Abstract
Background
Osteoprotegerin (OPG) inhibits bone resorption and binds with strong affinity to receptor activator of NF κB ligand (RANKL), thereby preventing RANKL from binding to its receptor RANK. Osteoclasts have documented effects on bone erosion of rheumatoid arthritis (RA). The aim of this study was to examine the role of miR-145-5p in the regulation of RA osteoclast differentiation and bone erosion.
Material/Methods
Expression of microRNA-145-5p in human peripheral blood mononuclear cells (PBMC) and synovial tissue was assayed by real-time polymerase chain reaction (RT-PCR). OPG, RANK, and RANKL expression in RAW-264.7 cells was examined by RT-PCR and Western blot analysis. Osteoclast formation was detected by tartrate-resistant acid phosphatase (TRAP) staining. The effect of miR-145-5p on predicted target mRNAs was examined by luciferase reporter assays. Collagen-induced arthritis (CIA) was induced by injecting DBA/1 mice with bovine type II collagen (CII), and miR-145-5p agomir was administered by intravenous injection. Morphological changes in the CIA joint were assessed by micro-computed tomography (CT) and histopathology.
Results
miR-145-5p levels significantly increased in RA PBMC and synovial tissue compared with normal PBMC and osteoarthritis (OA) tissue. After transfection of RAW-264.7 cells with miR-145-5p, RANK and RANKL expression increased significantly, while OPG expression decreased significantly. TRAP staining results showed osteoclast numbers increased. Micro-CT analysis of the arthritic joints showed that the miR-145-5p agomir caused bone erosion in mice, and histopathological analysis revealed that miR-145-5p agomir aggravates cartilage erosion.
Conclusions
Our findings indicate that administration of miR-145-5p aggravates joint erosion in CIA mice. This suggests that miR-145-5p is a potential target for the treatment of RA.
MeSH Keywords: Arthritis, Rheumatoid; MicroRNAs; Osteoprotegerin
Background
Rheumatoid arthritis (RA) represents a chronic autoimmune disease characterized by synovial lining cell hyperplasia and irreversible joint erosion [1,2]. Osteoclasts, the multinucleated giant cells, have a well-established effect on the progression of bone erosion [3,4]. It has been reported that osteoprotegerin (OPG) and receptor activator of NFκB ligand (RANKL) are new members of the tumor necrosis factor (TNF) receptor and TNF ligand families. In addition, OPG and RANKL are involved in regulation of osteoclast formation, activation, and apoptosis [5]. RANKL accelerates osteoclast differentiation, also called osteoclastogenesis, and increases osteoclast survival [6]. RANKL promotes osteoclastogenesis leans upon its receptor, RANK, which is present on osteoclast membranes [7]. However, OPG, as a decoy receptor for RANKL, can bind with RANKL, thereby weakening the regulation of RANKL and promoting osteoclast apoptosis [8]. Accumulating evidence suggests that OPG is involved in bone resorption in RA. In addition, RANKL and the osteoprotegerin system have regulatory effects on bone metabolism and may be pivotal to the pathogenesis of bone erosions in RA [9].
Micro-RNAs (miRNAs) are small, endogenous, single-stranded, non-coding RNAs that play a role on post-transcriptional regulation of gene expression. These have effects via weakening of mRNA translation and downregulation of targets expression [10]. Some studies have demonstrated the contribution of miRNAs to osteoclast differentiation [11,12]. Skeletal pathways predicted that miR-145-5p negatively regulate bone formation at erosion sites [13].
In the present study, miR-145-5p overexpression downregulated OPG, but increased expression of RANKL and RANK in RAW-264.7 cells, and increased osteoclast numbers. Administration of miR-145-5p aggravated bone erosion in collagen-induced arthritis mice (CIA) by targeting osteoprotegerin.
Material and Methods
RA and osteoarthritis (OA) patients
Thirty RA patients (22 women, 8 men; median age 56.2 years, range 21–61 years) who were admitted to the Department of Rheumatology and Immunology, Tianjin Medical University General Hospital, Tianjin, China, between October 2015 and January 2017 were enrolled in this study. The median duration of RA was 9.2±5.2 years. The control group consisted of 23 healthy blood donors. Twelve RA patients and 10 OA patients were admitted to the Department of Orthopedics of the hospital. All RA patients had been definitively diagnosed as having RA using the American College of Rheumatology criteria. All subjects gave written informed consent. The procedure was approved by the Ethics Committee of Tianjin Medical University General Hospital.
