Abstract
An ISES (In Situ Enzymatic Screening) lead pointed to conditions (PMP N-protecting group, Ni(cod)2 catalyst precursor) under which chiral, bidentate phosphines could promote Ni(0)-mediated allylic amination. Therefore, bidentate phosphines bearing central, axial and planar chirality were examined with two model substrates of interest for PLP-enzyme inhibitor synthesis. In the best case, with (R)-MeO-BIPHEP, vinylglycinol derivative 2 was obtained in 75% ee (97% ee, one recrystallization) from 1. Further manipulation provided a Ni(0)-mediated entry into L-vinylglycine.
Graphical Abstract
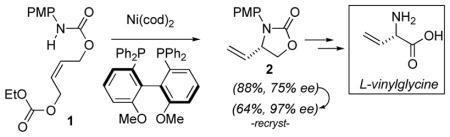
α-Vinyl amino acids are potential inactivators of pyridoxal phosphate (PLP) enzymes.1 ‘Suicide substrates’ bearing this vinylic trigger include the natural product, L-vinylglycine,2 and the anti-epileptic drug, (S)-vigabatrin (γ-vinyl-GABA),3 that may also have potential to treat substance abuse.4 This has led to considerable synthetic activity toward these targets.5,6,7 Among the most efficient and stereocontrolled approaches to appear are transition metal-mediated allylic amination routes by Hayashi,8 Alper,9 Trost10 and Overman.11 Heretofore, such ventures have been focused on palladium.
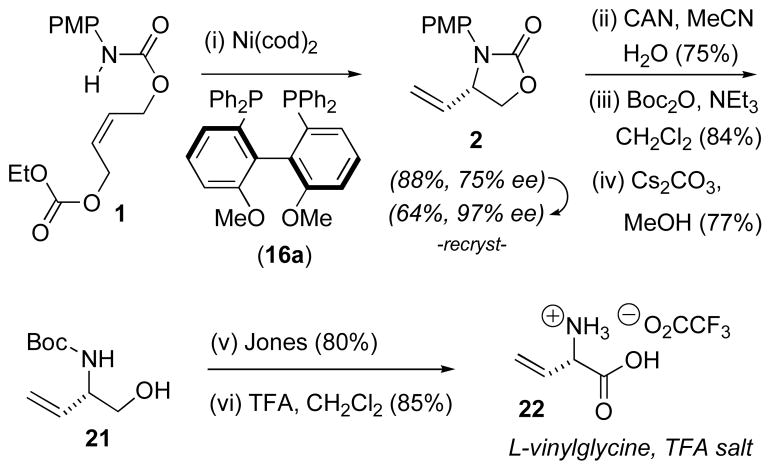
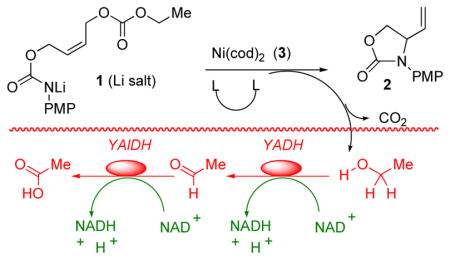
In developing an In Situ Enzymatic Screening (ISES) method for evaluating catalysts, we chose an intramolecular allylic amination route to a protected vinylglycinol (1→2) as a model reaction. Our initial findings demonstrated that, among non-Pd metals screened, Ni(0) showed particular promise for this transformation.12 Furthermore, the PMP nitrogen-protecting group and bidentate phosphine ligands were found to promote this chemistry.
