Abstract
Introduction
Renal venous graft restenosis is an uncommon event usually associated with significant clinical impact. Its treatment by endovascular stenting is seldom reported in the literature.
Report
Two cases of successful stenting for restenosis in aorto-renal venous grafts are described, detailing the technique and in one case reporting for the first time the use of a covered stent in this condition.
Discussion
Technical success may be achieved with proper material selection for the patient's anatomy and with dilation at relatively high pressures. The use of a covered stent may provide extra safety when treating vein grafts.
Keywords: Renal artery disease, Restenosis, Stent graft
Highlights
-
•
The first report of covered stenting for restenosis of aorto-renal venous bypass.
-
•
These type of restenosis require higher dilation pressures which can lead to rupture of the graft or the anastomosis.
-
•
Using stent-grafts may be safer in case of disruption.
Introduction
Based on Goldblatt's innovative work defining the pathophysiology of renovascular disease in 1934,1 surgical treatment has evolved from nephrectomy to open surgical procedures, mostly bypass techniques using venous or synthetic grafts.2, 3, 4 Presently, the preferred technique for renal artery revascularisation is endovascular angioplasty or stenting, a method introduced by Gruntzig in 1978,5 and currently used in many patients.
One complication of renal bypass grafts is restenosis, which has been reported as having an incidence of 3.4%.6 Restenosis of renal artery bypasses is always difficult to manage and, surprisingly, there are few descriptions of its treatment by an endovascular procedure, with only three cases reported in the literature.7, 8
The aim of this study is to report two cases of renal bypass graft stenoses successfully treated by endovascular stenting, and to detail and discuss the technical aspects of the procedure.
Case 1
In 2006, a 72 year old woman with arterial hypertension underwent an aorto-bifemoral bypass for occlusive aortic disease associated with prosthetic revascularisation of the superior mesenteric artery and bilateral venous bypass to both renal arteries due to tight ostial stenosis (inflow from the aortic graft). After the procedure, renal function and hypertension normalised. During follow up, bilateral ureteral stenting was needed to manage ureteral compression.