Isolation of peripheral blood mononuclear cells (PBMCs) from RA patients and normal controls
Fresh blood samples were collected from patients and normal donors, and then were added dropwise to Dulbecco’s phosphate-buffered saline/ethylenediaminetetraacetic acid (5 mM DPBS: 0.5 M EDTA), then blended well, followed by centrifuging at 400 g at 4°C (10 min). Then, we added DPBS-E reagent and blended the mixture. The viability of the purified PBMCs was assessed using the trypan blue exclusion method and was always greater than 90%. All procedures were performed in duplicate. The sedimentary cells were regarded as PBMCs.
Synovial tissue specimens of RA and OA patients
Synovial tissue samples were separated from OA and RA patients who underwent knee-joint replacement surgery. Sampling was performed promptly after opening the articular cavity. Briefly, RA and OA tissues were minced and mixed in 1 ml of TRIzol reagent (TaKaRa Bio, Shiga, Japan) and frozen at −80°C for later assay.
Cell culture and treatment
RAW-264.7 mouse macrophage cells were purchased from the BeNa Culture Collection (BNCC, Suzhou, China) and cultured in DMEM with 10% fetal bovine serum (FBS) and 100 IU/mL penicillin/streptomycin in an incubator with 5% CO2 at 37°C. RAW 264.7 cells were transfected with miR-145-5p mimic or inhibitor and respective control. After 24 h, the medium was replaced with test samples in differentiation medium supplemented with 50 ng/mL macrophage colony-stimulating factor (M-CSF, Sigma-Aldrich, St. Louis, MO, USA). The replacement was performed twice daily.
Osteoclasts differentiation and tartrate-resistant acid phosphatase (TRAP) staining
After 5 days, we removed the medium, and the cells were fixed, permeabilized, and then air-dried. For identification of osteoclasts, treated RAW 264.7 cells were stained for TRAP using an acid phosphatase kit (Sigma, St. Louis, MO, USA). TRAP+ cells were regarded as osteoclasts with more than 3 nuclei. TRAP activity and coloring data were calculated as the mean level of 3 replicates per condition per experiment.
Cell transfection with miR-145-5p mimic or miR-145-5p inhibitor
RAW-264.7 cells were transfected with miR-145-5p mimic or miR-145-5p inhibitor, and their negative control (Guangzhou RiboBio) using Lipofectamine 3000 (Invitrogen) according to the reagent manufacturer’s protocol, in 24- and 96-well plates (100 000 and 20 000 cells per well, respectively). The concentration of siRNA using for cell transfection were 50 or 100 nM. Growth of the transfected cells was observed for 48 h.
Real-time quantitative PCR
Total RNA isolated by TRIzol reagent (TaKaRa Bio, Shiga, Japan) was used to synthesize cDNA for the detection of mRNAs, and then the cDNAs were amplified. Real-time quantitative PCR (RT-qPCR) for mRNA was performed using SYBR-Green PCR Master Mix (TaKaRa Bio) and gene-specific primers (Sangon Biotech). The primers (Sangon Biotech) used were: OPG (forward, 5′-ACCCAGAAACTGGTCATCAGC-3′; reverse, 5′-CTGCAATACACACACTCATCACT-3′); and RANK (forward, 5′-GGAG GGAGCACGAAAAACTG-3′; reverse, 5′-GGAAGGGTTG GACACCTGAA-3′). RANKL (forward, 5′-CAGCATCGCTCT GTTCCTGTA-3′; reverse, 5′-CTGCGTTTTCATGGAGTCTCA-3′). GAPDH (forward, 5′-GGA GCGAGATCCCTCCA AAAT-3′; reverse, 5′-GGCTGTTGTC ATACTTCTCATGG-3′) served as the internal control. Relative expression was calculated using the comparative threshold cycle method.
MicroRNA target prediction
The miR-145-5p targets predictions based on TargetScan were downloaded from http://www.targetscan.org/. Prediction accuracy of target gene was assessed according to mirSVR scores. Binding sites of miR-145-5p with target genes were obtained from TargetScan.
Luciferase reporter activity assay
The 3′-UTR of OPG and their site-directed mutants were cloned into the pMIR-Report luciferase vector (RiboBio, Guangdong, China) between the NotI and HindIII sites. After being transfected, the cells were obtained, and we added 200 μl cell lysis buffer (Promega, USA). After being centrifuged, the supernatants were obtained. Then, luc activity in cell lysates (20 μl) was determined immediately by D-luciferin (100 μl; Promega) and the bioluminescence count obtained is expressed in relative light units (RLU).