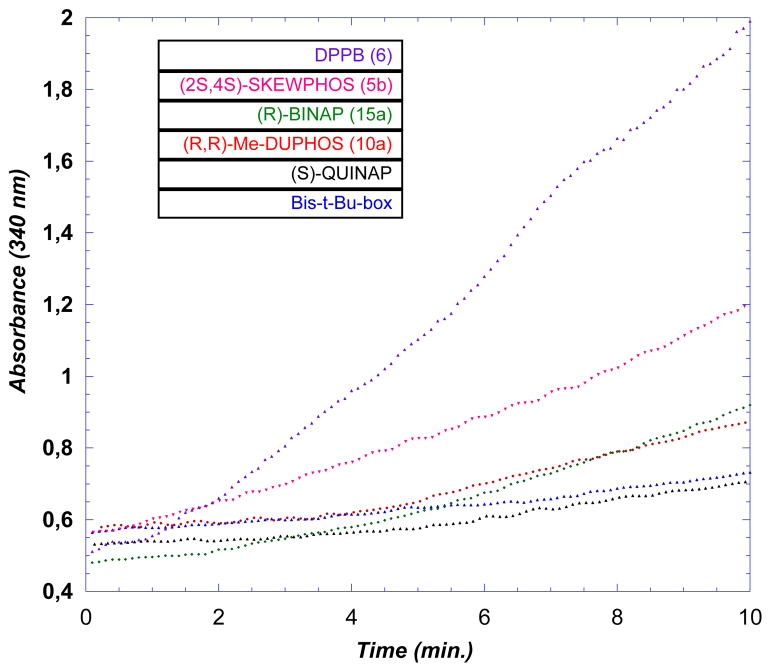
As can be seen in Figure 1, ISES reveals that the combination of Ni(cod)2 with chiral, bidentate phosphines also gives this chemistry. As before, relative ISES rates track well with relative rates of product formation under standard reaction conditions, as judged by NMR at short times (Table 1).
Figure 1.
UV/vis Traces for the ISES Data in Table 1.
Table 1.
ISES Evaluation of Bidentate Ligands for Ni(0)-Mediated Allylic Substitution.
| |||
|---|---|---|---|
| a no. | bidentate ligand | bΔO.D.340/time (mAbs/min) | c% conv |
| 1 | DPPB (6) | 132 ± 16 | 71 |
| 2 | (S,S)-SKEWPHOS (5b) | 64 ± 12 | 39 |
| 3 | (R)-BINAP (15a) | 45 ± 10 | 28 |
| 4 | (R,R)-Me-DUPHOS (10a) | 30 ± 1 | 22 |
| 5 | (S)-QUINAPd | 18 | f |
| 6 | (S,S)-Di-t-Bu-boxe | 15 ± 2 | f |
Conditions for the biphasic ISES screen (YADH = yeast alcohol dehydrogenase and YAlDH = yeast aldehyde dehydrogenase) as described in the Supporting Information.
Obs’d rates (10 min) of NADH formation in units of ΔO.D.340 min−1. Unless otherwise indicated, ISES slopes are reported as mean ± SD (duplicate runs).
Reaction conditions: 200 mM 1, 10 mol% Ni(cod)2, 20 mol% ligand, LiHMDS (1 eq.), THF, rt, 15 min. Product:educt ratio estimated by NMR following work-up.
Ligand is (S)-1-(2-diphenylphosphino-1-naphthyl)isoquinoline.
Ligand is 2,2′-methylenebis[(4S)-4-t-butyl-2-oxazoline].
Trace product (crude NMR).
