Abstract
Background
This study aims to investigate the role of miR-646 in hypoxia conditions in human periodontal ligament cells (hPDLCs), exploring the effect of hypoxia on hPDLCs proliferation and apoptosis. In addition, this study aimed to explore the potential mechanism of miR-646/IGF-1 signaling in hPDLCs in hypoxia conditions.
Material/Methods
hPDLCs (fifth passage) cultured by the tissue culture method were randomly assigned to the severe hypoxia (1% O2) group, the slight hypoxia (5% O2) group or the control (21% O2) group. Then reverse transcription quantitative real-time polymerase chain reaction and western blot analysis were used to detect the mRNA and protein expression of miR-646 and IGF-1. hPDLCs infected with lentivirus (LV)-pre-miR-646 or LV-anti-mR-646, and negative controls were cultured. MTT assay, caspase-3 ELISA assay, and wound healing assay were performed to evaluate how miR-646 was influenced by hypoxia. In addition, the relationship between miR-646 and IGF-1 was explored.
Results
The expression of miR-646 was downregulated and IGF-1 was upregulated in hypoxia conditions. MiR-646 was able to suppress hPDLCs proliferation and promote apoptosis in hypoxia conditions. The mRNA and protein expressions of IGF-1 were downregulated when miR-646 was overexpressed and upregulated when miR-646 was downregulated.
Conclusions
This finding identified a significant role of miR-646 in hPDLCs in suppressing cell proliferation and promoting apoptosis by inversely regulating IGF-1 expression. Meanwhile, the regulation of hPDLCs in hypoxia may be through the miR-646/IGF-1 signaling pathway, probably serving as a promising therapeutic target for periodontal diseases.
MeSH Keywords: Cell Hypoxia, Insulin-Like Growth Factor 1, MicroRNAs
Background
The periodontal ligament (PDL) is an important part of periodontium that not only attaches the tooth to the surrounding alveolar bone but also acts as a buffer by providing resistance to mechanical force [1,2]. Human periodontal ligament cells (hPDLCs) are the most numerous cells in the periodontium and play important roles in principal fibers and dental cement production, cell conglutination, extracellular matrix synthesis, mineralization, and transportation [3–5]. Oxygen is very important to functional metabolism as well as body growth and development [6]. In the oral cavity, the local periodontium hypoxia microenvironment happens in many situations, such as smoking [7], orthodontic treatment [8], and several periodontitis conditions [9].
MiR-646 was first found to be expressed in human cerebral cortical white and grey matter. MiR-646 belongs to the miR-15/107 gene group and is mammalian specific, only appearing in humans and chimpanzees [10]. Accumulation evidence has shown that the miR-15/107 gene group members have functions in cell division, metabolic pathways, and angiogenesis. In addition, miR-103 and miR-107 can be included in the group of 7 miRNAs (miR-30a-5p, miR-30b,c,d, miR-103/107, miR-191, and miR-195) that are possibly involved in neuronal migration, through the regulation of BDNF expression in human cortex. MiR-107 dysfunction may lead to neurodegeneration, cardiovascular dysfunction, neoplasia, etc. [11]. However, the possible relationship between miR-646 and hypoxia in hPDLCs has not yet been reported.
Insulin-like growth factors (IGFs) includes several family members such as IGF-1 and IGF-2 [12]. IGF-1 is involved in several kinds of cells and tissues. It plays important roles in cell proliferation, differentiation, survival, and cell cycle progression [13–16]. IGF-2 plays roles mainly during prenatal development [17]. Human periodontal ligament has been found to express the IGF-1 receptor, indicating that human periodontal ligament is able to stimulate IGF-1 [18]. Previous studies have reported that IGF-1 could enhance hPDLCs survival by inducing antiapoptotic molecules and downregulating proapoptotic molecules [19]. However, the specific molecular mechanism between IGF-1 and hypoxia in hPDLCs is still unknown.
Therefore, the present study aimed to investigate the role of miR-646 in hypoxia conditions in hPDLCs, exploring its impact on hPDLCs proliferation and apoptosis. Furthermore, we explored the relationship between miR-646 and IGF-1 underlying this process, to provide a better understanding of the etiology of periodontal diseases and find potential treatment for it.
Material and Methods
Cell culture
All research protocol was approved by the College of Clinical Medicine of Henan University of Science and Technology. The hPDLCs were isolated and cultured according to previous published protocols [20]. We obtained teeth from premolar teeth extracted from healthy patients (mean 14 years old) for orthodontic treatment. After extraction, the teeth were repeatedly washed with sterile PBS and placed into Dulbecco’s Modified Eagle’s medium (DMEM; Gibco-Invitrogen, Carlsbad, CA, USA) containing 5% fetal bovine serum (FBS; Thermo Scientific). Then the teeth were put on a super clean bench to obtain the periodontal ligament tissues from the middle third of the root surface. Subsequently, tissues were cultured in a T25 flask in DMEM (Gibco-Invitrogen, Carlsbad, CA, USA) containing 10% v/v FBS (Thermo Scientific), 50 U/mL penicillin, and 50 mg/mL streptomycin (Gibco-Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified atmosphere of 5% CO2 incubator. After the cells migrated from the tissue and became confluent, they were detached with 0.25% trypsin-EDTA and subcultured at a 1: 3 ratio. In each experiment, cells at the fifth to seventh passages were used.
Cell identification
The hPDLCs were fixed with 4% paraformaldehyde. After first incubation with vimentin/keratin antibody (Cell Signaling Technology, Danvers, MA, USA, 1: 100), fluorescent secondary antibody (Santa Curz Biotechnology, Santa Cruz, CA, USA) was added. Then the cells were washed and stained with propidium iodide (Santa Curz Biotechnology). Fluorescence was analyzed with a Zeiss Axiophot Photomicroscope (Carl Zeiss, Oberkochen, Germany).