Western blot analysis
The cells were detached with a cell lifter and lysed in radio-immunoprecipitation assay (RIPA) lysis buffer (Solarbio Science and Technology). We then isolated protein from the cell lysates, and the protein samples were separated and transferred to membranes (Pierce, Rockford, IL) using electrophoresis. We used sealing fluid (5% nonfat milk) to block the membrane for 2 h. Then, the membranes were incubated with primary antibody (rabbit anti-human catestatin region) and secondary antibody HRP in turn. The internal control was anti-β-actin.
Induction of CIA
Eight-week-old male DBA/1 mice obtained from Hua Fukang (Hua Fukang, Beijing, China) were kept and bred individually in Tianjin Medical University General Hospital (TMUGH). The experiment was approved by the Ethics Committee for Animals of TMUGH, and the treatment for all mice complied with the provisions of the Institutional Animal Care and Use Committee. We used acetic acid (0.01 M) to dissolve the Chicken type II collagen (CII), the concentration of which was adjusted for 2 mg/mL via churning overnight at 4°C. Then, CII was stored at −70°C for later use. We injected 0.1 ml of emulsion into the tails of mice on Day 0 and intraperitoneally injected collagen into mice for boosting on Day 21.
Intravenous injection of mir-145-5p agomir or scrambled miRNA agomir
An equal volume of mir-145-5p agomir or scrambled miRNA agomir (RiboBio, Guangdong, China) was injected in the tail vein of the mice on Day 30. Injected mir-145-5p agomir (n=4) or scrambled miRNA agomir (n=4) in the tail vein of mice at 1-week intervals. Four weeks after injection, the hind feet were used for micro-computed tomography (CT) analysis, and the ankles were used for histopathological analysis.
Histologic examinations
Histologic examinations were performed independently by 2 evaluators (Y.C. and X.W.). The samples underwent paraffin embedding and section processing. The slices were stained with hematoxylin and eosin (H&E) and fixed on the slides. Histological scores were graded as follows: cellular infiltration of sub-synovial tissue (0–5), synovial hyperplasia and pannus formation (0–3), cell exudate in joint space (0–3), and bone erosion (0–3).
Micro-CT images
The ankles of the mice were scanned and reconstructed using a micro-CT system (SkyScan-1076). Radiologic scores of joint destruction and bone erosion were calculated on a scale of 0 to 4 (with higher scores indicating greater severity).
Immunohistochemistry analysis
Routinely formalin-fixed and paraffin-embedded liver samples were immunostained with indirect immunohistochemistry using monoclonal antibodies to osteoprotegerin (OPG), followed by the appropriate secondary antibody. The primary antibody was OPG (Abcam). The secondary antibody was Alexa Fluor568-conjugated rabbit anti-goat immunoglobulin G (IgG; Molecular Probes/Invitrogen).
Statistical analysis
For RT-PCR experiments, as well as the luciferase reporter assays, differences between the 2 groups were analyzed using a two-tailed t test. Statistical significance was set as * P<0.05, ** P<0.01, *** P<0.001.
Results
Upregulation of miR-145-5p in PBMCs and synovial tissues of RA patients
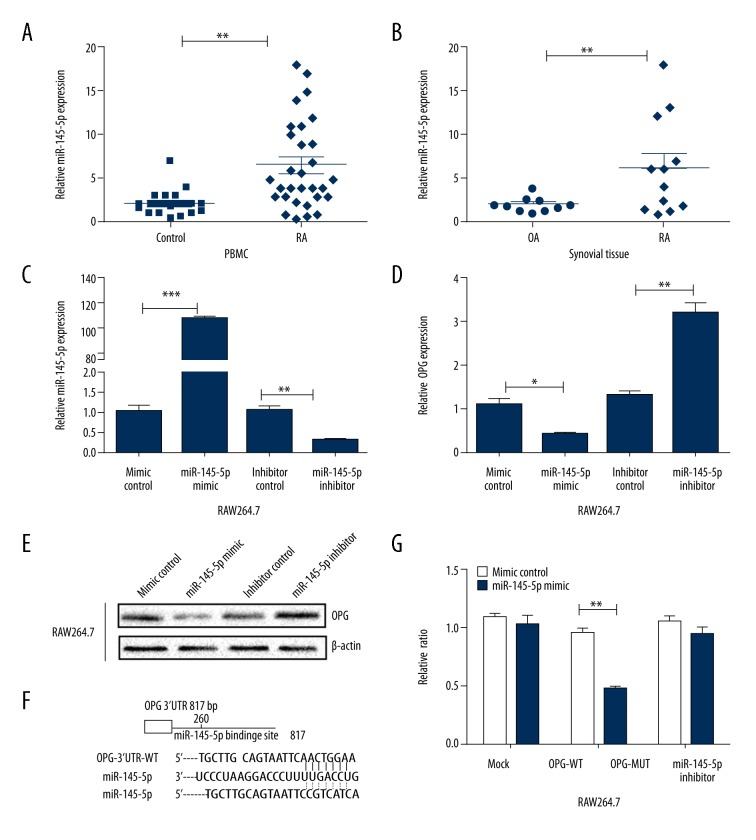
miR-145-5p expression levels were higher in RA PBMCs than in normal control PBMCs (Figure 1A). Increased miR-145-5p expression was observed in RA tissues when compared with OA tissues (Figure 1B).