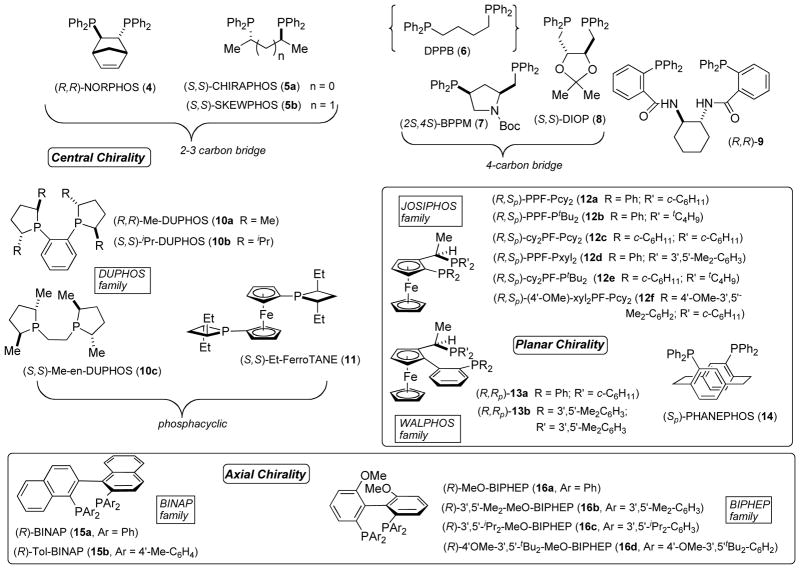
In our earlier studies, dppb had shown the fastest initial rates by ISES, and so it was retained as a standard here. Similarly, SKEWPHOS, a chiral, acyclic 3-carbon bridged bidentate P,P-ligand gives relatively rapid formation of 2. The initial ISES screen also pointed to slower, yet potentially useful conversions with chiral P,P-ligands of the DUPHOS and BINAP variety (Figure 1). On the contrary, neither QUINAP (P,N-BINAP analog) nor a prototypical bis(oxazoline) (N,N-ligand) gave significant ISES rates. Next, it was noted that although unconstrained ligands such as SKEWPHOS gave both very good rates and conversions, better ee’s could be obtained with BINAP or DUPHOS. Clearly, we were not seeing a direct correlation between rate and ee here. On the basis of these observations, it was decided to explore broadly relatively electron-rich chiral bidentate phosphines. The ligand array chosen includes elements of central, planar and axial chirality (Figure 2).
Figure 2.
Array of Chiral Bidentate Phosphine Ligands Examined
The results for the transformation 1→2 are collected in Table 2. A few patterns emerge. Indeed, all bis(diphenylphosphino) L’s with 2–4 sp3-carbon spacers (4, 5a, 5b, 7, 8) give excellent conversions, but low ee’s. The phospholane-based DUPHOS ligands all give excellent conversion, but only the methyl substituted ones (10a, 10c) lead to ee’s of some note, approaching 50%. The JOSIPHOS ligand family incorporates both central chirality and planar chirality. Best results, in terms of both catalysis and enantioselection, are seen with ligands bearing a (diarylphosphino)ferrocene in tandem with a (dialkylphosphino)ethyl moiety (i.e. 12a, 12b, & 12f). A similar structure/activity observation has been made with JOSIPHOS ligands in a model Pd-mediated allylic alkylation reaction.13 Interestingly, in comparing 12a & 12b, one sees that subtle changes in sterics can have significant consequences in ee and rate (Tables 2 & 3). Thus, 12a (R′ = cy), gives higher yields (55–65% vs. 17–23%) with both substrates 1 and 19 (vide infra), but 12b (R′ = tBu) gives higher ee’s (74–82%), indeed among the highest seen here.
Table 2.
Cyclizations of Allylic Amination Substrate 1
| a no. | ligand | yieldb | eec | confd |
|---|---|---|---|---|
| 1 | (R,R)-NORPHOS (4) | 80% | 28% | R |
| 2e | (S,S)-CHIRAPHOS (5a) | 75% | 0% | |
| 3f | (2S,4S)-SKEWPHOS (5b) | 94% | 13% | S |
| 4g | (2S,4S)-BPPM (7) | 87% | 6% | R |
| 5 | (S,S)-DIOP (8) | 79% | 0% | |
| 6 | Trost Ligand (9) | 37% | 4% | S |
| 7h | Trost Ligand (9) | nr | ||
| 8g | (R,R)-Me-DUPHOS (10a) | 89% | 48% | S |
| 9 | (S,S)-i-Pr-DUPHOS (10b) | 86% | 24% | S |
| 10g | (S,S)-Me-en-DUPHOS (10c) | 78% | 46% | R |
| 11 | (S,S)-Et-FerroTANE (11) | 60% | 7% | R |
| 12 | JOSIPHOS-type (12a) | 65% | 56% | S |
| 13 | JOSIPHOS-type (12b) | 17% | 82% | S |
| 14 | JOSIPHOS-type (12c) | 31% | 22% | S |
| 15 | JOSIPHOS-type (12d) | 38% | 0% | |
| 16 | JOSIPHOS-type (12f) | 79% | 43% | S |
| 17 | WALPHOS-type (13a) | nr | ||
| 18 | (Sp)-PHANEPHOS-type (14) | nr | ||
| 19 | (R)-BINAP (15a) | 90% | 46% | S |
| 20 | (R)-Tol-BINAP (15b) | 94% | 59% | S |
| 21 | (R)-MeO-BIPHEP (16a) | 86% | 72% | S |
| 22h | (R)-MeO-BIPHEP (16a) | 88% | 75% | S |
| 23 | (R)- Me2-OMe-BIPHEP (16b) | 32% | 38% | S |
| 24 | (R)- iPr2-OMe-BIPHEP (16c) | 74% | 44% | S |
| 25 | (R)- tBu2-OMe-BIPHEP (16d) | nr |
Reaction conditions: 10 mol% Ni(cod)2, 20 mol% ligand, LiHMDS (1 eq.), THF, rt, overnight (unless otherwise indicated).