Hypoxic mimic condition
The hPDLCs were randomly divided into 3 groups according to different hypoxia conditions: severe hypoxia group, 1% O2 content; slight hypoxia group, 5% O2 content; and the control group, 21% O2 content. Hypoxic mimic condition was generated through a 3-gas (CO2/O2/N2) incubator (NuAire Inc. Plymouth, MN, USA) while the normal O2 condition was generated in a common CO2 incubator (NuAire, Inc., Plymouth, MN, USA).
Total RNA extraction and quantitative real-time PCR
We investigated the mRNA expression of miR-646 and IGF-1 by quantitative real-time PCR (RT-qPCR). The hPDLCs in various O2 conditions were harvested on 7th day and then total RNA was isolated using TRIzol Reagent® (Molecular research Center, Cincinnati, Ohio, USA) according to the manufacturer’s instructions. From 1 mg total RNA, complementary DNA was synthesized using DyNAmoTM cDNA Synthesis Kit (Finnzymes, Genesearch, Australia) according to the manufacturer’s instructions. RT-qPCR was performed on an ABI Prism 7300 Thermal Cycler (Applied Biosystems, Australia) with SYBR Green detection reagent. Each sample was constructed in triplicate. The mean cycle threshold (Ct) value of each target gene was normalized against Ct value of GAPDH (for mRNA) or U6 SnRNA (for miRNAs). The relative expression was calculated using the following formula: 2−(normalized average Cts) ×104. The results are shown as fold-change values relative to the control group. The oligonucleotide sequences were as follows: miR-646, forward 5′-GAAGCAGCTGCCTCTGAGC-3′, reverse 5′-CAGAGCGCCAGCGAGGAGCC-3′; IGF-1, forward 5′-CATGCCT GCTCAGAAGGGTA-3′, reverse 5′-GCCTCTGATCCTTGAGGTGA-3′; GAPDH, forward 5′-GAAGGTGAAGGTCGGAGTC-3′, reverse 5′-GAAGATGGTGATGGGATTTC-3′.
Protein extraction and western blot analysis
We extracted proteins from hPDLCs in different O2 conditions and investigated the protein expression by using western blot analysis. Cells were lysed in lysis buffer (2 mM Tris HCl, pH 7.5, 15 mM NaCl, 0.1 mM Na2EDTA, 0.1 mM EGTA, 0.1% Triton, 0.25 mM sodium pyrophosphate, 0.1 mM β glycerophosphate, 0.1 mM Na3VO4, 0.1 μg/mL leupeptin) and resolved in 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). Then they were transferred onto an Immobilon-P membrane (Millipore, Billerica, MA, USA). The membrane was blocked with 5% nonfat milk in PBST for 1 hour at room temperature. The membrane was probed with primary antibody against IGF (1: 2000; Abcam, Cambridge, UK)) or GAPDH (1: 5000, Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and was incubated overnight at 4°C. After extensive washing, the membrane was incubated with secondary antibody conjugated with horseradish peroxidase (1: 10 000; Santa Cruz Biotechnology, Inc.) for 1 hour at room temperature. Odyssey Infrared Imaging System (LI COR, Inc) was used to visualize protein bands. The relative intensity of protein bands compared with GAPDH was quantified using ImageJ software version 1.47 (National Institutes of Health, Bethesda, MD, USA).
Cell infection
Lentivirus (LV)-pre-miR-646, LV-anti-mR-646 and their negative controls were purchased from GenePharma (Shanghai, China). The cells were cultured in a normal medium for 24 hours and then cultured in a medium containing Polybrene (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for cell infection. Then hPDLCs were infected with pre-miR-646, anti-miR-646, or their negative controls unit overnight. After that, the old medium was discarded and a new medium without polybrene was added and incubated overnight. Stable clones were selected with puromycin dihydrochloride (Santa Cruz Biotechnology, Inc.).
Plasmids constructs and dual-luciferase reporter assay
The cDNA fragment of 3′-UTR of IGF-1 containing the putative binding site of miR-646 was subcloned into a pGL3 luciferase promoter vector (Promega, Madison, WI, USA). The cells which firmly expresses pre-miR-646 were transfected with 0.1 μg of pGL3-IGF-1-3′-UTR and incubated for 48 hours. The cells were collected and lysed. The Dual-Luciferase Reporter assay kit (Promega) was used to quantify the luciferase activities according to the manufacturer’s instructions.
Cell proliferation assay
Cell proliferation was detected by 3-(4,5)-dimethylthiahiazol(-z-y1)-3,5-di-phenytetrazo-liumromide (MTT; Amresco, USA) assay. The hPDLCs infected with pre-miR-646, anti-miR-646, or their negative controls were seeded in 96-well culture plates according to their corresponding circumstance and 20 μL MTT solution (5 g/L) was added to each test well from 0 to 96 hours after cultivation. Subsequently, cultivation was continued for another 4 hours at 37°C. Then the culture solution was discarded, and 20 μL DMSO was added to each test well. The optical density (OD) of each tested well was measured with a microtiter plate reader (Thermo Electron Corporation, Vantaa, Finland).
Caspase-3 ELISA assay
Cell apoptosis was detected by caspase-3 ELISA assay. The hPDLCs infected with pre-miR-646, anti-miR-646, or their negative controls were seeded in 96-well culture plates according to their corresponding circumstance. 48 hours later, by calculating the activity of caspase-3 using the kit (Aibosi, Shanghai, China) according to manufacturer’s instructions, apoptosis of hPDLCs was detected. OD values were measured using a microplate reader (Bio-Rad).
