Brachioradial pruritus (BRP) is an unusual neuropathic condition that is characterized by itching, burning, stinging, or tingling of the upper extremities.1 Symptoms can be unilateral or bilateral and most frequently affect the dorsolateral surfaces of the arms (Fig 1), although there may be involvement of the face, neck, chest wall, or lower extremities.2 Skin findings can be minimal, often limited to secondary excoriations or lichenification created by scratching.3 Discomfort can be so severe that patients resort to placing ice packs on the skin, termed the “ice-pack sign.”4 Patients are most often in middle age with a female predominance.5
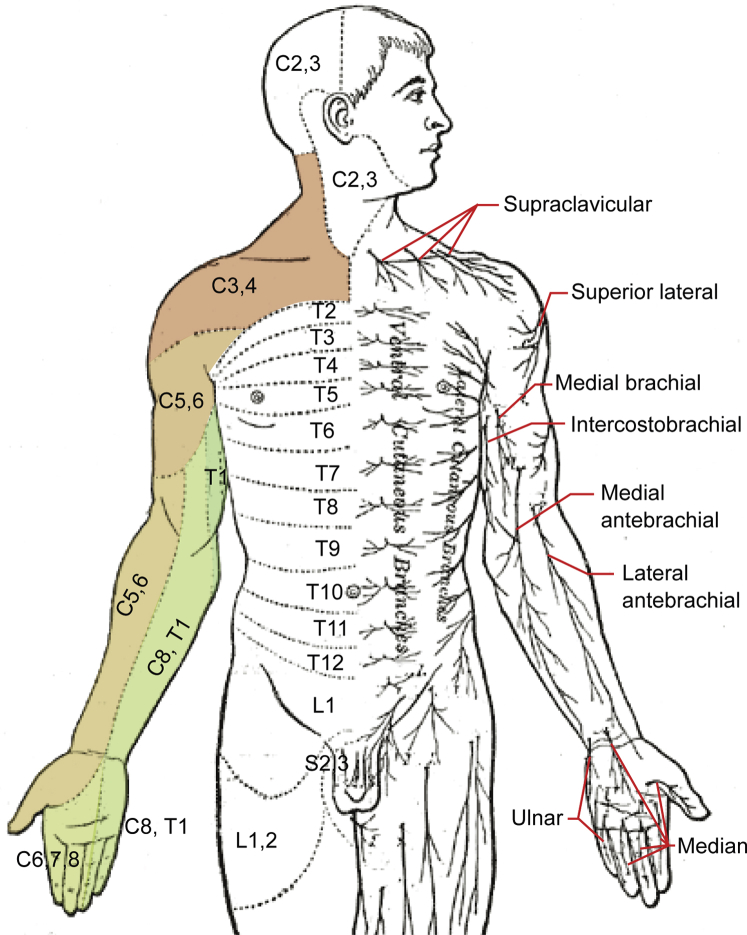
Fig 1.
Schematic of sensory dermatomes and sensory nerves of the upper extremity. The regions most commonly affected in brachioradial pruritus are shaded.
Modification of original work by Mikael Häggström.17 Used with permission.
The cause of BRP is unknown, although several mechanisms have been proposed, including sun exposure.6 Reports of cervical spine degenerative changes or spinal cord masses involving the symptomatic dermatome suggest that compressive neuropathy may play a role in disease pathogenesis.7, 8, 9 BRP is likely a result of a complex combination of factors,10 including light exposure, trauma,11 and nerve injury.12
Many treatments for BRP have been tried with varying degrees of success.5 Initial therapy consists of conservative symptomatic therapy, such as topical steroids, antihistamines, and antiinflammatory agents. Other treatments of neuropathic pain and itching, including capsaicin, amitriptyline, and gabapentin, can sometimes be successful.5, 12, 13 Many patients are refractory to multiple treatments.
Because of the association between BRP and cervical spine compression, some patients have been treated with minimally invasive steroid injections or surgical decompression.3 While these results have also been mixed,14 the positive outcomes have inspired interest in further investigating treatment of nerve root compression as a means of alleviating BRP symptoms.
We evaluate the use of minimally invasive computed tomography (CT)-guided steroid/anesthetic injections in the cervical spine as a treatment for BRP, a topic that has not been systematically addressed in the literature.