All yields are isolated yields of pure products. nr = no reaction observed.
ee’s were determined by chiral HPLC (Chiralcel OD: hexane/i-PrOH 80/20).
Configuration assigned by correlation with L-vinylglycine (Scheme 2).
t = 8 h,
t = 4 h,
t = 6 h,
Reaction carried out without exogenous base (i.e. no LiHMDS); t = 6 h.
Table 3.
Allylic Aminations to Vigabatrin Derivative 20
| ||||
|---|---|---|---|---|
| a no. | ligand | yieldb | eec | confd |
| 1 | (R,R)-Me-DUPHOS (10a) | 93% | 66% | R |
| 2 | (S,S)-iPr-DUPHOS (10b) | 90% | 47% | R |
| 3 | (S,S)-Et-FerroTANE (11) | 28% | 8% | S |
| 4 | JOSIPHOS-type (12a) | 55% | 31% | R |
| 5 | JOSIPHOS-type (12b) | 28% | 74% | R |
| 6 | JOSIPHOS-type (12c) | 23% | 22% | S |
| 7 | JOSIPHOS-type (12d) | 73% | 5% | S |
| 8 | JOSIPHOS-type (12e) | nr | ||
| 9 | JOSIPHOS-type (12f) | 46% | 36% | R |
| 10 | WALPHOS-type (13a) | 8% | 32% | S |
| 11 | WALPHOS-type (13b) | 60% | 53% | S |
| 12 | (R)-BINAP (15a) | 70% | 43% | R |
| 13 | (R)-Tol-BINAP (15b) | 84% | 44% | R |
| 14e | (S)-MeO-BIPHEP (16a) | 97% | 53% | S |
| 15 | (R)-Me2-OMe-BIPHEP (16b) | 65% | 54% | R |
| 16 | (R)-tBu2-OMe-BIPHEP (16d) | 60% | 23% | R |
Reaction conditions: 67 mM 19, 10 mol% Ni(cod)2, 20 mol% ligand, LiHMDS (1 eq.), THF, rt, overnight.
Isolated yields of pure products. nr = no reaction observed.
ee’s determined by chiral HPLC (Chiralcel OD: hexane/i-PrOH 73/27).
Configuration assigned by correlation with the known γ-lactam, following PMP deprotection (CAN, MeCN, H2O): [α]23D (66% ee-chiral HPLC-see SI) –23.7 (EtOH, c 2.0); Lit.: [α]23D {(S)-isomer} +50.4 (EtOH, c 2.2) - ref. 6e.
No exogenous base used (i.e. no LiHMDS).
The most practical results were obtained with ligands possessing axial chirality. Enantioselection steadily increases from BINAP (15a, 46% ee) to tol-BINAP (15b, 59% ee) to MeO-BIPHEP (16a, 72–75% ee),14,15,16 while yields are outstanding (86–94%) across the series. More sterically hindered BIPHEP ligands (16b–d) provided less satisfactory results.
