood of viral transmission during risky sexual behavior ( Quinn et al., 2000 ) and nonadherent individuals may be more likely to transmit drug resistant strains of virus ( Tang & Pillay, 2004 ). The consequence of suboptimal therapy for youth is a future population of HIV-infected adults who harbor multiple drug-resistant viruses and suffer from drug- and virus-induced metabolic complications ( Bangsberg, 2006 ). Conversely, treatment adherence decreases the pool of infectious individuals during a time when condom use is poor even among youth aware of their HIV status ( Outlaw et al., 2010 ). A review of >50 studies on pediatric HIV infection indicated that between 42% and 80% of youth reported suboptimal medication adherence ( Simoni et al., 2007 ). Results of the few studies focused on racial/ethnic minority youth with HIV suggest comparable rates of poor adherence to ART ( MacDonell, Naar-King, Murphy, Parsons, & Harper, 2010 ; Rudy, Murphy, Harris, Muenz, & Ellen, 2009 ), though little is known about the specific reasons underlying poor adherence in this population.
A growing body of research has explored predictors of ART adherence in youth living with HIV. Some of these findings may be generalizable to understanding adherence in racial and ethnic minority youth, particularly because minority youth are overrepresented in the population of youth living with HIV ( CDC, 2008 ). Psychosocial factors, particularly depression and anxiety, have been consistently associated with poor adherence to ART ( Reisner et al., 2009 ). In addition, results suggest that adherence should be considered within the broader contextual issues present in the lives of youth, such as HIV stigma and disclosure, peer relations, and mental health and substance use ( Rao et al., 2012 ; Rao, Kekwaletswe, Hosek, Martinez, & Rodriguez, 2007 ; Reisner et al., 2009 ). Ethnic or racial minority status ( Fogarty et al., 2002 ), lack of social support ( MacDonell, Naar-King, Murphy, Parsons, & Huszti, 2011 ), and low self-efficacy ( Naar-King et al., 2006 , 2013 ) have also been associated with poor medication adherence in youth living with HIV. Poor medication adherence has been linked to financial and structural challenges such as housing instability ( Martinez et al., 2000 ) and lower level of education ( Murphy et al., 2005 ). However, because few medication adherence studies have focused specifically on ethnic and racial minority youth, these factors need to be examined in exclusively racial and ethnic minority samples. In addition, empirically supported conceptual models of adherence in youth with HIV are needed to understand the complicated interrelationships among these multiple factors.
MacDonell et al. (2010) tested predictors of medication adherence in a sample of racial and ethnic minority youth living with HIV using a conceptual model including constructs from the Transtheoretical Model (TTM; Prochaska & Velicer, 1997 ). The TTM ( Prochaska & Velicer, 1997 ) has been suggested as one plausible framework for understanding medication adherence ( Riekert & Drotar, 2000 ). The TTM posits that motivational readiness to change behavior, or a continuum of a person’s perception of how ready they are to change, comes before actual behavior change. This was originally conceptualized as stages of change, but critiques of the stage model have suggested an alternative, continuous conceptualization of motivational readiness ( Migneault et al., 2005 ). There are also social and cognitive factors beyond motivational readiness, such as self-efficacy and social support, that might be included in a conceptual model of adherence behavior. In MacDonell et al. (2010) , the conceptual model included social cognitive predictors including a continuous measure of motivational readiness to take ART as prescribed. Higher social support for taking medications predicted motivational readiness and, in turn, higher readiness predicted optimal adherence. Thus, the authors recommended culturally competent interventions focused on motivational readiness and social support to improve adherence to ART in this population. However, this study was conducted with a small sample of youth with HIV who also engaged in risky sexual, substance use, and/or adherence behavior and may not be representative of the broader population of ethnic and racial minority youth living with HIV.
The purpose of the present study is to extend the work of MacDonell et al. (2010) and test a conceptual model of adherence behavior in a larger, more generalizable (i.e., not higher risk), multisite sample of ethnic and racial minority youth living with HIV. Because we are not directly comparing models of adherence for ethnic/minority versus White youth, we consider these analyses exploratory. We hypothesized that motivational readiness to take ART as prescribed would be associated with better adherence in ethnic/racial minority youth with HIV. We predicted that higher self-efficacy would predict increased motivational readiness and better ART adherence in this population. We also hypothesized that low levels of social support would be related to lower self-efficacy and suboptimal adherence to ART in ethnic/racial minority youth living with HIV. Problem-level substance use and psychological symptoms were also expected to significantly predict poor adherence in this minority sample.
Method
The goal of the present study was to test a model of social cognitive predictors of medication adherence in youth living with HIV/AIDS using data from protocols 086 and 106 from the Adolescent Trials Network for HIV/AIDS Interventions (ATN; Fernandez et al., 2015 ). For the purposes of the present analyses, data from ATN 086 (15 sites) and 106 (5 additional sites) were combined.
Participants
Participants in ATN 086 and 106 were behaviorally or perinatally HIV-infected youth ages 12–24 years ( N = 2,213; 1,704 in ATN 086, and 509 in ATN 106). Participants were aware of their HIV-positive serostatus, had at least one clinic visit during the 1-year study enrollment period, and understood written and/or spoken English or Spanish. Those with psychiatric symptoms that interfered with their ability to consent or complete questionnaires at the time of their clinic visit, appeared visibly distraught, or were under the influence of alcohol or other substances at the time of consent or data collection were excluded. In ATN 086/106, 59.3% of participants were currently prescribed ART and 69.5% were HIV-infected through sexual behavior or substance use.
