Abstract
Haemoptysis is concerning for both patient and healthcare provider and points to the presence of severe underlying lung disease warranting investigation. Approximately 8% of patients with pulmonary tuberculosis (PTB) infection will experience haemoptysis at some point during their life [1;2]. The aetiology of haemoptysis in the setting of PTB is diverse and may occur during active or following prior PTB infection due to pulmonary complications. We describe the case of a 33-year-old female who presented with massive haemoptysis on two separate occasions within a five-month period. Her background history included PTB 6 years prior and subsequent post-TB bronchiectasis with a destroyed left lung, and the development of apical mycetoma's. Despite numerous pre-existing aetiologies that could account for haemoptysis in this patient, on this admission, a newly identified ruptured Rasmussen's aneurysm was identified by angiography and successfully treated with arterial embolization. This report serves to highlight the multitude of reasons for haemoptysis in a patient with post PTB lung destruction and the associated diagnostic challenges that may be present. In particular, we highlight the Rasmussen's aneurysm, a rare entity, as a hidden cause of haemoptysis, where despite extensive parenchymal lung disease identified on chest radiography, specialised imaging is needed to confirm the diagnosis.
1. Background
Tuberculosis (TB), remains a significant health burden in the developing world despite medical and technological advances. As of 2015, South Africa remains in the top 6 of both the worldwide incidence and prevalence of TB, with haemoptysis being a closely associated symptom [3]. Despite 81% of PTB patients achieving cure, haemoptysis may present in the post - PTB patient, and may be related to parenchymal distortion and associated vascular complications associated with prior PTB [3]. This report aims to highlight the numerous aetiologies accounting for haemoptysis in a post-primary PTB patient, with emphasis on the Rasmussen's aneurysm, a rare but established entity with possible life-threatening consequences.
2. Case study
A 32-year-old HIV seronegative female patient, currently working as a research assistant in a healthcare facility presented with a second episode of massive haemoptysis within a 5-month period. She had been diagnosed with microbiologically proven PTB 6 years prior to her admission and completed 6 months of therapy. However, this was complicated by fibro-cavitatory destruction of her left lung with post-TB bronchiectasis (Fig. 1). Over the course of her follow up at the pulmonology clinic, and 2 years prior to this current presentation, she developed apical mycetoma's, which were identified on computed topographical scan of the chest (Fig. 2) and due to evident haemoptysis, she was considered for a left pneumonectomy. Her lung function test showed a restrictive lung pattern with a FEV1 of 64%, a FVC of 67% (FEV1/FVC of 95) and a DLCO of 74. Furthermore, a nuclear medicine split perfusion scan indicated no discernible perfusion to the affected lung. An electrocardiogram and echocardiogram were performed, indicating the presence of pulmonary hypertension.
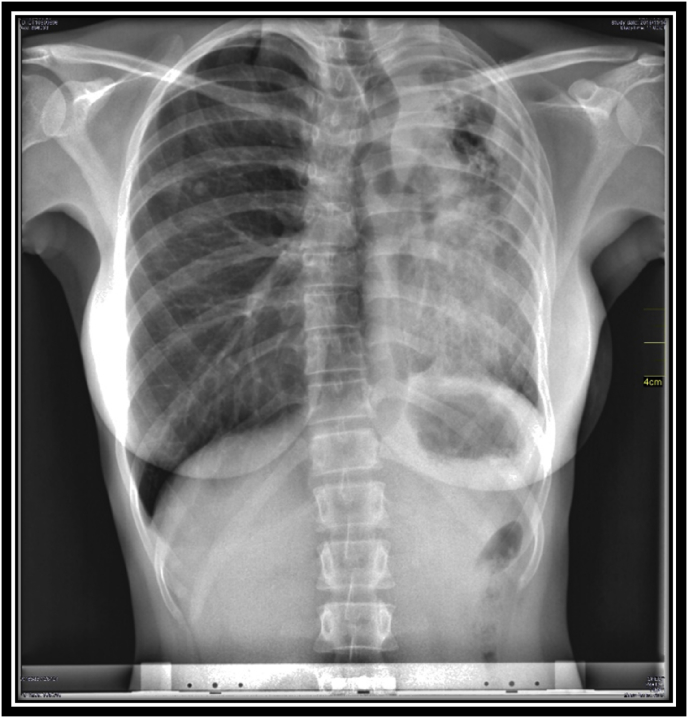
Fig. 1.