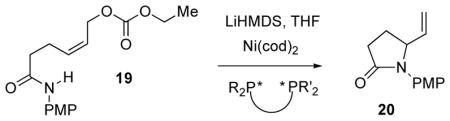
Given the importance of vigabatrin,4,6 we next set out to examine the Ni(0)-mediated transformation 19→20, which would serve as a formal synthesis of the drug. Note that substitution of a CH2 for the bridging O means that an N-PMP amidate replaces the N-PMP carbamate as the formal nitrogen nucleophile.
Pleasingly, it was found that the requisite substrate 19 could be efficiently assembled via C-alkylation of the dianion derived from N-PMP-acetamide (Scheme 1). The ligand survey (Table 3) revealed decreased enantioselection for the axially chiral ligands, with the exception of 16b. But, these ligands were eclipsed by DUPHOS ligand 10a (66% ee) and the PtBu2-bearing JOSIPHOS ligand 12b (74% ee), again, albeit with low conversion for 12b. Interestingly, one of the WALPHOS ligands (13b, 60%, 53% ee) also appears to show promise.
Scheme 1.
Synthesis of the Vigabatrin Precursor
While isolated examples of Ni(0)-mediated allylic amination have been reported,17 to our knowledge, these represent the first asymmetric examples. At this juncture, we chose to examine the best case more closely, namely the (R)-MeO-BIPHEP-Ni(0)-promoted synthesis of 2 (Table 2, entry no. 21). Unfortunately, additives found to be beneficial in other late transition metal-mediated allylic substitutions18 such as NEt3,18a HOAc,18a NBu4OAc,17a LiCl18b (<5% conv.), LiF18c (60%, 73% ee), NBu4F18d (60%, 57% ee), NBu4PF617b (83%, 70% ee) and NBu4BH418d (51%, 26% ee-R) were deleterious here. Changing the N-protecting group from PMP to OMP19 (46%, 64% ee) or TMP19 (83%, 67% ee) was not beneficial. The (E)-isomer of 1 gave the same sense of induction, though at lower ee (65%) and yield (69%). This result is reminiscent of Hayashi’s observations with Pd8 and may be evidence of rapidly equilibrating π-allyl metal intermediates.
However, whereas all of the initial screens had employed 10 mol% Ni(cod)2, 20 mol% L and a full equivalent of base (LiHMDS), it was found that, at least in this case, base could be completely eliminated and MeO-BIPHEP reduced to 10 mol% with essentially no consequence (83%, 75% ee). Gratifyingly, we also found that with a single recrystallization, the ee of the product could be increased from 75% to 97% @ 64% overall yield. This refinement then allowed for a practical entry into L-vinylglycine, centered around this new asymmetric Ni(0)-mediated intramolecular allylic amination (Scheme 2). Studies to further delineate the scope and limitations of this asymmetric Ni(0)-chemistry are in progress and will be described in due course.
Scheme 2.
A Ni(0)-Mediated Synthesis of L-Vinylglycine
Supplementary Material
Acknowledgments
The authors thank the NSF (CHE-0317083) for support. DBB acknowledges the Alfred P. Sloan Foundation for a fellowship. This research was facilitated by shared instrumentation grants for NMR (NIH SIG-1-510-RR-06301, NSF CHE-0091975, NSF MRI-0079750) & GC/MS (NSF CHE-9300831), respectively. We thank Rudolf Schmid (Roche AG), and Hans-Ulrich Blaser & Marc Thommen (Solvias AG), for providing BIPHEP- and Josiphos/Walphos-type ligands, respectively, and Kannan R. Karukurichi for assistance with ISES experiments.