Current Sample
Only racial minority youth (self-identified as Black, Asian/Pacific Islander, Native American/Alaskan Native, Mixed Race /Other) and/or ethnic minority youth (self-identified as Hispanic or Latino) ages 16–24 years prescribed HIV ART with complete data were included in the present study ( N = 956, 43.2% of the total sample and 91.9% of ethnic/minority youth). For racial identity, 72.0% self-identified as Black, 20.4% as mixed race/other, 4.6% as Hispanic or Latino/a White, 1.2% as Native American/Alaskan Native, and 0.8% as Asian/Pacific Islander. Twenty-three percent (23.6%) of the sample was Hispanic or Latino/a of any race. For sexual identity, 47.7% of youth identified themselves as heterosexual or straight, 36.8% as gay, 12.2% as bisexual, 1.3% as questioning, 0.9% as lesbian, 0.6% as other, and 0.4% as queer. Sexual identification was treated as a dichotomous variable (gay, bisexual, questioning, lesbian, queer, or other vs. heterosexual/straight) for subsequent analyses. A majority was biologically male (68.8%) and behaviorally infected (64.4%). Age ranged from 16 to 24 years, with M = 20.66 ( SD = 2.36).
Procedure
The protocol was approved by each ATN site’s institutional review board (IRB). A certificate of confidentiality was obtained from the National Institutes of Health. Participants were approached during a regularly scheduled clinic visit or during supportive activities. All participants were screened to confirm seropositivity or provided documented test results from a prior HIV screening. Informed consent was obtained from all participants, and a waiver of parental permission was obtained for youth ages 16 and 17 years. Data were collected through biomedical chart extraction and/or laboratory testing and by Audio Computer-Assisted Self-Interview. Participants received compensation and transportation in an amount determined by their local IRB.
Measures
Adherence to ART
ATN researchers adapted a questionnaire to assess adherence to ART ( Chesney et al., 2000 ) to be more appropriate for youth. This questionnaire included prompts to ask participants to report the total number of ART doses they were prescribed to take per day and to estimate how many times they had missed taking a dose in the past 7 days. “Dose” of ART was defined as “quantity of pills or medicines prescribed to be taken at one particular time (for example, 3 pills before bedtime).” ART adherence was defined as the percentage of doses taken in the past 7 days and was calculated as the total number of doses taken divided by total number prescribed doses per week. “Optimal adherence” was defined as adherence of ≥90% and is consistent with a number of recent studies of HIV medication adherence ( Pruitt, 2013 ; Viswanathan et al., 2015 ).
Viral Load
Viral load was obtained from medical chart review and abstraction. Participants who did not have plasma HIV RNA counts within 6 months of data collection had blood collected for these measurements. Plasma HIV RNA was assessed as a dichotomous variable (undetectable vs. detectable).
Self-Efficacy for Taking Medications
Three items assessed self-efficacy for taking medications as prescribed. The items included the following: “How sure are you that you can take the right amounts of your medicine at the right times?”; “How sure are you that you can do better with taking the right amounts of your medicine at the right times?”; and “How sure are you that you can take the right amounts of your medicine at the right times even if you were very tempted not to?” These items were scored using a 5-point Likert scale ranging from 1 ( very sure I can ) to 5 ( very sure I cannot ). For the current study, responses were reverse-coded and items summed to form a composite self-efficacy score (max possible score = 15.0). Reliability was acceptable with Cronbach’s alpha = .84.
Motivational Readiness for Medication Adherence
The adherence to medications item from Rollnick’s Readiness Ruler ( Stott, Rollnick, Rees, & Pill, 1995 ) was used to assess motivation for adherence. Respondents rated how ready they are to take HIV medications as prescribed on a scale from 1 ( not ready ) to 10 ( ready to change or already changed ). This measure has been recommended for use by clinicians to determine readiness to change in HIV care ( CDC, 1993 ), and has been used for adherence and other behaviors in youth living with HIV ( MacDonell et al., 2010 ; Naar-King, Parsons, Murphy, Kolmodin, & Harris, 2010 ).
Social Support for Taking Medications
A single item asking about social support specific to medication adherence (“There are people in my life that are supportive about taking HIV medication”) was rated on a 5-point Likert scale from 1 ( strongly agree ) to 5 ( strongly disagree ). This item was used because social support specific for reducing risk behaviors has been shown to be related to risk behaviors more than has general social support ( MacDonell et al., 2010 ; Naar-King et al., 2006 ).
Substance Use
The CRAFFT (Car, Relax, Alone, Forget, Friends, Trouble) Substance Abuse Screening Test is a screening tool that addresses indicators of problems related to alcohol and substance use ( Knight et al., 1999 ). “Yes” responses are assigned a score of 1, and “no” responses are assigned a score of 0. Total scores of ≥2 suggest problem-level substance use.
Symptoms of Emotional Distress
The Brief Symptom Inventory (BSI) measures nine primary symptom dimensions of physical and mental status combined to form the Global Severity Index (GSI) ( Derogatis & Spencer, 1982 ). The BSI asks respondents to rate how much they are distressed by a series of issues (e.g., lack of appetite, thoughts of ending your life) based on a 5-point response scale ranging from 1 ( not at all ) to 5 ( extremely ). We used the GSI T-score as a continuous variable and a dichotomous variable with a score of ≥63 and/or the presence of any two dimensions as the clinical cutoff for psychological distress( Derogatis, 2001 ). Internal consistency for the BSI GSI was excellent with Cronbach’s alpha = .98.
Data Analysis
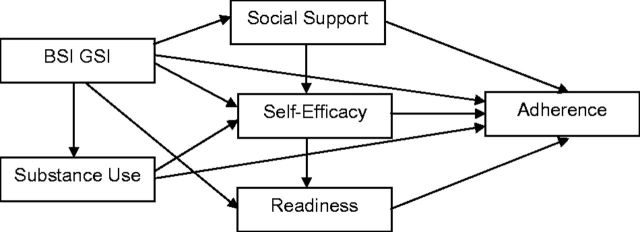
Descriptive statistics and bivariate analyses assessed simple associations between variables. Chi-square and t -tests were used to assess demographic differences across variables. Path analysis with bootstrapping (subsample of n = 200) was conducted using IBM SPSS AMOS 22.0 to examine the relations between the predictor variables in the model and adherence to HIV medication. Bootstrapping is useful for testing indirect effects and may reduce potential bias ( Bollen & Stine, 1992 ; Shrout & Bolger, 2002 ). Both direct and indirect relations were evaluated using standardized regression weights. Model testing involved consideration of the hypothesized model ( Figure 1 ) followed by modifications to improve parsimony. In the proposed path model, we tested whether self-efficacy and motivational readiness directly predicted medication adherence. We also tested if social support predicted self-efficacy and motivational readiness. Psychological symptoms and problem-level substance use were included in the proposed model as covariates. Standardized root mean square residual (SRMR), comparative fit index (CFI), and root mean square error of approximation (RMSEA) were used as fit indices. Values for the SRMR range from 0 to 1 with well-fitting models obtaining values <0.05 ( Byrne, 2013 ; Diamantopoulos, Siguaw, & Siguaw, 2000 ). For CFI, a cutoff criterion of ≥0.95 was used as indicative of good fit ( Hu & Bentler, 1999 ). For RMSEA, MacCallum, Browne, and Sugawara (1996) have used 0.01, 0.05, and 0.08 to indicate excellent, good, and mediocre fit, respectively.