Chest X-ray demonstrating mediastinal shift secondary to left lung fibro-cavitatory destruction.
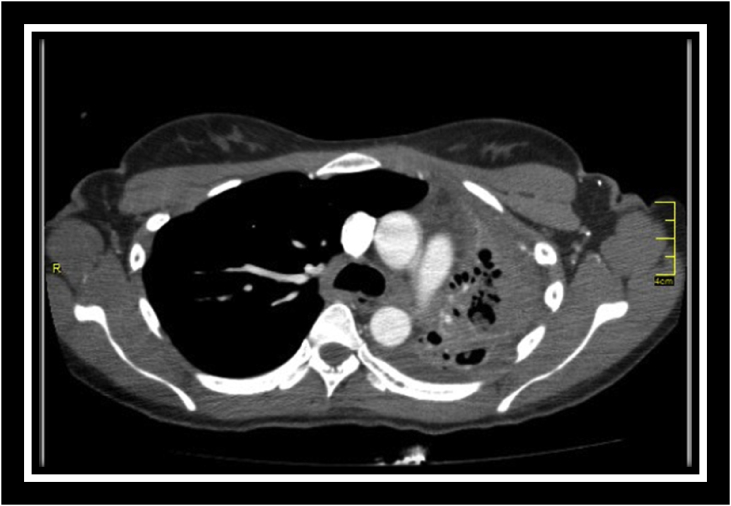
Fig. 2.
CT scan illustrating bronchiectactic changes with sub-centimetre mycetoma's.
On examination during this admission, she was haemodynamically stable with a blood pressure of 127/82 mmHg and a sinus tachycardia of 110 beats/min. She was afebrile, her respiratory rate measured 20 breaths/min and she was a-cyanotic on room air with an oxygen saturation of 93%. Marked digital clubbing and subconjunctival pallor were noted. On respiratory examination, evidence of left sided volume loss was present with decreased expansion of the left chest wall and compensatory hyperinflation of the right. The breath sounds on the right were vesicular in nature but the left side had breath sounds of reduced intensity with amphoric breath sounds and coarse crackles predominantly in the upper zone. No evidence of decompensated cor-pulmonale was present on cardiovascular examination, but pulmonary hypertension and mediastinal shift to the left were evident. A para-sternal heave with a palpable P2 were elicited, as was a loud pulmonary component of the second heart sound. Her apex beat was displaced to the anterior axillary line in the fifth intercostal space with tracheal deviation to the left side. Initial laboratory results showed a microcytic hypochromic anaemia with a haemoglobin of 7.7 g/dL (MCV = 73.8fl; MCHC = 31.4g/dL) and a platelet count of 487 × 109/l. Further haematological work-up demonstrated an iron-deficiency anaemia with a ferritin of 37 μg/l, percentage saturation of 5% and associated morphological features. Her renal and liver function tests were normal. Her C-Reactive Protein was 32mg/L and her INR measured 1.28. An Xpert MTB/RIF assay was performed and was reported as negative for mycobacterium tuberculosis.
She was admitted to medical high care. Transfused 1 unit of packed red blood cells and initiated on oral codeine phosphate. An emergency computed topographical pulmonary angiogram (CTPA) was performed and suggested the presence of a Rasmussen's aneurysm but formal angiography was deemed necessary for confirmation. Direct angiography indicated a pulmonary arterial blush adjacent to the left upper lung cavity confirming the presence of a Rasmussen's aneurysm (Fig. 3). Despite difficult initial ventilation due to her diseased lung, selective right bronchial intubation was performed and successful arterial embolization was achieved. Following 48 hours of post-procedural monitoring in a high care setting, she was stepped down to the general medical ward where after 6 days of remaining haemoptysis free, she was discharged home and is currently awaiting left sided pneumonectomy.