Figure 1.
miR-145-5p expression was analyzed by RT-qPCR in patients. (A) miR-145-5p expression in PBMC of RA and normal control (n=30, 23, respectively). (B) Comparison of miR-145-5p level in synovial tissues of RA patients and OA patients (n=12,10, respectively). (C) miR-145-5p mimic or inhibitor and the respective control transfected into RAW 264.7 cells. miR-145-5p levels were detected by RT-PCR. (D) OPG mRNA level in RAW 264.7 cells were assessed by RT-PCR. (E) OPG protein levels were detected by Western blot. (F) Binding sites of miR-145-5p and OPG. Sequence of the binding site, (wild-type, WT) and mutant (MUT) sites. (G) Effects of miR-145-5p on the 3′UTR of OPG mRNA (WT and MUT). * P<0.05; ** P<0.01; *** P<0.001 compared to the control group. Data are representative of 3 experiments. Values are means ±SD.
OPG expression in RAW264.7 after transfection with the miR-145 mimic or inhibitor
RAW264.7 were transfected with the miR-145 mimic or miR-145-5p inhibitor and respective controls (Figure 1C). Analysis by RT-PCR and Western blot showed high levels of miR-145-5p and significantly reduced the expression of OPG mRNA and protein compared with their control groups (both P<0.05) However, knocked-down expression of miR-145-5p increased OPG expression (Figure 1D, 1E).
OPG is a target gene of miR-145-5p
TargetScan was used to search for putative miRNA targets. Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway analysis demonstrated TNFRSF11B (OPG) to be involved in RA bone metabolism-related pathways. According to the bioinformatics database, we predicted that the miR-145-5p binding site was at the 3′-untranslated region (UTR) of OPG. The 3′-UTR of wild-type mRNA and mutation-type mRNA were cloned into a luciferase plasmid separately, and pre-miR-145-5p was co-transfected with each luciferase construct into cells (Figure 1F). Notably, miR-145-5p selectively suppressed the luciferase activity driven by the wild-type but not mutant forms of the 3′-UTR of OPG mRNA (Figure 1G). Our data demonstrate that miR-145-5p directly targets 3′-UTR of OPG.
Stimulative effect of miR-145-5p on the osteoclastic differentiation of RAW264.7 cells
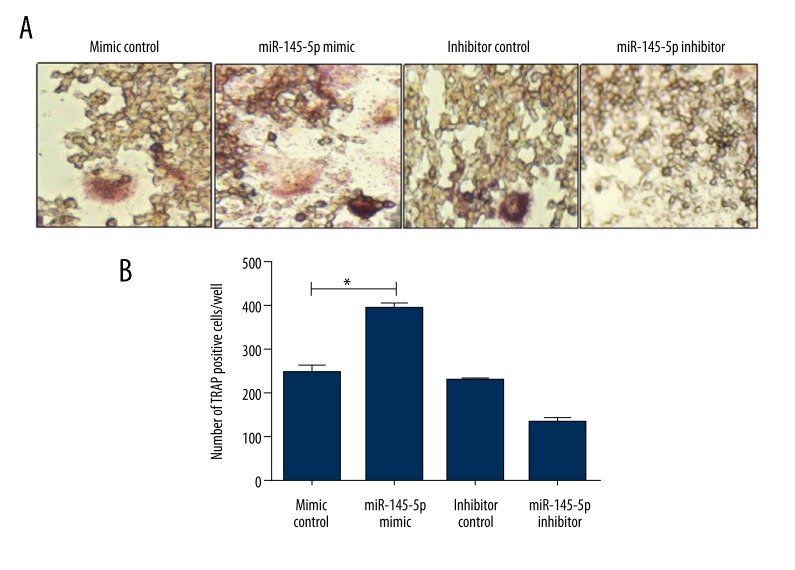
miR-145-5p has been reported to be involved in bone metabolism [14]. Murine macrophage RAW 264.7 cells were transfected with miR-145-5p mimic and control prior to M-CSF-induced osteoclast differentiation. Osteoclast formation demonstrated by TRAP staining was promoted by miR-145-5p mimics (Figure 2A, 2B).
Figure 2.