Figure 1.
Hypothesized model of medication adherence.
Results
Descriptive Analyses
Thirty-nine percent of youth ( n = 372) reported taking their prescribed antiretroviral therapy medications <90% of the time in the past 7 days. Percent ART doses taken in the past 7 days ranged from 0 to 100, with M = 87.27 ( SD = 21.76). The continuous measure of medication adherence was highly negatively skewed (56.8% reported 100% adherence) and subject to a logarithmic transformation for subsequent analyses. In addition, there were no substantive differences in our conclusions when the model was run defining the sample as adherent versus nonadherent. Forty-nine percent (48.6%, n = 465) of youth had detectable viral load. Youth with a detectable viral load had significantly lower medication adherence than youth with a viral load below detection, t (954) = 5.41, p ≤ .001, Cohen’s d = 0.35. Perinatally and behaviorally infected youth were not significantly different in medication adherence, t (941) = −1.19, p = .24, d = 0.08. Youth ranged from 5 to 24 years of age when they learned they were HIV positive, M = 15.81 ( SD = 4.89). The percentage of participants who were sexual minority differed by biological sex, χ2 (1, 1060) = 269.69, p < .001, so that biological men were more likely to be sexual minority. Youth who identified as gay, bisexual, lesbian, etc. (“sexual minority youth”) and those identified as heterosexual/straight did not differ significantly on medication adherence, t (954) = −1.87, p = .06, d = 0.12. Straight identifying youth reported M = 85.64% of doses taken in the past week ( SD = 23.16), while sexual minority youth reported M = 88.76% of doses taken ( SD = 20.30).
Forty-two percent (41.9%) of youth demonstrated clinically significant levels of psychological distress on the BSI GSI. Scores on this measure ranged from 0 to 3.81, with M = 0.89 ( SD = 0.77). Over half of the sample (52.8%) demonstrated problem-level substance use on the CRAFFT. Scores on this measure ranged from 0 to 6.0, with M = 2.07 ( SD = 1.84). Self-efficacy ranged from 3.0 to 15.0, with M = 13.97 ( SD = 1.64). Motivational readiness ranged from 1.0 to 10.0, M = 9.44 ( SD = 1.39). Social support ranged from 1.0 to 5.0, with M = 4.40 ( SD = 1.06). Because self-efficacy, social support, and motivational readiness were highly negatively skewed, transformed values (logarithmic) were used in all subsequent analyses.
Bivariate correlations were calculated for all variables and detailed in Table I .
Table I.
Bivariate Correlations
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| 1. | Adherence | – | .03 | .27 ** | .14 ** | −.11 ** | −.09 ** |
| 2. | Social support | – | .10 ** | .07 * | −.16 ** | −.004 | |
| 3. | Self-efficacy | – | .34 ** | −.19 ** | −.09 ** | ||
| 4. | Readiness | – | −.08 * | −.01 | |||
| 5. | BSI GSI | – | .33 ** | ||||
| 6. | Substance use | – |
Note . BSI GSI = brief symptom inventory global severity index.
*Correlation is significant at p < .05 level.
**Correlation is significant at p < .01 level.
Path Analysis
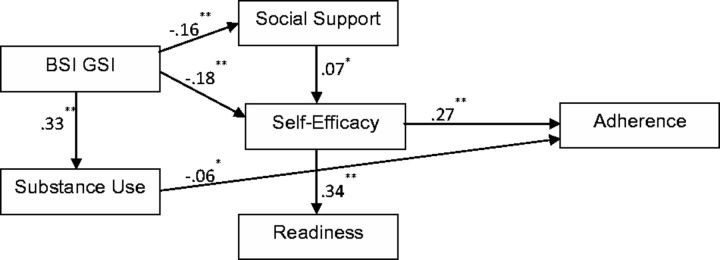
The hypothesized model resulted in a good fit, χ 2 (2, N = 956) = 3.31, p = .191, RMSEA = 0.026, CFI =0.996, SRMR = 0.012, but a number of paths were not significant. A reduced model was estimated with nonsignificant paths ( p > .05) deleted from the originally hypothesized model. Table II details the progression from the hypothesized model to the final, most parsimonious model. The final model ( Figure 2 ) had a good fit with the data, χ 2 (8, N = 956) = 10.12; p = .256; RMSEA = 0.017; CFI = 0.994; SRMR = 0.020).
Table II.
Progression of the Path Model
| Step | Path removed | χ 2 | p | df | CFI | RMSEA | SRMR |
|---|---|---|---|---|---|---|---|
| 1 | – | 3.31 | .191 | 2 | 0.996 | 0.026 | 0.012 |
| 2 | Social support → adherence | 3.31 | .346 | 3 | 0.999 | 0.010 | 0.012 |
| 3 | BSI GSI → readiness | 3.40 | .493 | 4 | 1.00 | 0.000 | 0.012 |
| 4 | Substance use → self-efficacy | 4.36 | .499 | 5 | 1.00 | 0.000 | 0.013 |
| 5 | BSI GSI → adherence | 5.56 | .475 | 6 | 1.00 | 0.000 | 0.014 |
| 6 | Social support → readiness | 6.93 | .436 | 7 | 1.00 | 0.000 | 0.017 |
| Final | Readiness → adherence | 10.12 | .256 | 8 | 0.994 | 0.017 | 0.020 |
Note . SRMR = standardized root mean square residual; CFI = comparative fit index; RMSEA = root mean square error of approximation; BSI GSI = brief symptom inventory global severity index.