Two years after the initial surgery, renal function deteriorated (blood creatinine increased from 0.7 mg/dL to 2.7 mg/dL) and, to achieve proper blood pressure control, the number of antihypertensive medications prescribed increased from two to four. An aortic angiogram documented severe stenosis of both venous renal grafts.
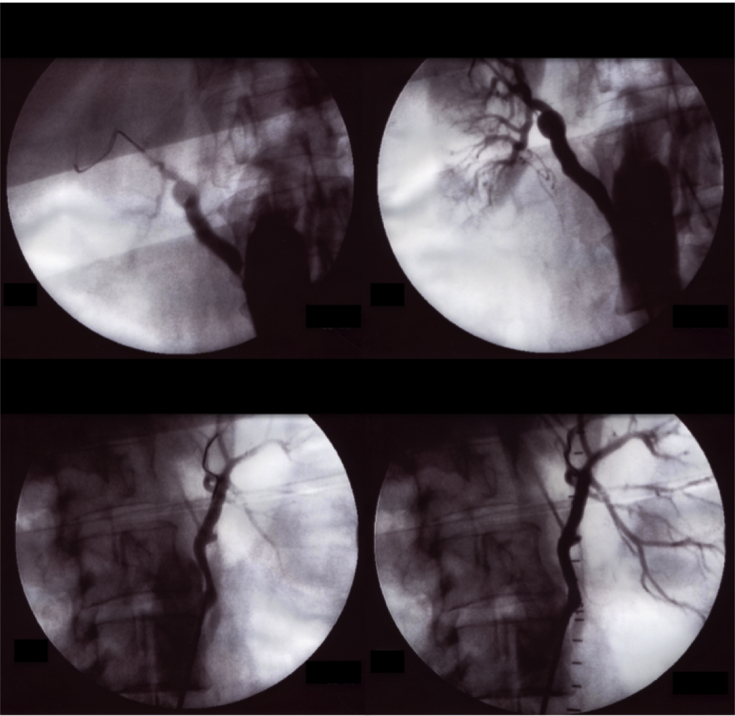
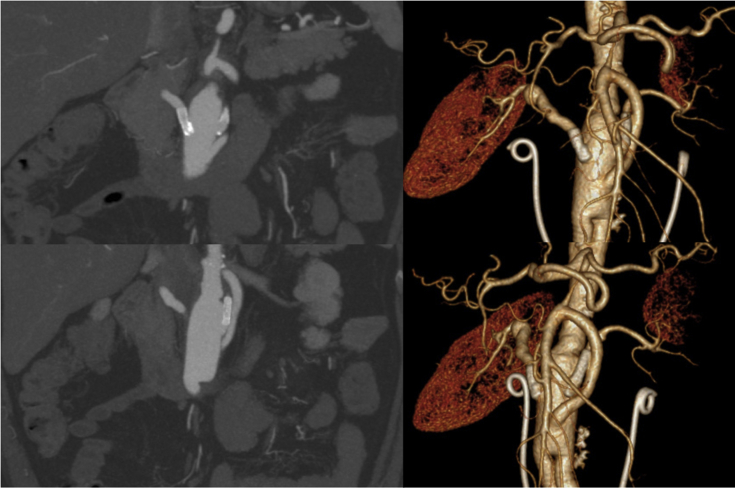
Under general anaesthesia and by exposure of the right superficial femoral artery to avoid exposure of the Dacron limb (thus reducing the chance of an infectious complication), a 7 Fr sheath was inserted and a guiding catheter (Mach1™ Guide Catheter; Boston Scientific, Natick, MA, USA) was advanced to the main body of the aortic graft. Catheterisation of the venous conduits was performed with a 0.035 Magic Torque™ Guidewire (Boston Scientific) followed by deployment of balloon expandable stents in the proximal anastomosis on the right and at the middle portion of the bypass on the left (Express™ SD Renal Monorail™ 6 × 18 mm on the right and 5 × 19 mm on the left; Boston Scientific [Fig. 1]). The procedure was uneventful. After 8 years of follow up the patient remains asymptomatic with blood creatinine stable at 1.4 mg/dL, and is currently taking two antihypertensive drugs. A recent computed tomographic angiogram (CTA) showed patency of both stents (Fig. 2).
Figure 1.
Pre-occlusive stenosis of the right aorto-renal bypass and moderate stenosis of the left aorto-renal bypass; the angiographic result after placement of balloon expandable stents.
Figure 2.
Computed tomography angiography showing patency of both stents.
Case 2
A 69 year old man with ischaemic heart disease (having undergone a coronary artery bypass graft 13 years previously), hypertension, dyslipidaemia, chronic kidney disease (stage III/V), and peptic ulcer had open surgery for treatment of a juxtarenal aortic aneurysm in 2013. This procedure included aorto-bifemoral graft interposition, associated with right renal artery bypass with the great saphenous vein (inflow from the aortic graft), owing to a severe stenosis in the origin of that artery (atrophic left kidney). The recovery was uneventful, with improvement of hypertension and stabilisation of renal function (blood creatinine level 1.4mg/dL [stage III/V]).