Cell apoptosis assay
The hPDLCs infected with pre-miR-646, anti-miR-646, or their negative controls were seeded in 96-well culture plates according to their corresponding circumstance. The hPDLCs were harvested 48 hours later and resuspended in fixation fluid. We added 2 mL propidium iodide and 5 mL Annexin VeFIFC to 500 mL cell suspensions. Flow cytometry was used to determine cell apoptosis (EPICS, XL-4, Beckman, CA, USA).
Statistical analysis
All the experiments were done in triplicate, and the results are given as the mean ± standard deviation of 3 independent experiments. Statistical comparisons were done using the single factor analysis of variance (ANOVA) and a paired t-test. All procedures were done using SPSS 19.0 (SPSS Inc., Chicago, IL, USA) with P<0.05 considered statistically significant.
Results
hPDLCs identification
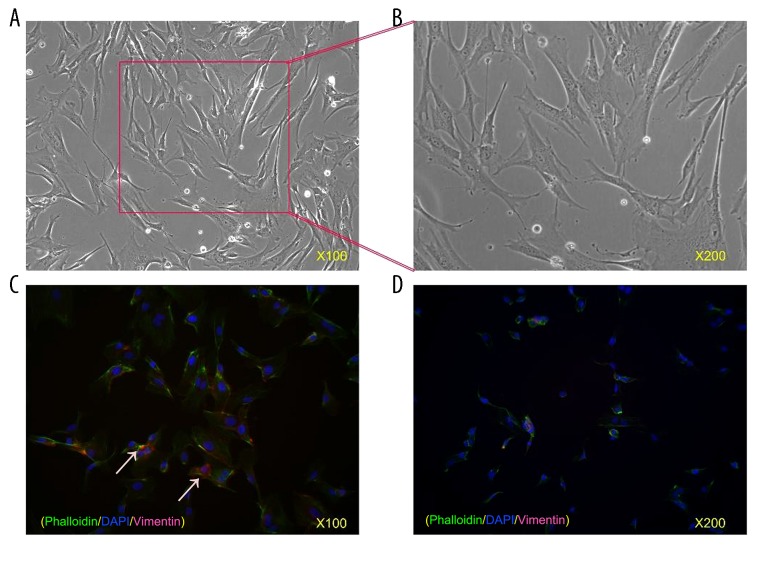
The cultivated hPDLCs were arranged in a sarciniform or swirl pattern and were fusiform in shape (Figure 1A). We first identified the immunofluorescence results of hPDLCs, finding that the cytoplasm of hPDLCs with a yellow-brown color was positive for vimentin (Figure 1B), while keratin was not found in the cytoplasm (Figure 1C). These results indicated that the hPDLCs were mesenchymal cells derived from the embryonic mesoderm.
Figure 1.
Cultivation and identification of hPDLCs. (A) Fifth passage of hPDLCs. Cells were arranged in a sarciniform or swirl pattern and were fusiform in shape. Original magnification, 100×. (B) Fifth passage of hPDLCs. Original magnification, 200×. (C) Immunofluorescence strains of hPDLCs. Vimentin was found in the cytoplasm with a red color whereas keratin was not found in hPDLCs, indicating hPDLCs are mesenchymal cells derived from the embryonic mesoderm. Original magnification, 100×. (D) Immunofluorescence strains of hPDLCs. Original magnification, 200×.
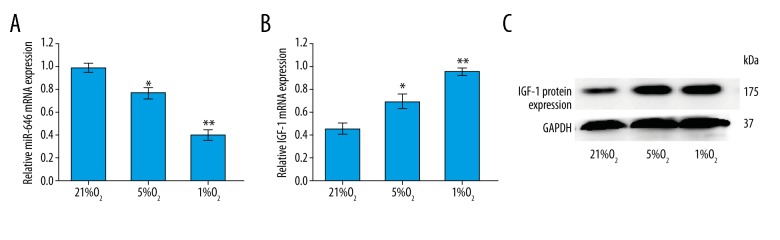
Downregulated expression of miR-646 and upregulated expression of IGF-1 in hypoxia conditions
RT-qPCR results showed that compared with the control group, the mRNA expression of miR-646 in the hypoxia groups was markedly decreased and IGF-1 significantly increased (Figure 2A, 2B). Similarly, the relative protein expression of IGF-1 was upregulated compared with the control group with regards to hypoxia conditions (Figure 2C). These observations showed that in hypoxia conditions, the expression of miR-646 was downregulated and the expression of IGF-1 was upregulated.
Figure 2.
Downregulated expression of miR-646 and upregulated expression of IGF-1 in hypoxia conditions. (A) The downregulation of miR646 mRNA in hypoxia conditions was analyzed by RT-qPCR (** P<0.01, * P=0.02). (B) The upregulation of IGF-1 mRNA in hypoxia conditions was analyzed by RT-qPCR (** P<0.01, * P=0.03). (C) The results of western blot shown IGF-1 protein expression was significantly increased.