Figure 2.
Final (reduced) model of medication adherence. Note . * p ≤ .05, ** p ≤ .01.
The reduced model demonstrated a number of significant associations among variables. Direct effects (standardized regression weights) are shown in Figure 2 . Higher self-efficacy strongly predicted higher motivational readiness and medication adherence. Higher levels of social support predicted higher self-efficacy. Psychological symptoms were associated with lower social support, lower self-efficacy, and higher levels of substance use. Psychological symptoms also had indirect effects on motivational readiness (−0.07, p ≤ .01) and medication adherence (−0.07, p ≤ .01). Higher problem substance use significantly predicted lower medication adherence. Details of direct effects obtained from bootstrapping are detailed in Table III .
Table III.
Final Path Model (Bootstrapping Results)
| Path | Standardized B | SE | Bias | Lower | Upper | p |
|---|---|---|---|---|---|---|
| BSI GSI → social support | −0.159 | 0.033 | 0.002 | −0.211 | −0.102 | .009 |
| Social support → self-efficacy | 0.069 | 0.033 | −0.002 | 0.013 | 0.114 | .077 |
| BSI GSI → self-efficacy | −0.180 | 0.029 | −0.003 | −0.221 | −0.123 | .025 |
| BSI GSI → substance use | 0.327 | 0.029 | 0.002 | 0.277 | 0.373 | .015 |
| Self-efficacy → readiness | 0.341 | 0.028 | −0.001 | 0.290 | 0.387 | .008 |
| Self-efficacy → adherence | 0.268 | 0.029 | 0.003 | 0.224 | 0.319 | .012 |
| Substance use → adherence | −0.064 | 0.030 | 0.002 | −0.108 | −0.012 | .053 |
Note. Bias refers to the difference between the average of estimates obtained from bootstrap samples ( n = 200) and the estimate obtained from the original sample. BSI GSI = brief symptom inventory global severity index.
Discussion
This study was among the first to explore predictors of adherence to ART in a large, multisite sample of racial and ethnic minority youth living with HIV. Nearly 40% of youth in the present study reported suboptimal (<90%) adherence to ART. This rate is lower than reported in another sample of ethnic/minority youth with HIV ( MacDonell et al., 2010 ), in which 79% of youth reported suboptimal adherence. However, MacDonell et al. (2010) included only youth who demonstrated sexual risk behavior, substance abuse, and/or poor adherence to ART and may not have been representative of all minority youth living with HIV. The current study’s finding is consistent with Rudy et al. (2009) , in which 37.4% of youth from a predominately minority sample identified as nonadherent. Results of the current study strongly supported the link between youth self-reported adherence to ART and viral load.
Our hypothesized model of medication adherence extends previous work with racial and ethnic minority youth with HIV using a larger and more representative (i.e., not higher risk) sample; moreover, the present study offers a feasible preliminary TTM-based framework for beginning to understand adherence to ART in minority youth. This framework is intended to serve as a foundation for future research focused on social cognitive predictors of medication adherence in racial and ethnic minority youth with HIV and, ultimately, to guide the development of adherence interventions for this population. Overall, results suggest that key constructs of the TTM may be applicable to minority populations to understand and predict adherence to ART. Youth with higher self-efficacy tended to have higher motivational readiness to take ART as prescribed and better adherence to ART. In turn, youth with higher levels of social support were likely to have higher self-efficacy. Youth with more psychological symptoms and problem-level substance use tended to have lower medication adherence in this study. In addition, youth with more psychological symptoms tended to have lower social support and self-efficacy. Previous research with youth living with HIV using diverse samples (i.e., not focused specifically on ethnic or racial minority youth) has fairly consistently found links between psychological symptoms and/or frequent substance use and lower medication adherence ( Hosek, Harper, & Domanico, 2005 ; Murphy, Wilson, Durako, Muenz, & Belzer, 2001 ; Reisner et al., 2009 ). This association appears to hold in the current ethnic and racial minority population.
In the present study, motivational readiness to take ART did not significantly predict adherence, contrary to findings from previous research using an ethnic and racial minority sample ( MacDonell et al., 2010 ). Instead, task-specific self-efficacy—to take ART medication as prescribed—was strongly related to adherence. In a recent meta-analysis of 207 adult studies of HIV adherence, self-efficacy to take medications was the strongest predictor of adherence to ART and the only predictor with a large effect size ( Langebeek et al., 2014 ). There is evidence that self-efficacy specifically for managing medications may be more strongly linked to treatment adherence than other types of self-efficacy in racial/ethnic minority populations ( Houston & Fominaya, 2015 ). This may suggest that interventions to promote medication adherence in racial and ethnic minority youth with HIV should aim to improve self-efficacy for taking ART.
Motivational Interviewing (MI) is a widely disseminated behavior change intervention that has demonstrated effectiveness in promoting behavior change among persons with a range of health issues ( Lundahl et al., 2013 ) including HIV. MI may be one useful approach to promote treatment adherence in youth with HIV because it supports self-efficacy through affirmation of client strengths ( Miller, Rollnick, & Moyers, 1998 ). Interventions based on the principles of MI have been shown to be effective in promoting ART adherence and/or self-efficacy in youth with HIV, including those tested with predominately racial and ethnic minority samples ( Naar-King et al., 2010 , 2013 ). However, there is still a need to go even further to improve intervention efforts to improve their effectiveness with minority groups, as rates of HIV transmission and health outcomes for minority youth are still significantly worse than for White youth. Innovative techniques are needed to increase the acceptability and effectiveness of intervention if improved adherence to ART is the goal, particularly given the known challenges in keeping ethnic and minority youth in clinic care and the mistrust some ethnic and racial groups tend to have for health providers ( Anderson et al., 2003 ; Snowden & Yamada, 2005 ; Whaley, 2001 ; Whetten, Reif, Whetten, & Murphy-McMillan, 2008 ). One possibility may be to use evidence-based practices such as MI via field outreach instead of traditional office-based intervention delivery. Field outreach involves face-to-face interaction between a worker/counselor (e.g., peer educator, community health worker) and an individual to promote behavior change such as taking ART as prescribed. MI through field outreach has been found to be successful in minority populations to promote HIV counseling and testing, and may prove effective for promoting adherence to ART ( Outlaw et al., 2010 ).