Methods
Our institutional review board approved this study. The electronic medical record from 2010 to 2016 was reviewed to identify patients with a clinical diagnosis of BRP who had also undergone epidural steroid injection of the cervical spine. Patient demographics, symptoms, diagnostic imaging, and procedural information regarding the cervical spine as well as outcomes of treatments were collected and reviewed. Pre- and postprocedure pruritus intensity was determined by a numeric rating scale in which patients rated their itch intensity from 0 (“no itch”) to 10 (“worst imaginable itch”).15
Transforaminal epidural steroid injections of the cervical spine were performed using CT guidance. Interventional targets were chosen based on diagnostic imaging combined with the distribution of symptoms. Moderate sedation was achieved with intravenous fentanyl and midazolam. After obtaining a low-dose planning CT, intermittent imaging was used to place 25-gauge, 6-cm spinal needles in the target neural foramina. A small amount of iodinated contrast diluted with sterile saline was injected to confirm epidural and extravascular positioning. After confirming positioning, a mixture of dexamethasone (10 mg/mL), bupivacaine (0.75%), and lidocaine (1%) in a 2:1:1 ratio was slowly injected with intermittent imaging guidance. Up to 1.0 mL of mixture was injected at each site in accordance with the standard procedure for treatment of cervical pain, with the full amount not injected if the patient began experiencing symptoms during the injection. Treatment was repeated as clinically appropriate for residual or recurrent symptoms.
Results
Three patients were identified who had a diagnosis of BRP and underwent CT-guided epidural steroid injection (Table I). All 3 patients were female, had bilateral symptoms, and were on average 66 years of age. After treatment, 2 patients had near complete resolution of symptoms after a single intervention. The third patient received a total of 3 injections with mild to moderate relief that continued to improve on mexiletine. There were no adverse events.
Table I.
Summary of patients with brachioradial pruritus treated with cervical nerve root blocks
| Patient no. | Age/sex | Symptom distribution | Foraminal stenosis | CT-guided treatments | Pruritus score |
|
|---|---|---|---|---|---|---|
| Preprocedure | Postprocedure | |||||
| 1 | 51/F | Bilateral shoulders, arms, and neck | C4-C5: Mild left | (1) C4-C5: Left; C6-C7: bilateral | 5 | 1 |
| C6-C7: Mild bilateral | ||||||
| 2 | 89/F | Bilateral posterior neck and upper back | C3-C4: Severe bilateral | (1) C3-C4: Bilateral | 10 | 3 |
| C4-C5: Moderate bilateral | ||||||
| 3 | 57/F | Bilateral entire upper extremities | C3-C4: Severe left | (1) C4-C5: Bilateral; C5-C6: bilateral | 10 | 7 |
| C4-C5: Moderate right | (2) C6-C7: Bilateral | 10 | 7 | |||
| C5-C6: Severe right | (3) C4-C5: Bilateral; C5-C6: bilateral | 10 | 0 | |||
| C6-C7: Mild bilateral | ||||||
CT, Computed tomography; F, female; M, male.
Patient 1 was a 51-year-old woman with a longstanding history of rheumatoid arthritis, atopy, and hypothyroidism. Her rheumatoid arthritis was well-controlled with methotrexate and rituximab requiring infrequent short courses of prednisone. Hypothyroidism was treated with levothyroxine. She presented with 3 years of bilateral shoulder and forearm pruritis with associated demonstrated erythema and dermatoheliosis on the face, neck, and arms. She obtained moderate relief from tricyclic antidepressants (doxepin and nortriptyline). A magnetic resonance imaging (MRI) scan of the cervical spine obtained for neck pain demonstrated neural foraminal stenosis on the left at C4-C5 and bilaterally at C6-C7. CT-guided transforaminal steroid injections were performed on the left at C4-C5 and bilaterally at C6-C7. She had near complete resolution of symptoms (itch score of 1) 2 months after the procedure. No further intervention was performed.
Patient 2 was an 89-year-old woman with a 3-year history of intense pruritus of the posterior neck and spreading to the upper back. Treatment with diphenhydramine, lorazepam, gabapentin, and topical agents provided little relief. CT of the cervical spine demonstrated degenerative changes from C3 to C6, with the greatest foraminal stenosis at C3-C4. This patient did not have an MRI performed. Because the symptom distribution corresponded to the C4 dermatome, CT-guided transforaminal steroid injections were performed bilaterally at C3-C4. The patient had near complete symptom resolution immediately after the procedure. Three months after the procedure, she had an itch score of 3 and continued to take pregabalin for her pruritis. No further procedures were performed.