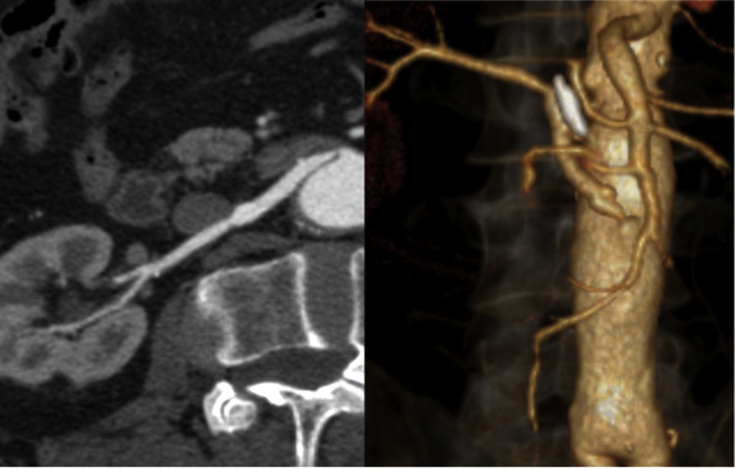
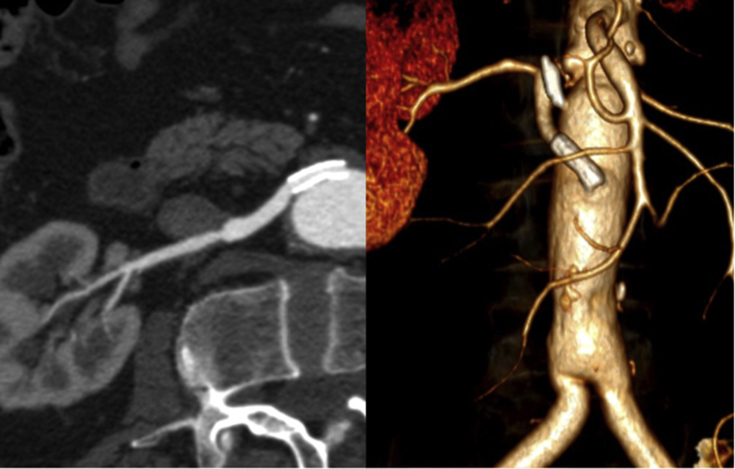
After three years of follow up, a deterioration in renal function was observed (blood creatinine increased from 1.4 mg/dL to 2.5mg/dL) and a CTA revealed severe stenosis of the renal venous bypass (Fig. 3). Owing to the recent decline of renal function and the patient's comorbidities (ischaemic heart disease with poor ejection fraction), it was decided to proceed with an endovascular intervention.
Figure 3.
Computed tomography angiography revealing a stenosis of the aorto-renal bypass.
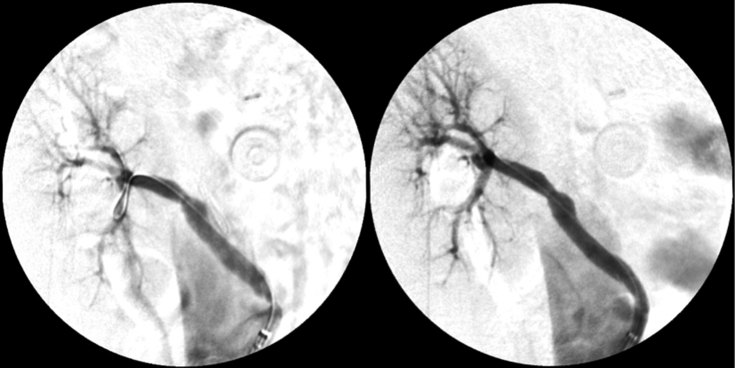
Under local anaesthesia, and using a percutaneous left femoral approach, a long 7 F introducer (Flexor® Introducer; Cook Medical, Bloomington, IN, USA) was placed for support. Catheterisation of the lesion was performed using a 0.014 inch guidewire (ProVia® 9 Guidewire; Medtronic, Minneapolis, MN, USA) supported by a TempoTMAqua 5 F Cobra C2 catheter (Cordis, Bridgewater, NJ, USA). Then, a 4 × 20 mm balloon (Advance®; Cook Medical) was advanced for predilation and, finally, an Atrium Advanta V12 5 × 22 mm covered stent (Maquet, Rastatt, Germany) was placed across the stenotic region (pre-rupture pressure was necessary for a good angiographic result; Fig. 4).
Figure 4.
Angiography documenting the stenosis of the aorto-renal bypass and the final result, after covered stenting.
The procedure was uneventful and the patient was discharged after 2 days. Currently (15 months later), the patient remains asymptomatic, with controlled blood pressure (while still taking the same three antihypertensive drugs) and with a baseline blood creatinine level of 1.5 mg/dL (stage III/V). The control CTA confirmed stent patency (Fig. 5).
Figure 5.
Computed tomography angiography demonstrating covered stent patency.