In the present study, sexual minority and straight-identifying youth were not significantly different in adherence to ART. We might expect marginalized groups such as sexual and racial/ethnic minority youth to face minority stress that may lower self-esteem, increase the sense of social isolation, and contribute to poor health outcomes ( Herrick et al., 2011 ). However, there is also rich evidence for strengths and resilience among sexual minority youth in both the scientific literature and in historical accounts of sexual minority (mainly gay male) culture ( Harper, Bruce, Hosek, Fernandez, & Rood, 2014 ; Herrick et al., 2011 ). Furthermore, members of the sexual minority in this sample may face significant stressors related to their HIV status and related to being a racial or ethnic minority resulting in similar levels of distress. Also, in the present study and other studies ( Koenig et al., 2010 ), perinatally infected youth were more likely to identify as straight/heterosexual. However, perinatally infected youth have been found to report more barriers to taking ART as prescribed than behaviorally infected youth ( MacDonell et al., 2013 ). Given this and our current findings, interventions may need to focus not only on addressing factors associated with poor adherence such as substance use and mental health, but also promote sources of self-efficacy and resiliency, particularly social support ( Herrick, Egan, Coulter, Friedman, & Stall, 2014 ; Herrick et al., 2011 ; Herrick, Stall, Goldhammer, Egan, & Mayer, 2014 ). Our findings also suggest that adherence interventions may be more effective if they are tailored based on characteristics of the individual (e.g., level of self-efficacy for taking medication, psychological health) rather than tailored based solely on sexual orientation or route of HIV infection.
This study is one of the first to explore predictors of adherence to ART in ethnic and racial minority youth, but there are notable limitations. The study was conducted with a clinic-based convenience sample already prescribed ART and may not represent community samples of youth with HIV. It also did not directly compare ethnic and racial minority youth versus White youth, which would be necessary to conduct confirmatory research. Thus, this model does not help to explain the underlying causes of health disparities and may or may not be applicable to other populations of youth with HIV. Another limitation is the study’s reliance on self-report measures, particularly for medication adherence and substance use, as well as the inclusion of viral load as a dichotomous measure (below detection vs. detectable). Because results support the link between self-reported adherence and viral load, researchers might consider using newer, emerging methodologies for further increasing the validity of self-report including Ecological Momentary Assessment ( Shiffman, Stone, & Hufford, 2008 ) along with objective measurements of adherence ( Haberer et al., 2010 ). Substance use was measured using a screener for all substances and did not differentiate between alcohol, marijuana, and other drugs, so results should be viewed with caution. Another limitation is that social support and motivational readiness were assessed using single items because of the necessary brevity of the assessment. Although these single item measures have demonstrated adequate validity in previous studies ( MacDonell et al., 2010 ; Stott et al., 1995 ), results could be viewed as preliminary and additional research using more comprehensive measures of these variables may be warranted. Finally, longitudinal data are required to truly predict adherence to ART in this population.
Future research should continue to explore adherence to ART in ethnic and racial minority youth. Interventions targeting adherence must be designed using culturally sensitive ( Harper, 2007 ), developmentally appropriate, and empirically supported frameworks that address the multiple factors that may influence adherence behavior in the individual. Our model suggests that boosting self-efficacy for taking medications as prescribed may directly and positively impact adherence behavior, though this must be explored further in future research. In the present study, we found that the presence of psychological symptoms and frequent problem-level substance use may have a major negative impact on medication adherence, and may interfere with positive influences on adherence such as self-efficacy. It is clear that interventions to address substance abuse and mental health continue to be a priority for youth living with HIV, though the current framework suggests that addressing self-efficacy for taking ART, possibly with interventions based in MI ( Naar-King et al., 2010 , 2013 ), are also important for minority youth. Finally, our model may offer a feasible framework for beginning to understand adherence to ART in ethnic and racial minority adolescents and young adults with chronic conditions other than HIV.
Acknowledgments
Clinics were located in the following cities: Los Angeles, California; San Francisco, California; Washington, DC; Baltimore, Maryland; Boston, Massachusetts; Chicago, Illinois; Philadelphia, Pennsylvania; New York City (Bronx and Manhattan), New York; San Juan, Puerto Rico; New Orleans, Louisiana; Memphis, Tennessee; Miami, Florida; Tampa, Florida; Ft. Lauderdale, Florida; Detroit, Michigan; Denver, Colorado; and Houston, Texas.
Funding
This work was supported by The Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) from the National Institutes of Health [U01 HD 040533 and U01 HD 040474] through the National Institute of Child Health and Human Development (B. Kapogiannis, S. Lee), with supplemental funding from the National Institutes on Drug Abuse (K. Davenny, S. Kahana) and Mental Health (P. Brouwers, S. Allison). The study was scientifically reviewed by the ATN’s Behavioral Leadership Group. Network, scientific and logistical support was provided by the ATN Coordinating Center (C. Wilson, C. Partlow) at The University of Alabama at Birmingham. Network operations and data management support was provided by the ATN Data and Operations Center at Westat, Inc. (J. Korelitz, B. Driver). We acknowledge the contribution of the investigators and staff at the following sites that participated in this study: The following ATN sites participated in this study: University of South Florida, Tampa (Emmanuel, Lujan-Zilbermann, Julian), Children’s Hospital of Los Angeles (Belzer, Flores, Tucker), Children’s National Medical Center (D’Angelo, Hagler, Trexler), Children’s Hospital of Philadelphia (Douglas, Tanney, DiBenedetto), John H. Stroger Jr. Hospital of Cook County and the Ruth M. Rothstein CORE Center (Martinez, Bojan, Jackson), University of Puerto Rico (Febo, Ayala-Flores, Fuentes-Gomez), Montefiore Medical Center (Futterman, Enriquez-Bruce, Campos), Mount Sinai Medical Center (Steever, Geiger), University of California-San Francisco (Moscicki, Auerswald, Irish), Tulane University Health Sciences Center (Abdalian, Kozina, Baker), University of Maryland (Peralta, Gorle), University of Miami School of Medicine (Friedman, Maturo, Major-Wilson), Children’s Diagnostic and Treatment Center (Puga, Leonard, Inman), St. Jude’s Children’s Research Hospital (Flynn, Dillard), Children’s Memorial Hospital (Garofalo, Brennan, Flanagan). Baylor College of Medicine (Paul, Calles, Cooper), Wayne State University (Secord, Cromer, Green-Jones), John Hopkins University School of Medicine (Agwu, Anderson, Park), The Fenway Institute—Boston (Mayer, George, Dormitzer), and University of Colorado Denver (Reirden, Hahn, Witte). The investigators are grateful to the youth who participated in this study.