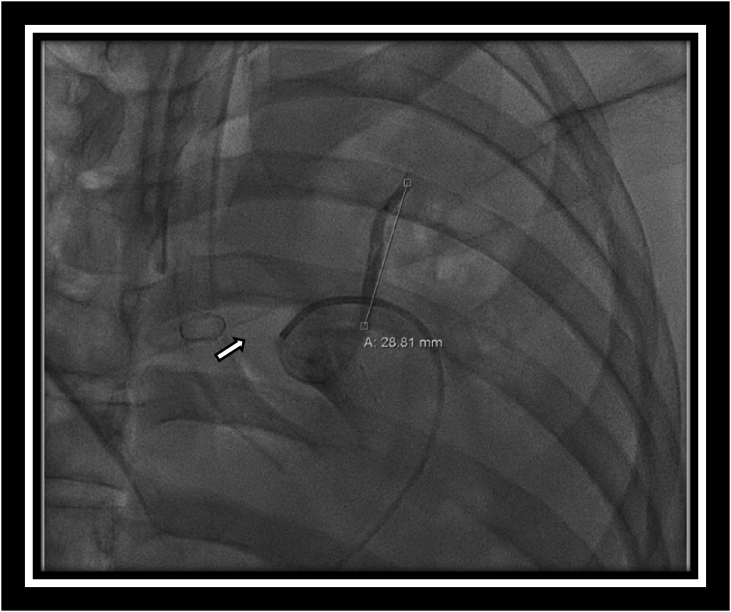
Fig. 3.
Direct angiography during coil embolization illustrating arterial blush secondary to ruptured aneurysm.
3. Discussion
Haemoptysis, the coughing up or expectoration of blood, is a worrying symptom and usually serves as an indicator of severe underlying lung pathology. Massive haemoptysis is a term reserved for a large volume of blood expectorated within a 24-h period, with the potential for morbidity and mortality stemming from haemodynamic instability, airway compromise and gaseous exchange abnormalities. There is no universal consensus on the precise volume of blood to define massive haemoptysis, and the most acceptable quantifiable definition refers to values ranging from 200 to 1000 ml over a 24-h period, to a bleeding rate of 100ml per hour [4].
South Africa is a WHO high burden country [3], but there is a dearth of literature assessing the prevalence of post-PTB related lung disease in South Africa, however, experts in South African pulmonary medicine believe PTB to be a significant contributor to chronic lung disease in the region, and is an important cause of haemoptysis. Active PTB may present with both trivial and significant degrees of haemoptysis and a sizeable proportion of haemoptysis is secondary to active PTB, as highlighted by an older South African case series where up to 73% of massive haemoptysis was attributable to active TB and this is supported by a recent study of HIV positive patients with diabetes who were 31 times more likely to have active TB if haemoptysis was a presenting symptom [5,6]. Although haemoptysis is strongly associated with active pulmonary TB infection, it may also present following resolution of acute disease due to subsequent pulmonary complications [1,2]. Unfortunately there is no data assessing the prevalence of haemoptysis amongst patients with active PTB compared to those with haemoptysis from post-primary PTB pulmonary complications. Thus even though the aetiologies of haemoptysis are varied and multi-systemic, in a high TB burden setting, its presence should always alert the physician to the possibility of active PTB.
Haemoptysis originates from local blood supply, being derived from both pulmonary and bronchial arteries. The pulmonary arterial system carries the entirety of the cardiac output through a relatively low-pressure system for oxygenation whilst the bronchial artery and its tributaries carry oxygenated blood to the lung parenchyma, and despite a much smaller volume are under high systemic pressures. In 90% of cases with massive haemoptysis, bleeding originates from these high-pressure bronchial artery networks [7]. Numerous possibilities are present when considering the aetiology of haemoptysis in the post-primary TB patient. Although the pathogenesis of haemoptysis, as a sequelae of PTB, occurs most commonly due to destruction and structural remodelling of the lung parenchyma and its vasculature, other concomitant disease processes may occur in these patients resulting in haemoptysis. This presents a diagnostic challenge to the physician when determining the cause in these patients, as well as, the necessary therapy. As such, despite clearly evident parenchymal lung destruction, multiple other possibilities may still explain haemoptysis.