Footnotes
Supporting Information Available. Experimental procedures, representative chiral HPLC traces and 1H NMR spectra for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Berkowitz DB, Jahng W-J, Pedersen ML. Bioorg Med Chem Lett. 1996;6:2151–2156. doi: 10.1016/0960-894X(96)00366-6. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inhibition studies: Liang F, Kirsch JF. Biochemistry. 2000;39:2436–2444. doi: 10.1021/bi9922704.Zheng L, White RH, Cash VL, Dean DR. Biochemistry. 1994;33:4714–20. doi: 10.1021/bi00181a031.Asada Y, Tanizawa K, Yonaha K, Soda K. Agric Biol Chem. 1988;52:2873–2878.Gehring H, Rando RR, Christen P. Biochemistry. 1977;16:4832–4836. doi: 10.1021/bi00641a012.Soper TS, Manning JM, Marcotte PA, Walsh CT. J Biol Chem. 1977;252:1571–1575.
- 3.Jung MJ, Palfreyman MG. Vigabatrin. In: Levy RH, Mattson RH, editors. Antiepileptic Drugs. 4. Raven; New York: 1995. [Google Scholar]
- 4.(a) Brodie JD, Figueroa E, Dewey SL. Synapse. 2003;50:261–265. doi: 10.1002/syn.10278. [DOI] [PubMed] [Google Scholar]; (b) Gerasimov MR, Dewey SL. Drug Dev Res. 2003;59:240–248. [Google Scholar]
- 5.L-Vinylglycine: Rose NGW, Blaskovich MA, Wong A, Lajoie GA. Tetrahedron. 2001;57:1497–1507.Campbell AD, Raynam TM, Taylor RJK. Synthesis. 1998:1707–1709.Badorrey R, Cativiela C, Diaz-de-Villegas MD, Galvez JA. Synthesis. 1997:747–749.Berkowitz DB, Smith MK. Synthesis. 1996:39–41. doi: 10.1055/s-1996-4177.Griesbeck AG. Hirt, J Liebigs Ann. 1995:1957–1961.Hallinan KO, Crout DHG, Errington W. J C S Perkin 1. 1994:3537–3543.Carrasco M, Jones RJ, Kamel S, Rapoport H, Truong T. Org Synth. 1991;70:29–34.Pellicciari R, Natalini B, Marinozzi M. Synthetic Commun. 1988;18:1715–1721.Duhamel L, Duhamel P, Fouquay S, Eddine JJ, Peschard O, Plaquevent JC, Ravard A, Solliard R, Valnot JY, Vincens H. Tetrahedron. 1988;44:5495–5506.Barton DHR, Crich D, Herve Y, Potier P, Thierry J. Tetrahedron. 1985;41:4347–4357.Hanessian S, Sahoo SP. Tetrahedron Lett. 1984;25:1425–1428.Schöllkopf U, Nozulak J, Groth U. Tetrahedron. 1984;40:1409–1417.
- 6.(S)-Vigabatrin: Anderson CE, Overman LE. J Am Chem Soc. 2003;125:12412–12413. doi: 10.1021/ja037086r.Masson G, Zeghida W, Cividino P, Py S, Vallee Y. Synlett. 2003:1527–1529.Chandrasekhar S, Mohapatra S. Tetrahedron Lett. 1998;39:6415–6418.Alcón M, Poch M, Moyano A, Pericàs MA, Riera A. Tetrahedron: Asymmetry. 1997;8:2967–2974.Wei ZY, Knaus EE. Tetrahedron. 1994;50:5569–78.Wei ZY, Knaus EE. J Org Chem. 1993;58:1586–8.
- 7.Enantioenriched quaternary, α-vinylic AA’s: Ma D, Zhu W. J Org Chem. 2001;66:348–350. doi: 10.1021/jo0013711.Berkowitz DB, McFadden JM, Chisowa E, Semerad CL. J Am Chem Soc. 2000;122:11031–11032. doi: 10.1021/ja0055110.Berkowitz DB, McFadden JM, Sloss MK. J Org Chem. 2000;65:2907–2918. doi: 10.1021/jo9918091.Avenoza A, Cativiela C, Corzana F, Peregrina JM, Zurbano MM. J Org Chem. 1999;64:8220–8225. doi: 10.1021/jo990957o.Berkowitz DB, Pumphrey JA, Shen Q. Tetrahedron Lett. 1994;35:8743–8747.Colson PJ, Hegedus LS. J Org Chem. 1993;58:5918–5924.Seebach D, Bürger HM, Schickli CP. Liebigs Ann Chim. 1991:669–684.Weber T, Aeschimann R, Maetzke T, Seebach D. Helv Chim Acta. 1986;69:1365–1377.Groth U, Schöllkopf U, Chiang YC. Synthesis. 1982:864–866.