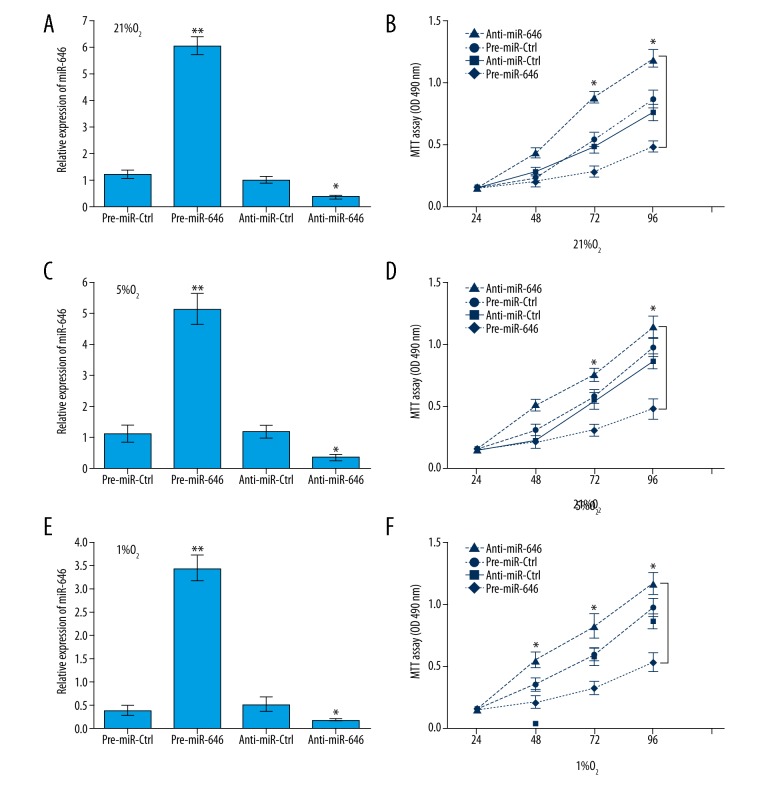
miR-646 suppresses proliferation of hPDLCs in hypoxia conditions
Considering the downregulated expression of miR-646 in hPDLCs in hypoxia conditions, we investigated the function of miR-646 in hPDLCs by infecting with pre-miR-646, anti-miR-646, or their negative control units. The expression of miR-646 was significantly upregulated or downregulated in hPDLCs (Figure 3A, 3C, 3E). MTT assay results showed that hPDLCs with miR-646 that was upregulation significantly suppressed the proliferation in hypoxia conditions from the 0 to the 96 hours. On the contrary, hPDLCs with miR-646 that was downregulation remarkedly promoted the proliferation in hypoxia conditions (Figure 3B, 3D, 3F). In each group, the proliferation of hPDLCs was increased in a time-dependent manner and the growth rate of hPDLCs was decreased by degree with the reduction of O2 content. These data reveal that miR-646 suppressed hPDLCs proliferation in hypoxia conditions.
Figure 3.
RT-qPCR analysis of miR-646 expression in (A) 21% O2 condition (** P<0.01 pre-miR-646 vs. pre-miR-Ctrl, * P=0.02 anti-miR 646 vs. anti-miR-Ctrl); (C) 5% O2 condition (** P<0.01 pre-miR-646 vs. pre-miR-Ctrl, * P=0.02 anti-miR-646 vs. anti-miR-Ctrl); and (E) 1% O2 condition (** P<0.01 pre-miR-646 vs. pre-miR-Ctrl, * P=0.02 anti-miR-646 vs. anti-miR-Ctrl). The hPDLCs proliferation was significantly suppressed with miR-646 overexpression and remarkedly promoted with miR-646 downregulation in (B) 21% O2 condition (* P=0.03 pre-miR-646 vs. pre-miR-Ctrl, * P=0.02 anti-miR-646 vs. anti-miR-Ctrl); (D) 5% O2 condition (* P=0.03 pre-miR-646 vs. pre-miR-Ctrl, * P=0.02 anti-miR-646 vs. anti-miR-Ctrl); and (F) 1% O2 condition (* P=0.02 pre-miR-646 vs. pre-miR-Ctrl, * P=0.02 anti-miR-646 vs. anti-miR-Ctrl).
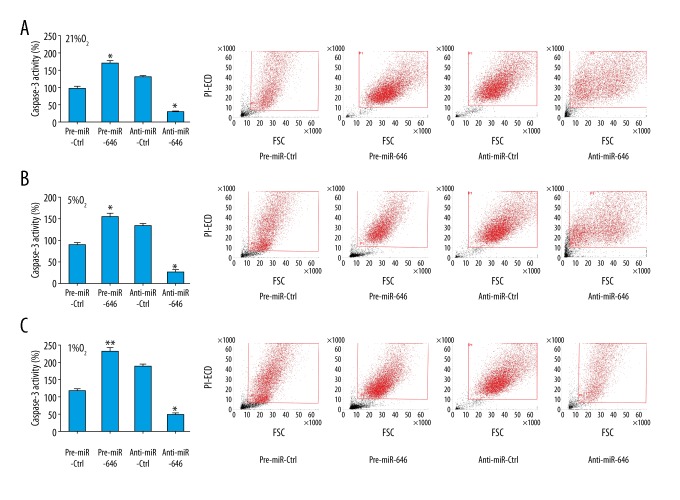
miR-646 promotes apoptosis of hPDLCs in hypoxia conditions
The relative caspase-3 activity was decreased when miR-646 was downregulated and increased when miR-646 was upregulated in hypoxia conditions by ELISA assay. In addition, cell apoptosis induction was observed in hPDLCs infected with pre-miR-646, anti-miR-646, or their negative controls by using flow cytometry analysis. The results were consistent with the caspase-3 ELISA assay (Figure 4). These results indicated that miR-646 promotes hPDLCs apoptosis in hypoxia conditions.
Figure 4.
MiR-646 promote apoptosis of hPDLCs in hypoxia conditions. The relative caspase-3 activity was decreased with miR-646 downregulation and increased with miR-646 overexpression in (A) 21% O2 condition (* P=0.03 pre-miR-646 vs. pre-miR-Ctrl, * P=0.02 anti-miR-646 vs. anti-miR-Ctrl); (B) 5% O2 condition (* P=0.03 pre-miR-646 vs. pre-miR-Ctrl, * P=0.02 anti-miR-646 vs. anti-miR-Ctrl); and (C) 1% O2 condition (* P<0.01 pre-miR-646 vs. pre-miR-Ctrl, * P=0.02 anti-miR-646 vs. anti-miR-Ctrl). The results of flow cytometry analysis results were consistent with the caspase-3 ELISA assay.