Conflicts of interest : None declared.
References
- Anderson L. M., Scrimshaw S. C., Fullilove M. T., Fielding J. E., Normand J. ; Task Force on Community Preventive Services . ( 2003. ). Culturally competent healthcare systems: A systematic review . American Journal of Preventive Medicine , 24 , 68 – 79 . [DOI] [PubMed] [Google Scholar]
- Bangsberg D. R. ( 2006. ). Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression . Clinical Infectious Diseases , 43 , 939 – 941 . doi: 10.1086/507526 [DOI] [PubMed] [Google Scholar]
- Benator D. A., Elmi A., Rodriguez M. D., Gale H. B., Kan V. L., Hoffman H. J., Tramazzo S., Hall K., McKnight A., Squires L. ( 2015. ). True durability: HIV virologic suppression in an urban clinic and implications for timing of intensive adherence efforts and viral load monitoring . AIDS and Behavior , 19 , 594 – 600 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen K. A., Stine R. A. ( 1992. ). Bootstrapping goodness-of-fit measures in structural equation models . Sociological Methods & Research , 21 , 205 – 229 . doi: 10.1177/0049124192021002004 [Google Scholar]
- Byrne B. M. ( 2013. ). Structural equation modeling with AMOS: Basic concepts, applications, and programming (2nd ed.) . New York, NY: : Routledge; . [Google Scholar]
- CASCADE . ( 2003. ). Determinants of survival following HIV-1 seroconversion after the introduction of HAART . The Lancet , 362 , 1267 – 1274 . doi: 10.1016/S0140-6736(03)14570-9 [DOI] [PubMed] [Google Scholar]
- CDC . ( 1993. ). Recommendations for HIV testing services and outpatients in acute-care hospital settings and technical guidance on HIV counseling . Morbidity and Mortality Weekly Report , 42 ( RR-2 ). [PubMed] [Google Scholar]
- CDC . ( 2008. ). Subpopulation estimates from the HIV incidence surveillance system–United States, 2006 . MMWR. Morbidity and Mortality Weekly Report , 57 , 985 – 989 . [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . ( 2013. ). HIV Surveillance Report, 2013 ; vol. 25 . http://www.cdc.gov/hiv/library/reports/surveillance . Accessed October 12, 2015 . [Google Scholar]
- Chesney M. A., Ickovics J., Chambers D., Gifford A. L., Neidig J., Zwickl B., Wu A.W. ; Patient Care Committee &Adherence Working Group of the Outcomes Committee of the Adult AIDSClinical Trials Group (AACTG) . ( 2000. ). Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments . AIDS Care , 12 , 255 – 266 . [DOI] [PubMed] [Google Scholar]
- Derogatis L. R. ( 2001. ). BSI 18, brief symptom inventory 18: Administration, scoring and procedures manual . Minneapolis, MN: : NCS Pearson, Inc; . [Google Scholar]
- Derogatis L. R., Spencer P. M. ( 1982. ). The brief symptom inventory (BSI): Administration, and procedures manual-I . Baltimore, MD: : Clinical Psychometric Research; . [Google Scholar]
- Diamantopoulos A., Siguaw J. A., Siguaw J. A. ( 2000. ). Introducing LISREL: A guide for the uninitiated . London: : Sage; . [Google Scholar]
- Eaton E. F., Saag M. S., Mugavero M. ( 2014. ). Engagement in human immunodeficiency virus care: linkage, retention, and antiretroviral therapy adherence . Infectious Disease Clinics of North America , 28 , 355 – 369 . [DOI] [PubMed] [Google Scholar]
- Fernandez M. I., Huszti H., Wilson P. A., Kahana S., Nichols S., Gonin R., Xu J., Kapogiannis B.G. ( 2015. ). Profiles of risk among HIV-infected youth in a clinic setting . AIDS and Behavior , 19 , 918 – 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty L., Roter D., Larson S., Burke J., Gillespie J., Levy R. ( 2002. ). Patient adherence to HIV medication regimens: A review of published and abstract reports . Patient Education and Counseling , 46 , 93 – 108 . doi: 10.1016/S0738-3991(01)00219-1 [DOI] [PubMed] [Google Scholar]
- Haberer J. E., Kahane J., Kigozi I., Emenyonu N., Hunt P., Martin J., Bangsberg D. R. ( 2010. ). Real-time adherence monitoring for HIV antiretroviral therapy . AIDS and Behavior , 14 , 1340 – 1346 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper G. W. ( 2007. ). Sex isn't that simple: Culture and context in HIV prevention interventions for gay and bisexual male adolescents . The American Psychologist , 62 , 803 – 819 . doi: 10.1037/0003-066x.62.8.806 [DOI] [PubMed] [Google Scholar]
- Harper G. W., Bruce D., Hosek S. G., Fernandez M. I., Rood B. A. ( 2014. ). Resilience processes demonstrated by young gay and bisexual men living with HIV: Implications for intervention . AIDS Patient Care STDS , 28 , 666 – 76 . doi: 10.1089/apc.2013.0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick A. L., Egan J. E., Coulter R. W. S., Friedman M. S., Stall R. ( 2014. ). Raising sexual minority youths' health levels by incorporating resiliencies into health promotion efforts . American Journal of Public Health , 104 , 206 – 210 . doi: 10.2105/AJPH.2013.301546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick A. L., Lim S. H., Wei C., Smith H., Guadamuz T., Friedman M. S., Stall R. ( 2011. ). Resilience as an untapped resource in behavioral intervention design for gay men . AIDS and Behavior , 15 , 25 – 29 . [DOI] [PubMed] [Google Scholar]
- Herrick A. L., Stall R., Goldhammer H., Egan J. E., Mayer K. H. M. D. ( 2014. ). Resilience as a research framework and as a cornerstone of prevention research for gay and bisexual men: Theory and evidence . AIDS and Behavior , 18 ( 1 ), 1 – 9 . [DOI] [PubMed] [Google Scholar]