- 8.Hayashi T, Yamamoto A, Ito Y. Tetrahedron Lett. 1988;29:99–102. [Google Scholar]
- 9.Larksarp C, Alper H. J Am Chem Soc. 1997;119:3709–3715. [Google Scholar]
- 10.Trost BM, Bunt RC, Lemoine RC, Calkins TL. J Am Chem Soc. 2000;122:5968–5976. [Google Scholar]
- 11.Overman uses a Pd(II) catalyst and invokes an intramolecular, aminopalladation mechanism that preserves the Pd(II) oxidation state throughout the catalytic cycle: Overman LO, Remarchuk TP. J Am Chem Soc. 2002;124:12–13. doi: 10.1021/ja017198n.
- 12.Berkowitz DB, Bose M, Choi S. Angew Chem Int Ed. 2002;41:1603–1607. doi: 10.1002/1521-3773(20020503)41:9<1603::aid-anie1603>3.0.co;2-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Togni A, Breutel C, Schnyder A, Spindler F, Landert H, Tijani A. J Am Chem Soc. 1994;116:4062–4066. [Google Scholar]
- 14.Schmid R, Foricher J, Cereghetti M, Schoenholzer P. Helv Chim Acta. 1991;74:370–389. [Google Scholar]
- 15.BIPHEP ligands have been utilized mostly for asymmetric Ru-mediated ketone reductions. For a leading reference, see: Jeulin S, Duprat de Paule S, Ratovelomanana-Vidal V, Genêt J-P, Champion N, Dellis P. Angew Chem Int Ed. 2004;43:320–325. doi: 10.1002/anie.200352453.
- 16.For examples of Pd-mediated allylic substitutions using BIPHEP, see: Sinou D, Rabeyrin C, Nguefack C. Advanced Synthesis & Catalysis. 2003;345:357–363.Bolm C, Kaufmann D, Gessler S, Harms K. J Organomet Chem. 1995;502:47–52.Barbara PS, Pregosin PS, Salzmann R, Albinati A, Kunz RW. Organometallics. 1995;14:5160–5170.
- 17.Bricout H, Carpentier JF, Mortreux A. Tetrahedron. 1998;54:1073–1084.Bricout H, Carpentier JF, Mortreux A. Chem Commun. 1995:1863–1864.For evidence of π-allyl nickel intermediates, see: Moberg C. Tetrahedron Lett. 1980;21:4539–4542.Yamamoto T, Ishizu J, Yamamoto A. J Am Chem Soc. 1981;103:6863–6869.Tolman CA. J Am Chem Soc. 1970;92:6785–6790.For examples of substrate-directed, regio- and stereoselective Ni(0)-mediated substitutions of allylic ethers with Grignard reagents, see: Didiuk MT, Morken JP, Hoveyda AH. Tetrahedron. 1998;54:1117–1130.Farthing CN, Kocovsky P. J Am Chem Soc. 1998;120:6661–6672.
- 18.(a) Trost BM, Shen HC, Dong L, Surivet J-P. J Am Chem Soc. 2003;125:9276–9277. doi: 10.1021/ja036052g. [DOI] [PubMed] [Google Scholar]; (b) Bartels B, García-Yebra C, Rominger F, Helmchen G. Eur J Inorg Chem. 2002:2569–2586. [Google Scholar]; (c) Welter C, Koch O, Lipowsky G, Helmchen G. Chem Commun. 2004:896–897. doi: 10.1039/b400023d. [DOI] [PubMed] [Google Scholar]; (d) Burckhardt U, Baumann M, Togni A. Tetrahedron: Asymmetry. 1997;8:155–159. [Google Scholar]
- 19.OMP = o-methoxyphenyl. TMP = 3,4,5-trimethoxyphenyl.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.