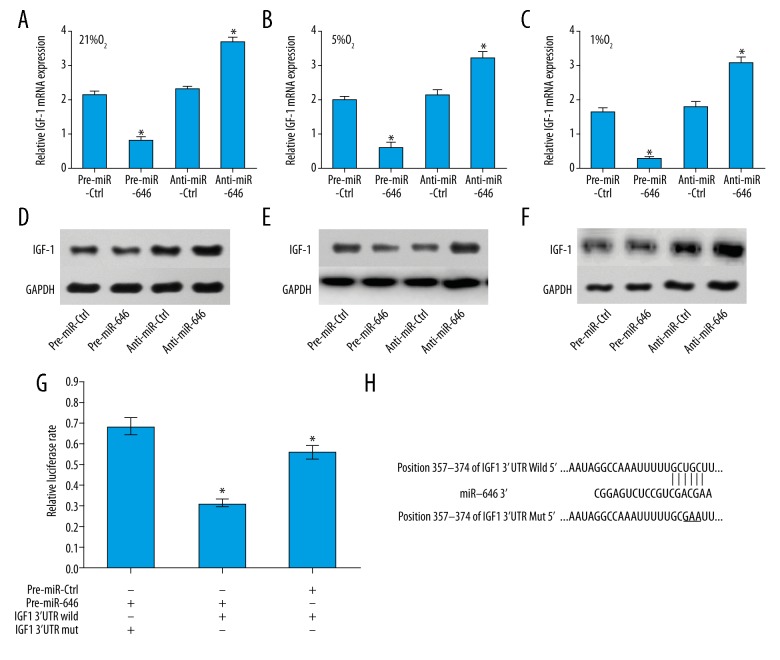
IGF-1 was inversely regulated by miR-646
To investigate the potential interaction between miR-646 and IGF-1 in hPDLCs, we detected the mRNA and protein expressions of IGF-1 when miR-646 was overexpressed or downregulated in different O2 conditions. We found that the mRNA expression of IGF-1 was upregulated when miR-646 was decreased whereas it was downregulated when miR-646 was increased (Figure 5A–5C). The results for protein expression of IGF-1 were similar to the results for mRNA expressions (Figure 5D–5F). These results indicated that IGF-1 was inversely regulated by miR-646.
Figure 5.
MiR-646 inversely regulated IGF-1 mRNA and protein expression. The mRNA expression of IGF-1 was upregulated with miR-646 downregulation and downregulated with miR-646 overexpression in (A) 21% O2 condition (* P=0.03 pre-miR-646 vs. pre-miR-Ctrl, * P=0.02 anti-miR-646 vs. anti-miR-Ctrl); (B) 5% O2 condition (* P=0.03 Pre-miR-646 vs. pre-miR-Ctrl, * P=0.02 anti-miR-646 vs. anti-miR-Ctrl); and (C) 1% O2 condition (* P=0.02 pre-miR-646 vs. pre-miR-Ctrl, * P=0.02 anti-miR 646 vs. anti-miR-Ctrl). The results of western blot showed that miR-646 significantly repressed IGF-1 protein expression in (D) 21% O2 condition; (E) 5% O2 condition; and (F) 1% O2 condition. (G) The direct binding between miR-646 and 3′-UTR of IGF-1 was detected by luciferase activity assay. The wild-type or mutated 3′-UTR of IGF-1 containing binding sites in luciferase reporters was transfected into hPDLCs with miR-646 overexpression or negative control (* P=0.02 pre-miR-646 vs. pre-miR-Ctrl). (H) The predicted miR-646-binding site in the IGF-1 3′-UTR.
IGF-1 was the direct target gene of miR-646
We further explored the potential mechanisms miR-646/IGF-1 signaling in hPDLCs in hypoxia conditions. We verified that IGF-1 was a likely target of miR-646 as it contained a putative miR-646 target site in the 3′-UTR by using TargetScan software (Lewis et al., 2005) (Figure 5H). Then to confirm whether IGF-1 was a direct target gene of miR-646, the wild- type and the mutants of 3′-UTR of IGF-1 in the predicted binding sequences were constructed into luciferase reporters. After that, these reporters were transfected into hPDLCs with miR-646 overexpression or negative controls by infection with and pre-miR-646 or pre-miR-Ctrl. The results showed that miR-646 overexpression significantly inhibited the luciferase activity in wild-type transfected cells. Additionally, in the mutated transfected cells, the altered expression of miR-646 had no obvious effect on the luciferase activity. These results indicate that IGF-1 was the direct target gene of miR-646. However, to determine whether miR-646 regulates hPDLCs proliferation and apoptosis through IGF-1, requires further experiments.
Discussion
Small non-coding RNA have drawn increasing attention in recent years because of their roles in gene transcription and post-transcription, especially in the study of cancer. It has been estimated that nearly 30% of gene expression in the human body is regulated by miRNAs [21]. PDL is an important part of periodontium, which connects the tooth and the surrounding tissues. hPDLCs, also named as human periodontal ligament fibroblasts (hPLFs), is dominate in the periodontium. It is a type of heterogeneous pluripotent cell with self-renewal ability [22–24]. Hypoxia can seriously affect periodontal tissue reconstruction, leading to serious periodontal diseases, but the underlying mechanisms remain unknown. This study investigated the influence of hypoxia on miR-646 in hPDLCs. We reported that the expression of miR-646 was decreased in hypoxia conditions and miR-646 could suppress hPDLCs proliferation and promote apoptosis in hypoxia conditions. Furthermore, we found that IGF-1 was inversely regulated by miR-646. These findings suggest, for the first time, that the interaction between hypoxia and miR-646 and the association between miR-646 and IGF-1 are mapped in hPDLCs, which may be involved in regulation of cellular functions and mechanisms of hPDLCs under oxygen conditions.