Discussion
Two cases of renal bypass graft stenosis successfully treated by endovascular stenting are reported. Surprisingly, despite its common use in the renal territory, there are, to the best of the authors' knowledge, only two papers (with three cases) published in the literature describing the endovascular treatment of stenosis in aorto-renal bypasses.7, 8
Restenosis is a cause of recurrence of difficult to control hypertension and decrease of renal function and these clinical features are the main reason to support its treatment.
In both of the present cases, restenosis developed after 2–3 years of follow up, and intimal hyperplasia is the most likely aetiology.
Like other arterial territories, endovascular dilatation should be the first line management option and restenosis in vein grafts usually requires higher dilation pressures,9 which is what was observed in the cases reported herein. However, excessive pressures can lead to rupture of the graft or the anastomosis. Therefore, selection of the correct stent size is crucial and the use of a covered stent may be safer in case of disruption. To the authors' knowledge, this is the first report of a covered stent graft used for this purpose.
The need to extend the experience to support the safety and efficacy of this technique is acknowledged, although the follow up of case 1 has been long (8 years) with an excellent clinical and CTA result.
Conclusion
A large number of patients with renal artery stenosis/aneurysm have been treated by open surgery using venous or synthetic grafts. Vein graft restenosis seems to be an uncommon occurrence and awareness of this potential problem is important. The endovascular treatment of vein graft restenosis is seldom reported in the literature and two cases, where a good result was achieved with stenting, are reported.
Conflict of interest
None.
Funding
None.
References
- 1.Goldblatt H., Lynch J., Hanzal R.F., Summerville W.W. Studies on experimental hypertension. J Exp Med. 1934;59:347–379. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman N.E., Leeds F.H., Elliott W.G., Roland M.D. Thromboendarterectomy for hypertension due to renal artery occlusion. JAMA. 1954;156:1077–1079. doi: 10.1001/jama.1954.02950110039012. [DOI] [PubMed] [Google Scholar]
- 3.Decamp P.T., Birchall R. Recognition and treatment of renal arterial stenosis associated with hypertension. Surgery. 1958;43:134–151. [PubMed] [Google Scholar]
- 4.Luke J.C., Levitan B.A. Revascularization of the kidney in hypertension due to renal artery stenosis. AMA Arch Surg. 1959;79:269–275. doi: 10.1001/archsurg.1959.04320080105012. [DOI] [PubMed] [Google Scholar]
- 5.Grüntzig A., Kuhlmann U., Vetter W., Lütolf U., Meier B., Siegenthaler W. Treatment of renovascular hypertension with percutaneous transluminal dilatation of a renal-artery stenosis. Lancet. 1978;1:801–802. doi: 10.1016/s0140-6736(78)93000-3. [DOI] [PubMed] [Google Scholar]
- 6.Cherr G.S., Hansen K.J., Craven T.E., Edwards M.S., Ligush J., Jr., Levy P.J. Surgical management of atherosclerotic renovascular disease. J Vasc Surg. 2002;35:236–245. doi: 10.1067/mva.2002.120376. [DOI] [PubMed] [Google Scholar]
- 7.Kusakabe M., Sasaki H., Sato J., Akahane M., Miyata T., Ohtomo K. Percutaneous transluminal renal angioplasty with stenting for stenotic venous bypass grafts: report of two cases. SpringerPlus. 2013;2:456. doi: 10.1186/2193-1801-2-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garfinkel H.B., Rohr R.E., Rottenberg R.W. Angioplasty of a stenotic aorto-renal artery saphenous vein bypass graft to a single kidney. Am J Kidney Dis. 1984;4:171–174. doi: 10.1016/s0272-6386(84)80067-0. [DOI] [PubMed] [Google Scholar]
- 9.Trerotola S.O., Kwak A., Clark T.W., Mondschein J.I., Patel A.A., Soulen M.C. Prospective study of balloon inflation pressures and other technical aspects of hemodialysis access angioplasty. J Vasc Interv Radiol. 2005;16:1613–1618. doi: 10.1097/01.RVI.0000183588.57568.36. [DOI] [PubMed] [Google Scholar]