Stimulative effect of miR-145-5p on the osteoclastic differentiation of RAW264.7 cells. (A, B) TRAP staining showing the effects of miR-145-5p mimic and inhibitor on human osteoclast generation. (A) TRAP staining showing osteoclast generation; (B) Numbers of TRAP-positive cells after transfection. * P<0.05. Values are means ±SD.
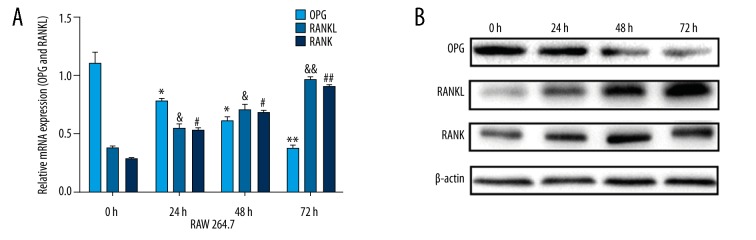
OPG, RANK, and RANKL mRNA expression in RAW 264.7 cells at different time points
Analysis of RT-PCR showed OPG, RANK, and RANKL mRNA expression after transfection of the miR-145-5p mimic. As shown in Figure 3A, there was a high level of miR-145-5p, the mRNA level of OPG gradually decreased, and the mRNA levels of RANK and RANKL significantly increased compared with control groups. These changes were time-dependent.
Figure 3.
After RAW264.7 cells were transfected miR-145-5p mimic and control, expression of OPG, RANKL, and RANK were detection at 4 time points. (A) Analysis of OPG, RANKL, and RANK mRNA expression via RT-PCR. (B) Analysis of OPG, RANKL, and RANK protein expression via Western blot. * Compared to 0 h, * P<0.05; ** P<0.01; & compared to 0 h, + P<0.05; && P<0.01; # compared to 0 h, # P<0.05; ## P<0.01; Values are means ±SD.
OPG, RANK, and RANKL protein expression in RAW 264.7 cells at different time points
Western blot analysis demonstrated that for OPG, RANK, and RANKL protein expression after transfection with miR-145-5p mimic, OPG gradually decreased while RANK and RANKL gradually increased, and these changes were time-dependent. As shown in Figure 3B, gene protein expression was detected by at 0 h, 24 h, 48 h, and 72 h.
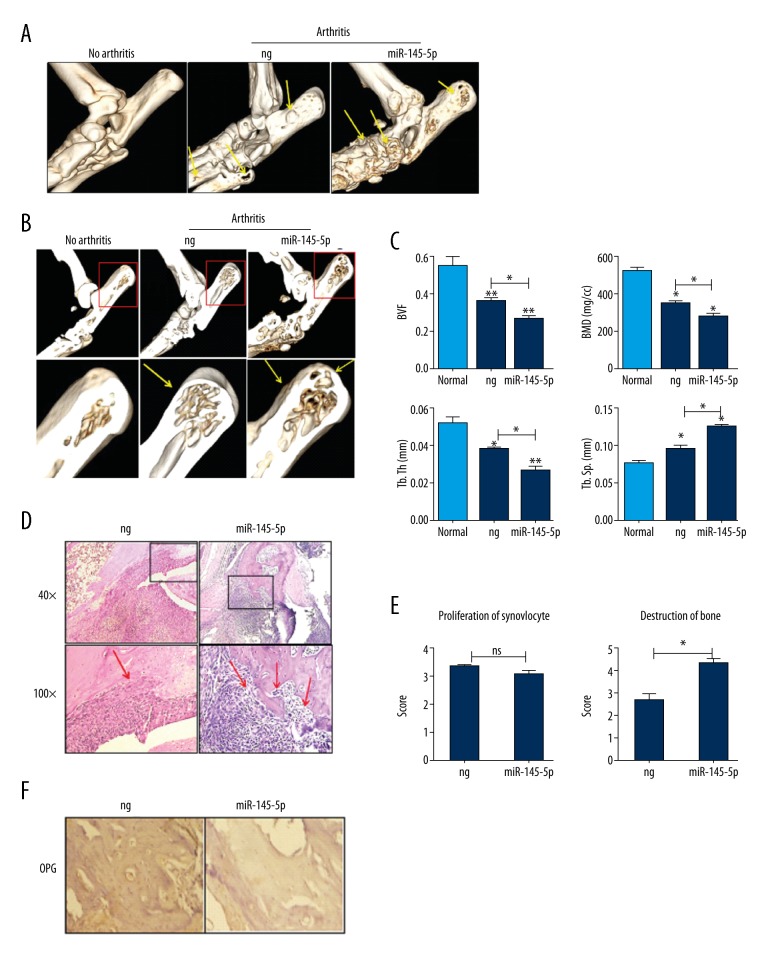
miR-145-5p agomir aggravates bone erosion in CIA mice
Four weeks after the first agomir injection, a micro-CT was used to assess regulation of miR-145-5p agomir in CIA mice. Figure 4A shows the micro-CT datasets of bones of ankle joints in the 2 groups. Compared with nonspecific miRNA agomir-treated, miR-145-5p markedly increased the extent of bone surface erosion in miR-145-5p agomir-treated CIA mice.