MicroRNAs (miRNAs) which belong to small non-coding RNA, are capable of modulating the expression of many functional proteins. The expression level of miRNAs can be regulated by several microenvironments like hypoxia, oxidative stress, or nutrient deprivation. MiR-646 is one kind of miRNAs that has been reported to be aberrantly expressed in various human cancers like gastric cancer, lung cancer, renal cancer, hepatocellular carcinoma, and osteosarcoma [25–29]. However, the underlying molecular mechanisms of miR-646 and hypoxia in hPDLCs have not yet been investigated. In the present study, we investigated whether hypoxia can have an impact on miRNA expression. We found that in severe, slight, and normal O2 hypoxia conditions, the expression of miR-646 was decreased compared with a control group. In addition, the downregulation of miR-646 significantly suppressed proliferation and promoted apoptosis of hPDLCs in hypoxia conditions, while on the contrary, overexpression of miRNA-646 markedly suppressed proliferation and promoted apoptosis of hPDLCs in hypoxia conditions.
IGF-1 consists of 70 amino acid residues accompanied by 3 disulfide bridges [30]. Accumulating studies have illustrated that IGF-1 could bind with the IGF-1 receptor (IGF-1R) and then trigger multiple downstream signaling pathways. Among them, the MAPK/ERK and phosphatidylinositol-3-kinase PI3K/AKT pathways have been associated with cell proliferation and survival [31,32]. It has been proven that IGF-1 is one of the neurotrophins that modulate cell growth [33]. It has also been shown that IGF-1 can influence early embryonic development via increasing the number of cells [34]. Moreover, IGF-1 plays an important role in bone growth and development, promoting cell proliferation and osteogenic differentiation in human PDLs [13,35]. The expression of IGF-1 in hypoxia conditions has been studied recently. Zhao et al. illustrated that IGF-1 increased cell viability while decreasing apoptosis in hypoxic neural stem cells through the PI3K/AKT and the MAPK/ERK pathways [36]. Liu et al. reported that IGF-1R may increase cell viability under hypoxic conditions by promoting autophagy and scavenging ROS production, which is closed with PI3K/Akt/mTOR signaling pathway [37]. In this study, we found the expression of IGF-1 was increased in hypoxia conditions, indicating that IGF-1 play a critical role in hypoxia conditions in hPDLCs. Nevertheless, the underlying mechanisms of IGF-1 and hypoxia in hPDLCs remain to be elucidated.
We further explored the interaction between miR-646 expression and IGF-1 expression in hypoxia conditions. Previous studies have reported on the association between microRNAs and IGF-1. For example, Ho et al. found that miR-181d exhibited an inverse correlation with IGF-1 in gliomas [38]. Wang et al. reported that miR-422a was downregulated in glioma tissues and was negatively correlated with the expression levels of IGF1/IGF1R [39]. Li et al. demonstrated that miR-379 plays an important role in regulating vascular smooth muscle cells proliferation, invasion, and migration by targeting IGF-1 [40]. Budzinska et al. found that miR-96, miR-145, and expression were increased while IGF-1R was decreased in peripheral blood mononuclear cells of aging humans. The age-associated higher expression of miR-96 and miR-145 might contribute to the lower expression of IGF-1R [41]. In this study, we first investigated the relationship between miR-646 and IGF-1, revealing that IGF-1 was the direct target of miR-646 and there was a converse relationship between them. However, further investigations are needed to explore whether miR-646 regulates proliferation and apoptosis of hPDLCs in hypoxia conditions through directly targeting on IGF-1.
In addition, there are different new treatments for periodontitis. Recent Isola et al. study showed that diode laser therapy, and changes in microbial and inflammatory mediators are closely related to the efficacy of invasive periodontitis; also scaling and root planning (SRP) plus the desiccant resulted in a greater reduction in clinical, microbial, and inflammatory mediators compared to SRP alone [42,43]. It has been suggested that microRNAs (miRNAs) are involved in the immune regulation of periodontitis. Recent research shows miR-146a inhibits inflammatory cytokine production in B cells through directly targeting IRAK1, suggesting a regulatory role of miR-146a in B cell-mediated periodontal inflammation [44].
Conclusions
We identified a significant role played by miR-646 in hPDLCs in that it suppressed cell proliferation and promoted apoptosis by inversely regulating IGF-1 expression. Meanwhile, hypoxia may regulate hPDLCs proliferation and apoptosis via the miR-646/IGF-1 signaling pathway, as such, interference with miR-646 may be a target for the treatment of periodontal disease.
Acknowledgements
We would like to thank Pro. Hua Tan and Dr. Yu Xiao for reviewing the article and providing helpful advice.