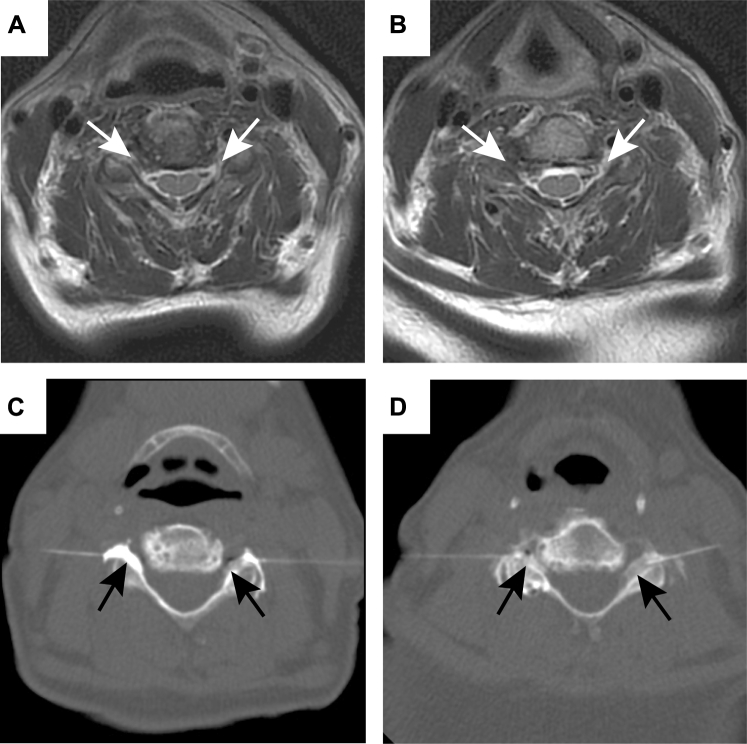
Patient 3 was a 57-year-old woman with a 20-year history of bilateral upper extremity pruritus. Her symptoms were initially intermittent but became progressively worse and peaked 2 years before presentation. Initial itch severity was 10, and she got little relief from oral or topical medications. She underwent >25 superficial trigger point injections with moderate relief. MRI of the cervical spine demonstrated multilevel degenerative changes with and neural foraminal narrowing from C4 to C7. Because her symptoms were worse in higher dermatomes, initial CT-guided transforaminal steroid injections were performed bilaterally at C4-C5 and C5-C6 (Fig 2). After 2 months, the patient had an overall itch severity of 7, with improvement of symptoms in the neck and shoulders with continued symptoms in the arms. Because of the incomplete relief, 3 months after the initial injections, bilateral injections were performed at C6-C7, again with moderate relief of symptoms. Two months after the second injections, the patient continued to have intense flares of 10 of 10 itching, although decreased in frequency. Six months after the initial injections, a third round of injections was performed bilaterally at C4-C5 and C5-C6. At this time, the patient began oral mexiletine and had complete resolution of itch (itch score of 0) that was maintained 15 months postprocedure.
Fig 2.
Magnetic resonance imaging and computed tomography procedural images from a 57-year-old woman with brachioradial pruritus (patient 3). Magnetic resonance imaging through the C4-C5 (A) and C5-C6 (B) neural foramina showing severe bilateral narrowing at both levels (white arrows). Intraprocedural images from her first treatment show the injection along the bilateral C5 (C) and C6 exiting nerve roots (D). Injected steroid and anesthetic mixture is seen flowing into the neural foramina bilaterally at each injection site (black arrows).
Discussion
BRP is a poorly understood condition that has been associated with cervical spine degenerative disease and cervical spine tumors.16 However, the relationship between minimally invasive treatment of cervical spine disease and improvement of pruritus has been only anecdotal. In this report, we present 3 patients with BRP and concurrent cervical degenerative disease who were treated with CT-guided transforaminal cervical nerve root blocks. Two of 3 patients experienced near complete relief from a single procedure, while the third got moderate relief from multiple procedures and further improved with medical management.
While other reports have also shown improvement of BRP after surgery for degenerative disease3 or spinal tumor,16 this is the largest series of patients showing benefit from minimally invasive treatment of cervical nerve root compression. The treatment mechanism remains uncertain and is somewhat unexpected given relief continues beyond the expected time of action of the steroids and anesthetics. It is possible that the injections mediate the inflammatory component of nerve compression or even disrupt a positive feedback loop in these patients with chronic neurogenic pain, although further study is warranted.
The limitations of this paper are its relatively small number of patients and retrospective design. Patients were also selected by retrospectively evaluating patients who had both BRP and minimally invasive nerve blocks, which can lead to bias in patient selection and limit conclusions about the prevalence of cervical spine disease in contributing to BRP in general. Further prospective studies including a wider range of patients may better elucidate the effects of this treatment, including on other broader outcome measures, such as patient quality of life.