Figure 4.
miR-145-5p agomir aggravates bone destruction in CIA mice. (A, B) Comparison of bone erosion: micro-CT images of the ankles of non-CIA mice and of CIA mice treated with negative control (ng) or with miR-145-5p. (A) Bone surface; (B) Bone cross-section. Yellow arrows indicate bone erosion. Red boxed areas are shown the trabecular bone. (C) Four parameters – BVF, BMD, Tb.Th, and Tb.Sp – of proximal end of the tibia facing the articular cavity in these groups. (D) Hematoxylin and eosin staining of sections from the hind paws of the CIA mice (n=4, per group). Boxed areas in the top panels (original magnification×40) are shown at a higher magnification in the middle panels (original magnification×100). Red arrows indicate cartilage erosion. (E) Histopathological scores of CIA mice (n=4, per group). (F) IHC and quantification of OPG in CIA mice (n=4, per group). Bars=200 μm at 40×original magnification. * P<0.05, ** P<0.01, and *** P<0.001. Values are means ±SD.
Figure 4B shows the bone cross-section of ankle joints in the 3 groups. Compared to nonspecific agomir-treated mice, miR-145-5p also increased the extent of joint erosion in miR-145-5p agomir-treated CIA mice. Moreover, 4 parameters – bone volume(BVF), bone mineral density (BMD), trabecular thickness (Tb.Th), and trabecular separation (Tb.Th) – of the ankle joints in 2 groups can be used to quantify the extent of joint erosion (Figure 4C). Administration of the miR-145-5p agomir decreased BVF, BMD, and Tb.Th of ankle joints.
Although histopathological analysis of ankle joints of the 2 groups of CIA mice showed the same degree of inflammatory cell infiltration and synovial hyperplasia, there was more severe erosion of the cartilage and bone in miR-145-5p agomir-treated mice (Figure 4D, 4E).
OPG levels in miR-145-5p–treated mouse joints
Immunohistochemical (IHC) analysis showed an increase in OPG protein expression in the ankles of mice treated with miR-145-5p agomir. The results also showed a reduction in OPG protein in the ankles of mice treated with miR-145-5p agomir compared with the ankles of the control mice (Figure 4F).
Discussion
RA is a systemic autoimmune inflammatory disease which predominantly affects multiple peripheral joints. Bone erosion is an important feature of RA. Arend also reported that macrophages and many cytokines of synovial tissue contribute to the pathophysiology of rheumatoid, and they deciphered the mechanisms of the apparent autonomous and aggressive behavior of RA-FLS [15]. Various drugs for the treatment of RA have been developed to prevent inflammation and bone erosion, but the definite mechanism is unknown.
Bone remodeling is a very complex process of tightly coordinated action involving bone resorption and osteoclastogenesis [16]. Bone plays an important role in hemopoiesis, and recent reports show their vital influence as a component of the immune system [17]. Several investigators have shown RA is characterized by bone erosion, and interactions between the RANKL/RANK/OPG system and the T cells may partly explain the lesions [18]. Osteoprotegerin (OPG) functions as an endogenous decoy receptor that competitively weakens osteoclasts differentiation and prevents excessive bone resorption via its interaction with RANKL [19]. RANKL promotes osteoclastogenesis and enhances osteoclastic activation by interacting with its receptor, RANK, locating on the osteoclast membrane [20]. However, when the balance of the RANKL/RANK/OPG system is broken, the risk of RA patients can be increased. Hairul-Islam et al. [21] revealed that dysfunction of the RANKL/RANK/OPG system induces the differentiation and activation of osteoclasts to cause joint erosion in RA. Shen et al. [22] showed that OPG/RANKL ratio and joint erosion index were positively correlated in RA patients. In addition, to increasing or decreasing the OPG/RANKL ratio might slow or accelerate the deterioration of RA.
miRNAs also appear to affect osteoblast differentiation and proliferation in vivo through identifiable pathways [23]. Jeong et al. [24] reported that upregulated of miR-194 facilitated osteoblast differentiation of primary osteoblasts and might be a key regulator in maintaining the balance between osteogenesis and adipogenesis. Wu et al. [25] reported that down-regulation of miR-126-5p affects osteoclast differentiation and bone resorption by decreasing MMP-13 expression in multinucleated giant cells. Jun et al. demonstrated that there is a potential targeting site in the OPG 3′-UTR for miR-145 in metabolic bone diseases, which relates to osteoblast differentiation [26]. Furthermore, miR-145-5p mediated steroid-induced necrosis of the femoral head by targeting the OPG/RANK/RANKL signaling pathway [27]. However, the mechanism by which miR-145 regulates OPG/RANK/RANKL pathway and RA bone erosion is not clear.