Abbreviations
- DAB
3, 3′-diaminobenzidine
- DMEM
Dulbecco Modified Eagle’s Medium
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate-dehydrogenase
- hPDLC
human periodontal ligament cell
- hPLF
human periodontal ligament fibroblast
- IGF
insulin-like growth factor
- IGF-1R
IGF-1 receptor
- LV
lentivirus
- miRNAs
microRNAs
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- OD
optical density
- PDL
periodontal ligament
- RT-qPCR
reverse transcription quantitative real-time polymerase chain reaction
- SDS-PAGE
sodium dodecyl sulphate polyacrylamide gel electrophoresis
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Shuttleworth CA, Smalley JW. Periodontal ligament. Int Rev Connect Tissue Res. 1983;10:211–47. doi: 10.1016/b978-0-12-363710-9.50010-1. [DOI] [PubMed] [Google Scholar]
- 2.Xiao Z, Han Y, Zhang Y, et al. Hypoxia-regulated human periodontal ligament cells via Wnt/β-catenin signaling pathway. Medicine. 2017;96:e6562. doi: 10.1097/MD.0000000000006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchesan JT, Scanlon CS, Soehren S, et al. Implications of cultured periodontal ligament cells for the clinical and experimental setting: A review. Arch Oral Biol. 2011;56:933–43. doi: 10.1016/j.archoralbio.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu Y, Mu J, Fan Z, et al. Insulin-like growth factor 1 enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells via ERK and JNK MAPK pathways. Histochem Cell Biol. 2012;137:513–25. doi: 10.1007/s00418-011-0908-x. [DOI] [PubMed] [Google Scholar]
- 5.Choe Y, Yu J-Y, Son Y-O, et al. Continuously generated H2O2 Stimulates the proliferation and osteoblastic differentiation of human periodontal ligament fibroblasts. J Cell Biochem. 2012;113:1426–36. doi: 10.1002/jcb.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis JS, Lee JA, Underwood JC, et al. Macrophage responses to hypoxia: Relevance to disease mechanisms. J Leukoc Biol. 1999;66:889–900. doi: 10.1002/jlb.66.6.889. [DOI] [PubMed] [Google Scholar]
- 7.Hanioka T, Tanaka M, Takaya K, et al. Pocket oxygen tension in smokers and non-smokers with periodontal disease. J Periodontol. 2000;71:550–54. doi: 10.1902/jop.2000.71.4.550. [DOI] [PubMed] [Google Scholar]
- 8.Cattaneo PM, Dalstra M, Melsen B. The finite element method: A tool to study orthodontic tooth movement. J Dent Res. 2005;84:428–33. doi: 10.1177/154405910508400506. [DOI] [PubMed] [Google Scholar]
- 9.Pradeep AR, Prapulla DV, Sharma A, et al. Gingival crevicular fluid and serum vascular endothelial growth factor: Their relationship in periodontal health, disease and after treatment. Cytokine. 2011;54:200–4. doi: 10.1016/j.cyto.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Finnerty JR, Wang WX, Hébert SS, et al. The miR-15/107 group of microRNA genes: Evolutionary biology, cellular functions, and roles in human diseases. J Mol Biol. 2010;402:491–509. doi: 10.1016/j.jmb.2010.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gatsiou A, Boeckel JN, Randriamboavonjy V, et al. MicroRNAs in platelet biogenesis and function: Implications in vascular homeostasis and inflammation. Curr Vasc Pharmacol. 2012;10:524–31. doi: 10.2174/157016112801784611. [DOI] [PubMed] [Google Scholar]
- 12.Raja S, Byakod G, Pudakalkatti P. Growth factors in periodontal regeneration. Int J Dent Hyg. 2009;7:82–89. doi: 10.1111/j.1601-5037.2009.00380.x. [DOI] [PubMed] [Google Scholar]
- 13.Abreu FA, Ferreira CL, Silva GA, et al. Effect of PDGF-BB, IGF-I growth factors and their combination carried by liposomes in tooth socket healing. Braz Dent J. 2013;24:299–307. doi: 10.1590/0103-6440201302238. [DOI] [PubMed] [Google Scholar]
- 14.Yu Y, Mu J, Fan Z, et al. Insulin-like growth factor 1 enhances the proliferation and osteogenic differentiation of human periodontal ligament stem cells via ERK and JNK MAPK pathways. Histochem Cell Biol. 2012;137:513–25. doi: 10.1007/s00418-011-0908-x. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Yang Z, Li Z, et al. Exogenous IGF-1 promotes hair growth by stimulating cell proliferation and down regulating TGF-β1 in C57BL/6 mice in vivo. Growth Horm IGF Res. 2014;24:89–94. doi: 10.1016/j.ghir.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Pais RS, Moreno-Barriuso N, Hernández-Porras I, et al. Transcriptome analysis in prenatal IGF1-deficient mice identifies molecular pathways and target genes involved in distal lung differentiation. PLoS One. 2013;8:e83028. doi: 10.1371/journal.pone.0083028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Götz W, Kunert D, Zhang D, et al. Insulin-like growth factor system components in the periodontium during tooth root resorption and early repair processes in the rat. Eur J Oral Sci. 2006;114:318–27. doi: 10.1111/j.1600-0722.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 18.Götz W, Heinen M, Lossdörfer S, et al. Immunohistochemical localization of components of the insulin-like growth factor system in human permanent teeth. Arch Oral Biol. 2006;51:387–95. doi: 10.1016/j.archoralbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Han X, Amar S. IGF-1 signaling enhances cell survival in periodontal ligament fibroblasts vs. gingival fibroblasts. J Dent Res. 2003;82:454–59. doi: 10.1177/154405910308200610. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Wu C, Xiao Y. The stimulation of proliferation and differentiation of periodontal ligament cells by the ionic products from Ca7Si2P2O16 bioceramics. Acta Biomater. 2012;8:2307–16. doi: 10.1016/j.actbio.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Shen J, Stass SA, Jiang F. MicroRNAs as potential biomarkers in human solid tumors. Cancer Lett. 2013;329:125–36. doi: 10.1016/j.canlet.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 23.Murakami Y, Kojima T, Nagasawa T, et al. Novel isolation of alkaline phosphatase-positive subpopulation from periodontal ligament fibroblasts. J Periodontol. 2003;74:780–86. doi: 10.1902/jop.2003.74.6.780. [DOI] [PubMed] [Google Scholar]