- Hosek S. G., Harper G. W., Domanico R. ( 2005. ). Predictors of medication adherence among HIV-infected youth . Psychology, Health and Medicine , 10 , 166 – 179 . doi: 10.1080/1354350042000326584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston E., Fominaya A. W. ( 2015. ). Antiretroviral therapy adherence in a sample of men with low socioeconomic status: The role of task-specific treatment self-efficacy . Psychology, Health & Medicine , 20 , 896 – 905 . doi: 10.1080/13548506.2014.986137 [DOI] [PubMed] [Google Scholar]
- Hu L. T., Bentler P. M. ( 1999. ). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives . Structural Equation Modeling: A Multidisciplinary Journal , 6 ( 1 ), 1 – 55 . [Google Scholar]
- Knight J. R., Shrier L. A., Bravender T. D., Farrell M., Vander Bilt J., Shaffer H. J. ( 1999. ). A new brief screen for adolescent substance abuse . Archives of Pediatrics & Adolescent Medicine , 153 , 591 – 596 . [DOI] [PubMed] [Google Scholar]
- Koenig L. J., Pals S. L., Chandwani S., Hodge K., Abramowitz S., Barnes W., D'Angelo L. ( 2010. ). Sexual transmission risk behavior of adolescents with HIV acquired perinatally or through risky behaviors . JAIDS Journal of Acquired Immune Deficiency Syndromes , 55 , 380 – 390 . doi: 10.1097/QAI.1090b1013e3181f1090ccb1096 [DOI] [PubMed] [Google Scholar]
- Langebeek N., Gisolf E. H., Reiss P., Vervoort S. C., Hafsteinsdottir T. B., Richter C., Sprangers M. A., Nieuwkerk P. T. ( 2014. ). Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: A meta-analysis . BMC Medicine , 12 , 142 . doi: 10.1186/preaccept-1453408941291432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundahl B., Moleni T., Burke B. L., Butters R., Tollefson D., Butler C., Rollnick S. ( 2013. ). Motivational interviewing in medical care settings: A systematic review and meta-analysis of randomized controlled trials . Patient Education and Counseling , 93 , 157 – 168 . doi: 10.1016/j.pec.2013.07.012 [DOI] [PubMed] [Google Scholar]
- MacCallum R. C., Browne M. W., Sugawara H. M. ( 1996. ). Power analysis and determination of sample size for covariance structure modeling . Psychological Methods , 1 , 130 . [Google Scholar]
- MacDonell K., Naar-King S., Huszti H., Belzer M. ( 2013. ). Barriers to medication adherence in behaviorally and perinatally infected youth living with HIV . AIDS and Behavior , 17 , 86 – 93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonell K., Naar-King S., Murphy D. A., Parsons J. T., Harper G. W. ( 2010. ). Predictors of medication adherence in high risk youth of color living with HIV . Journal of Pediatric Psychology , 35 , 593 – 601 . doi: 10.1093/jpepsy/jsp080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonell K., Naar-King S., Murphy D. A., Parsons J. T., Huszti H. ( 2011. ). Situational temptation for HIV medication adherence in high-risk youth . AIDS Patient Care STDS , 25 , 47 – 52 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Bell D., Camacho R., Henry-Reid L., Bell M., Watson C., Rodriguez F. ( 2000. ). Adherence to antiviral drug regimens in HIV-infected adolescent patients engaged in care in a comprehensive adolescent and young adult clinic . Journal of the National Medical Association , 92 , 55 . [PMC free article] [PubMed] [Google Scholar]
- Migneault J. P., Adams T. B., Read J. P. ( 2005. ). Application of the transtheoretical model to substance abuse: Historical development and future directions . Drug and Alcohol Review , 24 , 437 – 448 . doi: 10.1080/09595230500290866 [DOI] [PubMed] [Google Scholar]
- Miller W. R., Rollnick S. ( 2012. ). Motivational interviewing: Helping people change , (3rd ed.) . New York: : The Guilford Press; . [Google Scholar]
- Murphy D. A., Belzer M., Durako S. J., Sarr M., Wilson C. M., Muenz L. R. ; Adolescent Medicine HIV/AIDS Research Network . ( 2005. ). Longitudinal antiretroviral adherence among adolescents infected with human immunodeficiency virus . Archives of Pediatrics & Adolescent Medicine , 159 , 764 – 770 . doi: 10.1001/archpedi.159.8.764 [DOI] [PubMed] [Google Scholar]
- Murphy D. A., Wilson C. M., Durako S. J., Muenz L. R., Belzer M. ( 2001. ). Antiretroviral medication adherence among the REACH HIV-infected adolescent cohort in the USA . AIDS Care , 13 , 27 – 40 . doi: 10.1080/09540120020018161 [DOI] [PubMed] [Google Scholar]
- Naar-King S., Outlaw A. Y., Sarr M., Parsons J. T., Belzer M., MacDonell K., Tanney M., Ondersma S. J. ; Adolescent Medicine Network for HIV/AIDS Interventions . ( 2013. ). Motivational Enhancement System for Adherence (MESA): Pilot randomized trial of a brief computer-delivered prevention intervention for youth initiating antiretroviral treatment . Journal of Pediatric Psychology , 38 , 638 – 648 . doi: 10.1093/jpepsy/jss132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naar-King S., Parsons J. T., Murphy D., Kolmodin K., Harris D. R. ( 2010. ). A multisite randomized trial of a motivational intervention targeting multiple risks in youth living with HIV: Initial effects on motivation, self-efficacy, and depression . Journal of Adolescent Health , 46 , 422 – 428 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naar-King S., Wright K., Parsons J. T., Frey M., Templin T., Ondersma S. ( 2006. ). Transtheoretical model and substance use in HIV-positive youth . AIDS Care , 18 , 839 – 845 . [DOI] [PubMed] [Google Scholar]