In this study we revealed a novel miRNA – miR-145-5p – associated with RA, and found that its effects on osteoblast differentiation in RA involves the OPG/RANK/RANKL pathway. miR-145-5p overexpression was shown to aggravate osteoclastic differentiation of RAW264.7 cells by down-regulating OPG levels. The results of micro-CT analysis also support administration of miR-145-5p in vitro. In addition, we showed that miR-145-5p decreased the ratio of OPG/RANKL by reducing OPG expression. Taken together, these findings support the role of miR-145-5p in the OPG/RANK/RANKL system in osteoclastic differentiation and bone erosion in RA.
Although miRNAs are burgeoning as an important contributory factor in the pathophysiology of RA, the potential of a single miRNA species to modulate many distinct disease-regulatory pathways simultaneously reminds us that the pathway based on OPG/RANK/RANKL should be considered. Time and budget permitting, we will do further related research.
Conclusions
Our study provides mechanistic evidence that miR-145 regulates osteoblast differentiation in RA by targeting the OPG/PANK/PANKL signaling pathway, which may highlight new insights regarding the roles of miRNAs in RA and may facilitate the development of new therapies for RA.
Abbreviations
- RA
rheumatoid arthritis
- OA
osteoarthritis
- FLS
fibroblast-like synovial cells
- OPG
osteoprotegerin
- RANKL
receptor activator of NFκB ligand
- CIA
collagen-induced arthritis
- miRNAs
microRNAs
- PBMC
peripheral blood mononuclear cells
- micro-CT
micro-computed tomography
- IHC
immunohistochemical
- TRAP
tartrate-resistant acid phosphatase
Footnotes
Source of support: This work was supported in part by the Medical Scientific Research Foundation of Tianjin, China (Grant/Award Number: ‘Project No. 13KG140’) and the National Natural Science Foundation of China (Grant/Award Number: ‘Project No. 81330029’)
References
- 1.Böhler C, Radner H, Ernst M, et al. Rheumatoid arthritis and falls: The influence of disease activity. Rheumatology. 2012;51(11):2051–57. doi: 10.1093/rheumatology/kes198. [DOI] [PubMed] [Google Scholar]
- 2.Goldring SR. The final pathogenetic steps in focal bone erosions in rheumatoid arthritis. Ann Rheum Dis. 2000;59(Suppl 1):i72–74. doi: 10.1136/ard.59.suppl_1.i72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gravallese EM. Bone wasn’t built in a day: Destruction and formation of bone in the rheumatic diseases. Trans Am Clin Climatol Assoc. 2017;128:24–43. [PMC free article] [PubMed] [Google Scholar]
- 4.Liu FL, Chen CL, Lee CC, et al. The Simultaneous inhibitory effect of niclosamide on RANKL-induced osteoclast formation and osteoblast differentiation. Int J Med Sci. 2017;14(9):840–52. doi: 10.7150/ijms.19268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Remuzgomartínez S, Genre F, Lópezmejías R, et al. Expression of osteoprotegerin and its ligands, RANKL and TRAIL, in rheumatoid arthritis. Sci Rep. 2016;6:29713. doi: 10.1038/srep29713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baum R, Gravallese EM. Impact of inflammation on the osteoblast in rheumatic diseases. Curr Osteoporos Rep. 2014;12(1):9–16. doi: 10.1007/s11914-013-0183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hidayat R, Isbagio H, Setyohadi B, Setiati S. Correlation between receptor activator of nuclear factor-κβ ligand (RANKL), and osteoprotegerin (OPG) with cartilage degradation in rheumatoid arthritis patients. Acta Med Indones. 2014;46(1):24–29. [PubMed] [Google Scholar]