Up to 40–60% of patients with prior pulmonary TB suffer from airway and parenchymal destruction with fibrosis and cavitation, the hallmark being apical and posterior traction bronchiectasis with upper lobe fibro-cavitation and mediastinal shift toward the diseased lung [8]. Post tuberculous fibro-cavitatory disease and bronchiectasis leads to irreversible damage and dilation of the airways and its associated vasculature. The bronchial vasculature becomes hypertrophied, ectatic and exposed. The hyper vascularity, disrupted architectural support and open exposure of these vessels make them easily prone to bleeding and these abnormalities account for the majority of clinically evident haemoptysis in the post-PTB patient [9].
Significant post-primary PTB parenchymal lung damage with cavitation serves as the ideal milieu for fungal colonisation and infection with pathogenic species such as Aspergillus spp. Pulmonary aspergillosis may manifest as cavities containing fungal balls referred to as mycetoma's or as chronic cavitatory or fibrosing aspergillosis [10]. Occurring in 7–11% of patients after previous PTB, the presence of both a mycetoma on radiological imaging (with CT demonstrating greater diagnostic accuracy than a standard chest x-ray [11]) as well as serum aspergillus antibodies leads to an increase incidence of clinically evident haemoptysis [12]. Erosion of adjacent vasculature and intra-cavitatory bleeding serve as the basis for haemoptysis in these patients [6].
Mycobacterial mediated granulomatous inflammation and lymphadenitis may progress to calcification of tissue known as broncholiths. The presentation of post-tuberculous broncholiths may be vague but rarely calcified nodes may disrupt and erode adjacent bronchi and when bronchial vascular networks are involved, haemoptysis may be apparent [8,13]. Although rare, lung cancer and TB may co-exist, with post-primary PTB lung scarring predisposing to the growth of malignancy (scar carcinoma), as such haemoptysis with suspicious lesions on radiological imaging should not be overlooked and require further evaluation [8,13].
Direct vascular involvement by PTB may also occur, as both arteries and veins may demonstrate an active mycobacterial induced necrotising granulomatous vasculitis, resulting in inflammation with aneurysmal changes, broncho-pulmonary and arterio-venous connections [8]. The Rasmussen's aneurysm, refers to a pseudo-aneurysm of the pulmonary artery adjacent to a tuberculous cavity. Named after Danish physician Fritz Valdemar Rasmussen, an autopsy series reported a prevalence of up to 5% in patients with cavitatory tuberculous disease [14]. Its development stems from granulomatous inflammation of the arterial wall leading to vascular remodelling involving fibrin and subsequent caseation, with progressive weakening of vascular lining with aneurysmal dilation [2,8]. Subsequent vessel wall rupture or leaking from a Rasmussen's aneurysm, may result in massive haemoptysis carrying a mortality exceeding 50% [15]. Chest radiography and clinical examination may highlight the presence of post-TB sequelae but may not account for vascular lesions, and if suspected, radiological or formal angiography is necessary to confirm the diagnosis.