- Outlaw A. Y., Naar-King S., Parsons J. T., Green-Jones M., Janisse H., Secord E. ( 2010. ). Using motivational interviewing in HIV field outreach with young African American men who have sex with men: A randomized clinical trial . American Journal of Public Health , 100 ( Suppl 1 ), S146 – S151 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska J. O., Velicer W. F. ( 1997. ). The transtheoretical model of health behavior change . American Journal of Health Promotion , 12 , 38 – 48 . doi: 10.4278/0890-1171-12.1.38 [DOI] [PubMed] [Google Scholar]
- Pruitt Z. ( 2013. ). The determinants of antiretroviral therapy adherence and the relationship of healthcare expenditures to adherence among Florida medicaid-insured patients diagnosed with HIV or AIDS. Graduate Theses and Dissertations, University of South Florida. http://scholarcommons.usf.edu/etd/4834 [Google Scholar]
- Quinn T. C., Wawer M. J., Sewankambo N., Serwadda D., Li C., Wabwire-Mangen F., Meehan M. O., Lutalo T., Gray R. H. ( 2000. ). Viral load and heterosexual transmission of human immunodeficiency virus type 1 . New England Journal of Medicine , 342 , 921 – 929 . [DOI] [PubMed] [Google Scholar]
- Rao D., Feldman B. J., Fredericksen R. J., Crane P. K., Simoni J. M., Kitahata M. M., Crane H. M. ( 2012. ). A structural equation model of HIV-related stigma, depressive symptoms, and medication adherence . AIDS and Behavior , 16 , 711 – 716 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao D., Kekwaletswe T. C., Hosek S., Martinez J., Rodriguez F. ( 2007. ). Stigma and social barriers to medication adherence with urban youth living with HIV . AIDS Care , 19 , 28 – 33 . doi: 10.1080/09540120600652303 [DOI] [PubMed] [Google Scholar]
- Reisner M. S. L., Mimiaga M. J., Skeer M. M., Perkovich M. B., Johnson M. C. V., Safren S. A. ( 2009. ). A review of HIV antiretroviral adherence and intervention studies among HIV–infected youth . Topics in HIV Medicine: A Publication of the International AIDS Society, USA , 17 , 14 . [PMC free article] [PubMed] [Google Scholar]
- Riekert K. A., Drotar D. ( 2000. ). Adherence to medical treatment in pediatric chronic illness: Critical issues and answered questions . In Drotar D. (Ed.), Promoting adherence to medical treatment in chronic childhood illness: Concepts, methods, and interventions . Hillsdale, NJ: : Lawrence Erlbaum Associates; . [Google Scholar]
- Rudy B. J., Murphy D. A., Harris D. R., Muenz L., Ellen J. ( 2009. ). Patient-related risks for nonadherence to antiretroviral therapy among HIV-infected youth in the United States: A study of prevalence and interactions . AIDS Patient Care STDS , 23 , 185 – 194 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S., Stone A. A., Hufford M. R. ( 2008. ). Ecological momentary assessment . Annual Review of Clinical Psychology , 4 , 1 – 32 . [DOI] [PubMed] [Google Scholar]
- Shrout P. E., Bolger N. ( 2002. ). Mediation in experimental and nonexperimental studies: New procedures and recommendations . Psychological Methods , 7 , 422 . [PubMed] [Google Scholar]
- Simoni J. M., Huh D., Wilson I. B., Shen J., Goggin K., Reynolds N. R., Remien R. H., Rosen M. I., Bangsberg D. R., Liu H. ( 2012. ). Racial/Ethnic disparities in ART adherence in the United States: Findings from the MACH14 study . Journal of Acquired Immune Deficiency Syndromes , 60 , 466 – 472 . doi: 10.1097/QAI.0b013e31825db0bd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni J. M., Montgomery A., Martin E., New M., Demas P. A., Rana S. ( 2007. ). Adherence to antiretroviral therapy for pediatric HIV infection: A qualitative systematic review with recommendations for research and clinical management . Pediatrics , 119 , e1371 – e1383 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden L. R., Yamada A.-M. ( 2005. ). Cultural differences in access to care . Annual Review of Clinical Psychology , 1 , 143 – 166 . [DOI] [PubMed] [Google Scholar]
- Stott N. C., Rollnick S., Rees M., Pill R. ( 1995. ). Innovation in clinical method: Diabetes care and negotiating skills . Family Practice , 12 , 413 – 418 . [DOI] [PubMed] [Google Scholar]
- Stricker S. M., Fox K. A., Baggaley R., Negussie E., de Pee S., Grede N., Bloem M. W. ( 2014. ). Retention in care and adherence to ART are critical elements of HIV care interventions . AIDS and Behavior , Suppl 5 , S465 – S475 . [DOI] [PubMed] [Google Scholar]
- Tang J. W., Pillay D. ( 2004. ). Transmission of HIV-1 drug resistance . Journal of Clinical Virology , 30 ( 1 ), 1 – 10 . [DOI] [PubMed] [Google Scholar]
- Viswanathan S., Detels R., Mehta S., Macatangay B. C., Kirk G., Jacobson L. ( 2015. ). Level of adherence and HIV RNA Suppression in the current era of highly active antiretroviral therapy (HAART) . AIDS and Behavior , 19 , 601 – 611 . doi: 10.1007/s10461-014-0927-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley A. L. ( 2001. ). Cultural mistrust and mental health services for african americans: a review and meta-analysis . The Counseling Psychologist , 29 , 513 – 531 . doi: 10.1177/0011000001294003 [Google Scholar]
- Whetten K., Reif S., Whetten R., Murphy-McMillan L. K. ( 2008. ). Trauma, mental health, distrust, and stigma among HIV-positive persons: Implications for effective care . Psychosomatic Medicine , 70 , 531 – 538 . [DOI] [PubMed] [Google Scholar]