- 24.Kaneda T, Miyauchi M, Takekoshi T, et al. Characteristics of periodontal ligament subpopulations obtained by sequential enzymatic digestion of rat molar periodontal ligament. Bone. 2006;38:420–26. doi: 10.1016/j.bone.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 25.Zhang P, Tang WM, Zhang H, et al. MiR-646 inhibited cell proliferation and EMT-induced metastasis by targeting FOXK1 in gastric cancer. Br J Cancer. 2017;117(4):525–34. doi: 10.1038/bjc.2017.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan Y, Chen Y, Ma D, et al. miR-646 is a key negative regulator of EGFR pathway in lung cancer. Exp Lung Res. 2016;42:286–95. doi: 10.1080/01902148.2016.1207726. [DOI] [PubMed] [Google Scholar]
- 27.Azam AT, Bahador R, Hesarikia H, et al. Downregulation of microRNA-217 and microRNA-646 acts as potential predictor biomarkers in progression, metastasis, and unfavorable prognosis of human osteosarcoma. Tumour Biol. 2016;37:5769–73. doi: 10.1007/s13277-015-3821-4. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Liu M, Feng Y, et al. Downregulated miR-646 in clear cell renal carcinoma correlated with tumour metastasis by targeting the nin one binding protein (NOB1) Br J Cancer. 2014;111:1188–200. doi: 10.1038/bjc.2014.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang R, Zhang J, Jiang W, et al. Association between a variant in microRNA-646 and the susceptibility to hepatocellular carcinoma in a large-scale population. ScientificWorldJournal. 2014;2014:312704. doi: 10.1155/2014/312704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978;253:2769–76. [PubMed] [Google Scholar]
- 31.Ma J, Sawai H, Matsuo Y, et al. IGF-1 mediates PTEN suppression and enhances cell invasion and proliferation via activation of the IGF-1/PI3K/Akt signaling pathway in pancreatic cancer cells. J Surg Res. 2010;160:90–101. doi: 10.1016/j.jss.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Valenciano A, Moreno M, Lloret M, Lara PC. Role of IGF-1 receptor in radiation response. Transl Oncol. 2012;5:1–9. doi: 10.1593/tlo.11265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pucilowska JB, Davenport ML, Kabir I, et al. The effect of dietary protein supplementation on insulin-like growth factors (IGFs) and IGF-binding proteins in children with shigellosis. J Clin Endocrinol Metab. 1993;77:1516–21. doi: 10.1210/jcem.77.6.7505287. [DOI] [PubMed] [Google Scholar]
- 34.Supeno NE, Pati S, Hadi RA, et al. IGF-1 acts as controlling switch for long-term proliferation and maintenance of EGF/FGF-responsive striatal neural stem cells. Int J Med Sci. 2012;10:522–31. doi: 10.7150/ijms.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Locatelli V, Bianchi VE. Effect of GH/IGF-1 on bone metabolism and osteoporsosis. Int J Endocrinol. 2015;2014:235060. doi: 10.1155/2014/235060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao B, Zheng Z. Insulin growth factor 1 protects neural stem cells against apoptosis induced by hypoxia through Akt/mitogen-activated protein kinase/extracellular signal-regulated kinase (Akt/MAPK/ERK) pathway in hypoxia-ishchemic encephalopathy. Med Sci Monit. 2017;23:1872–79. doi: 10.12659/MSM.901055. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Liu Q, Guan JZ, Sun Y, et al. Insulin-like growth factor 1 receptor-mediated cell survival in hypoxia depends on the promotion of autophagy via suppression of the PI3K/Akt/mTOR signaling pathway. Mol Med Rep. 2017;15:2136–42. doi: 10.3892/mmr.2017.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho KH, Chen PH, Hsi E, et al. Identification of IGF-1-enhanced cytokine expressions targeted by miR-181d in glioblastomas via an integrative miRNA/mRNA regulatory network analysis. Sci Rep. 2017;7:732. doi: 10.1038/s41598-017-00826-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Tang C, Na M, et al. miR-422a inhibits glioma proliferation and invasion by targeting IGF1 and IGF1R. Oncol Res. 2017;25:187–94. doi: 10.3727/096504016X14732772150389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li K, Wang Y, Zhang A, et al. miR-379 inhibits cell proliferation, invasion, and migration of vascular smooth muscle cells by targeting insulin-like factor-1. Yonsei Med J. 2017;58:234–40. doi: 10.3349/ymj.2017.58.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Budzinska M, Owczarz M, Pawlik-Pachucka E, et al. miR-96, miR-145 and miR-9 expression increases, and IGF-1R, and FOXO1, expression decreases in peripheral blood mononuclear cells of aging humans. BMC Geriatr. 2016;16:200. doi: 10.1186/s12877-016-0379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matarese G, Ramaglia L, Cicciu M, et al. The effects of diode laser therapy as an adjunct to scaling and root planing in the treatment of aggressive periodontitis: A 1-year randomized controlled clinical trial. Photomedicine and Laser Surgery. 2017;35(12):702–9. doi: 10.1089/pho.2017.4288. [DOI] [PubMed] [Google Scholar]
- 43.Isola G, Matarese G, Williams RC, et al. The effects of a desiccant agent in the treatment of chronic periodontitis: A randomized, controlled clinical trial. Clin Oral Investig. 2018;22(2):791–800. doi: 10.1007/s00784-017-2154-7. [DOI] [PubMed] [Google Scholar]
- 44.Jiang S, Hu Y, Deng S, et al. miR-146a regulates inflammatory cytokine production in Porphyromonas gingivalis lipopolysaccharide-stimulated B cells by targeting IRAK1 but not TRAF6. Biochim Biophys Acta. 2018;1864(3):925–33. doi: 10.1016/j.bbadis.2017.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]