These promising positive results in a small set of patients suggest the need for further study to validate the clinical utility of percutaneous CT-guided epidural steroid injections to treat BRP symptoms. Because of the potential role of cervical nerve root compression in BRP, MRI of the cervical spine should be considered in patients with refractory disease, especially if those patients have neck pain or radiculopathy. If patients have degenerative narrowing at relevant levels, subsequent minimally invasive nerve blocks may be an option to provide symptomatic relief by supplementing conventional treatment methods.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
Presented as an electronic exhibit at the 2017 American Society of Spine Radiology Annual Symposium, San Diego, CA, February 22-26, 2017.
References
- 1.Lott M.E., Dudelzak J., Sheehan D. What is your diagnosis? Brachioradial pruritus. Cutis. 2007;80(21):3–4. [PubMed] [Google Scholar]
- 2.Kwatra S.G., Stander S., Bernhard J.D., Weisshaar E., Yosipovitch G. Brachioradial pruritus: a trigger for generalization of itch. J Am Acad Dermatol. 2013;68:870–873. doi: 10.1016/j.jaad.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Binder A., Folster-Holst R., Sahan G. A case of neuropathic brachioradial pruritus caused by cervical disc herniation. Nat Clin Pract Neurol. 2008;4:338–342. doi: 10.1038/ncpneuro0807. [DOI] [PubMed] [Google Scholar]
- 4.Bernhard J.D., Bordeaux J.S. Medical pearl: the ice-pack sign in brachioradial pruritus. J Am Acad Dermatol. 2005;52:1073. doi: 10.1016/j.jaad.2005.02.056. [DOI] [PubMed] [Google Scholar]
- 5.Mirzoyev S.A., Davis M.D. Brachioradial pruritus: Mayo Clinic experience over the past decade. Br J Dermatol. 2013;169:1007–1015. doi: 10.1111/bjd.12483. [DOI] [PubMed] [Google Scholar]
- 6.Waisman M. Solar pruritus of the elbows (brachioradial summer pruritus) Arch Dermatol. 1968;98:481–485. [PubMed] [Google Scholar]
- 7.Goodkin R., Wingard E., Bernhard J.D. Brachioradial pruritus: cervical spine disease and neurogenic/neuropathic [corrected] pruritus. J Am Acad Dermatol. 2003;48:521–524. doi: 10.1067/mjd.2003.203. [DOI] [PubMed] [Google Scholar]
- 8.Marziniak M., Phan N.Q., Raap U. Brachioradial pruritus as a result of cervical spine pathology: the results of a magnetic resonance tomography study. J Am Acad Dermatol. 2011;65:756–762. doi: 10.1016/j.jaad.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 9.Veien N.K., Laurberg G. Brachioradial pruritus: a follow-up of 76 patients. Acta Derm Venereol. 2011;91:183–185. doi: 10.2340/00015555-1006. [DOI] [PubMed] [Google Scholar]
- 10.Veien N.K., Hattel T., Laurberg G., Spaun E. Brachioradial pruritus. J Am Acad Dermatol. 2001;44:704–705. doi: 10.1067/mjd.2001.112912. [DOI] [PubMed] [Google Scholar]
- 11.Crane D.A., Jaffee K.M., Kundu A. Intractable pruritus after traumatic spinal cord injury. J Spinal Cord Med. 2009;32:436–439. doi: 10.1080/10790268.2009.11753261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barry R., Rogers S. Brachioradial pruritus—an enigmatic entity. Clin Exp Dermatol. 2004;29:637–638. doi: 10.1111/j.1365-2230.2004.01642.x. [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz S., Ceyhan A.M., Baysal Akkaya V. Brachioradial pruritus successfully treated with gabapentin. J Dermatol. 2010;37:662–665. doi: 10.1111/j.1346-8138.2010.00830.x. [DOI] [PubMed] [Google Scholar]
- 14.Crevits L. Brachioradial pruritus—a peculiar neuropathic disorder. Clin Neurol Neurosurg. 2006;108:803–805. doi: 10.1016/j.clineuro.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Pereira M.P., Stander S. Assessment of severity and burden of pruritus. Allergol Int. 2017;66:3–7. doi: 10.1016/j.alit.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Kavak A., Dosoglu M. Can a spinal cord tumor cause brachioradial pruritus? J Am Acad Dermatol. 2002;46:437–440. doi: 10.1067/mjd.2002.113674. [DOI] [PubMed] [Google Scholar]
- 17.Häggström M. Medical gallery of Mikael Häggström 2014. WikiJournal of Medicine. 2014:1. [Google Scholar]