In the management of haemoptysis in a post primary-TB patient, recognising the potential for life-threatening haemoptysis is critical, with emphasis on monitoring the patients bleeding volume, bleeding rate, and underlying vital parameters. Presently there is a lack of local guidelines or recommendations addressing post-PTB haemoptysis, as such management is guided by hospital protocols based on international literature. Airway protection and haemodynamic stability is the initial step prior to investigation, especially given that this group of patients have poor underlying lung reserve [16]. Should on-going haemoptysis occur; further investigation is warranted beyond that of the gross abnormalities identified on chest radiograph. Fibre-optic bronchoscopy benefits those with active haemorrhage in an adjacent bleeding bronchial vessel. Direct visualisation of the bleed may be possible and controlling measures can be immediately utilised such as direct coagulation or tamponade effects [17,18]. Aneurysmal lesions require detailed characterisation, as such, CTPA is utilised with a sensitivity of 80% reported [19]. Diagnostic uncertainty may require direct angiography, its use is of particular value when arterial embolization is indicated, such as in our patient [17,18]. While TB arteritis due to necrotising granulomatous change plays the key role in development, vessel biopsy and microscopic visualisation is neither practical nor feasible. Arterial embolization carries up to a 90% success rate in cessation of haemoptysis but repeated embolization's may be required. If unsuccessful, surgical lung resection may be required, and while elective resection may carry a mortality of 18%, arterial embolization is a critical bridge in therapy [7,20]. Emergency lung resection for life threatening haemoptysis has been utilised, but mortality in this setting is up to 40%. As such, initial arterial angiography with the intention and utilisation of embolization remains a mainstay of therapy in the emergency setting particularly given that it may serve as a bridge to definitive therapy in patients with significant peri-operative challenges [17]. Interventional methods remain the mainstay in therapy for massive haemoptysis but case reports and series highlight the use of chest radiation therapy in patients with haemoptysis from a mycetoma [21], currently a trial in South Africa is underway to further validate its use in inoperable patients. In resource limited settings, the necessary diagnostics tools and management expertise are unlikely to be available, the mortality for untreated massive haemoptysis exceeds 50% [22], thus patients' with massive haemoptysis should be resuscitated and urgently referred to a centre were the required diagnostic modalities and management expertise are available.
4. Conclusion
Haemoptysis in a patient with previous PTB is a diverse and concerning entity. In the setting of a grossly abnormal chest radiogram, vascular lesions may be missed. Due to the potential for massive haemoptysis to cause haemodynamic instability and airway compromise, detailed imaging is necessary. The Rasmussen's aneurysm is a known, albeit rare, complication of PTB as a result of granulomatous vasculature inflammation and should be considered in massive haemoptysis in a post-primary TB patient even in the setting of extensive radiographic parenchymal abnormalities.
Author contributions
Both authors contributed to the discussions around this report. UFS wrote the initial draft of the manuscript that was reviewed by FS.
Funding
None.
Conflicts of interest
None.
Acknowledgements
The authors would like to thank the patient in this case report.
References
- 1.Middleton J.R., Sen P., Lange M. Death-producing haemoptysis in tuberculosis. Chest. 1977;72:601–604. doi: 10.1378/chest.72.5.601. [DOI] [PubMed] [Google Scholar]
- 2.Leung A.N. Pulmonary tuberculosis: the essentials. Radiology. 1999;210(2):307–322. doi: 10.1148/radiology.210.2.r99ja34307. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organisation: reportGlobal TB Report (Country Fact Sheet and High Burden Country List in the Post 2015 Era.).