- 8.Ren H, Ren H, Li X, et al. Effects of intermedin on proliferation, apoptosis and the expression of OPG/RANKL/M-CSF in the MC3T3-E1 osteoblast cell line. Mol Med Rep. 2015;12(5):113–17. doi: 10.3892/mmr.2015.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geusens P. The role of RANK ligand/osteoprotegerin in rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2012;4(4):225–33. doi: 10.1177/1759720X12438080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schanen BC, Li X. Transcriptional regulation of mammalian miRNA genes. Genomics. 2011;97:1–6. doi: 10.1016/j.ygeno.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao H, Zhang J, Shao H, et al. MiRNA-340 inhibits osteoclast differentiation via repression of MITF. Biosci Rep. 2017;37(4) doi: 10.1042/BSR20170302. pii: BSR20170302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun KT, Chen MYC, Tu MG, et al. MicroRNA-20a regulates autophagy related protein-ATG16L1 in hypoxia-induced osteoclast differentiation. Bone. 2015;73:145–53. doi: 10.1016/j.bone.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Maeda Y, Farina NH, Matzelle MM, et al. Synovium-derived microRNAs regulate bone pathways in rheumatoid arthritis. J Bone Miner Res. 2017;32:461–72. doi: 10.1002/jbmr.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang M, Lin H, Luo M, et al. Integrated miRNA and mRNA expression profiling of tension force-induced bone formation in periodontal ligament cells. In Vitro Cell Dev Biol Anim. 2015;51(8):797–807. doi: 10.1007/s11626-015-9892-0. [DOI] [PubMed] [Google Scholar]
- 15.Arend WP. The mode of action of cytokine inhibitors. J Rheumatol Suppl. 2002;65(65):16–21. [PubMed] [Google Scholar]
- 16.Kwon JO, Yong DL, Kim H, et al. Tetraspanin 7 regulates sealing zone formation and the bone-resorbing activity of osteoclasts. Biochem Biophys Res Commun. 2016;477(4):1078–84. doi: 10.1016/j.bbrc.2016.07.046. [DOI] [PubMed] [Google Scholar]
- 17.Anastassova-Kristeva M. The origin and development of the immune system with a view to stem cell therapy. J Hematother Stem Cell Res. 2003;12(2):137–54. doi: 10.1089/152581603321628287. [DOI] [PubMed] [Google Scholar]
- 18.Zhao D, Cui D, Wang B, et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50(1):325–30. doi: 10.1016/j.bone.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Kohli SS, Kohli VS. Role of RANKL-RANK/osteoprotegerin molecular complex in bone remodeling and its immunopathologic implications. Indian J Endocrinol Metab. 2011;15:175–81. doi: 10.4103/2230-8210.83401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samara S, Dailiana Z, Chassanidis C, et al. Expression profile of osteoprotegerin, RANK and RANKL genes in the femoral head of patients with avascular necrosis. Exp Mol Pathol. 2014;96:9–14. doi: 10.1016/j.yexmp.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Hairulislam MI, Saravanan S, Thirugnanasambantham K, et al. Swertiamarin, a natural steroid, prevent bone erosion by modulating RANKL/RANK/OPG signaling. Int Immunopharmacol. 2017;53:114–24. doi: 10.1016/j.intimp.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Shen Y, Jiang T, Wang R, et al. (5R)-5-Hydroxytriptolide (LLDT-8) inhibits osteoclastogenesis via RANKL/RANK/OPG signaling pathway. BMC Complement Altern Med. 2015;15:77. doi: 10.1186/s12906-015-0566-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baum R, Gravallese EM. Impact of inflammation on the osteoblast in rheumatic diseases. Curr Osteoporos Rep. 2014;12(1):9–16. doi: 10.1007/s11914-013-0183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeong BC, Kang IH, Hwang YC, et al. MicroRNA-194 reciprocally stimulates osteogenesis and inhibits adipogenesis via regulating COUP-TFII expression. Cell Death Dis. 2014;5(5):e1532. doi: 10.1038/cddis.2014.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Z, Yin H, Liu T, et al. MiR-126-5p regulates osteoclast differentiation and bone resorption in giant cell tumor through inhibition of MMP-13. Biochem Biophys Res Commun. 2014;443(3):944–49. doi: 10.1016/j.bbrc.2013.12.075. [DOI] [PubMed] [Google Scholar]
- 26.Jia J, Zhou H, Zeng X, Feng S. Estrogen stimulates osteoprotegerin expression via the suppression of miR-145 expression in MG-63 cells. Mol Med Rep. 2017;15(4):1539–46. doi: 10.3892/mmr.2017.6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao JJ, Wu ZF, Wang L, et al. MicroRNA-145 mediates steroid-induced necrosis of the femoral head by targeting the OPG/RANK/RANKL signaling pathway. PLoS One. 2016;11:e0159805. doi: 10.1371/journal.pone.0159805. [DOI] [PMC free article] [PubMed] [Google Scholar]