- 4.Ibrahim W.H. Massive haemoptysis: the definition should be revised. Eur. Respir. J. 2008;32:1131–1132. doi: 10.1183/09031936.00080108. [DOI] [PubMed] [Google Scholar]
- 5.Knott-Craig C.J., Oostuizen G., Rossouw G., Joubert J.R., Barnard P.M. Management and prognosis of massive hemoptysis: recent experience with 120 patients. J. Thorac. Cardiovasc. Surg. 1993;105:394–397. [PubMed] [Google Scholar]
- 6.Berkowitz N., Okorie A., Goliath R., Levitt N., Wilkinson R.J., Oni T. The prevalence and determinants of active tuberculosis among diabetes patients in Cape Town, South Africa, a high HIV/TB burden setting. Diabetes Res. Clin. Pract. 2018;138:16–25. doi: 10.1016/j.diabres.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit. Care Med. 2000;28:1642–1647. doi: 10.1097/00003246-200005000-00066. [DOI] [PubMed] [Google Scholar]
- 8.Kim H.Y., Song K.S., Goo J.M., Lee J.S., Lee K.S., Lim T.H. Thoracic sequelae and complications of tuberculosis. Radiographics. 2001;21:839–860. doi: 10.1148/radiographics.21.4.g01jl06839. [DOI] [PubMed] [Google Scholar]
- 9.Song J.W., Im J.G., Shim Y.S., Park J.H., Yeon K.M., Han M.C. Hypertrophied bronchial artery at thin-section CT in patients with bronchiectasis: correlation with CT angiographic findings. Radiology. 1998;208:187–191. doi: 10.1148/radiology.208.1.9646812. [DOI] [PubMed] [Google Scholar]
- 10.Denning D.W. Chronic aspergillosis. In: Latge J.P., Steinbach W.J., editors. Aspergillus fumigatus and Aspergillosis. ASM Press; Washington: 2009. [Google Scholar]
- 11.Adil A., el Amraoui F., Kadiri R. Role of computed tomography in pulmonary aspergillosis. 20 cases. Presse Med. (Paris, France 1983) 2001;30:621–625. [PubMed] [Google Scholar]
- 12.Chu C.M., Woo P.C., Chong K.T., Leung W.S., Chan V.L., Yuen K.Y. Association of presence of Aspergillus antibodies with hemoptysis in patients with old tuberculosis or bronchiectasis but no radiologically visible mycetoma. J. Clin. Microbiol. 2004;42:665–669. doi: 10.1128/JCM.42.2.665-669.2004. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.De S., De S. Broncholithiasis. Lung India. 2008;25:152–154. doi: 10.4103/0970-2113.45280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santelli E.D., Katz D.S., Goldschmidt A.M., Thomas H.A. Embolization of multiple Rasmussen aneurysms as a treatment of haemoptysis. Radiology. 1994;193:396–398. doi: 10.1148/radiology.193.2.7972750. [DOI] [PubMed] [Google Scholar]
- 15.Garzon A.A., Gourin A. Surgical management of massive hemoptysis. A ten-year experience. Ann. Surg. 1978;187(3):267–271. doi: 10.1097/00000658-197803000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lordan J.L., Gascoigne A., Corris P.A. The pulmonary physician in critical care. Illustrative case 7: assessment and management of massive haemoptysis. Thorax. 2003;58:814–819. doi: 10.1136/thorax.58.9.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernando H.C., Stein M., Benfield J.R., Link D.P. Role of bronchial artery embolization in the management of hemoptysis. Arch. Surg. 1998;133(8):862–865. doi: 10.1001/archsurg.133.8.862. [DOI] [PubMed] [Google Scholar]
- 18.Bruzzi J.F., Rémy-Jardin M., Delhaye D. Multi-detector row CT of hemoptysis. Radiographics. 2006;26(1):3–22. doi: 10.1148/rg.261045726. [DOI] [PubMed] [Google Scholar]
- 19.Yoon W., Kim Y.H., Kim J.K., Kim Y.C., Park J.G., Kang H.K. Massive hemoptysis: prediction of nonbronchial systemic arterial supply with chest CT. Radiology. 2003;227:232–238. doi: 10.1148/radiol.2271020324. [DOI] [PubMed] [Google Scholar]
- 20.Sopko D.R., Smith T.P. Bronchial artery embolization for hemoptysis. Semin. Intervent. Radiol. 2011;28(1):48–62. doi: 10.1055/s-0031-1273940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falkson C., Sur R., Pacella J. External beam radiotherapy: a treatment option for massive haemoptysis caused by mycetoma. Clin. Oncol. (Roy. Coll. Radiol. (G.B.)) 2002;14:233–235. doi: 10.1053/clon.2002.0063. [DOI] [PubMed] [Google Scholar]
- 22.Larici A.R., Franchi P., Occhipinti M., Contegiacomo A., del Ciello A., Calandriello L., Storto M.L., Marano R., Bonomo L. Diagnosis and management of hemoptysis. Diagn. Interventional Radiol. 2014;20:299–309. doi: 10.5152/dir.2014.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]