Abstract
OBJECTIVE: Asian melanoma patients, predominantly comprised of acral and mucosal subtypes, might not benefit from immunotherapy and targeted therapy as much as Caucasian patients. Novel treatment strategies are demanded after conventional treatment failure. This was a prospective, single-arm, and single-center dose escalation study to investigate the safety and preliminary efficacy of apatinib combined with temozolomide in heavily treated advanced melanoma patients. METHODS: Patients were sequentially admitted to four dose-escalating groups of apatinib and temozolomide (three cases in each group) using a traditional 3 + 3 dose escalation design method. RESULTS: Twelve patients were enrolled between December 2016 and August 2017. Most patients with an acral or mucosal primary origin progressed after immunotherapy or targeted therapy. Dose escalation had been completed without dose-limiting toxicity. Common adverse events included hypertension, hand-foot syndrome, proteinuria, neutropenia, nausea, and fatigue. All adverse events were grade 1 or 2, while the maximum tolerated dose was not reached. Up to January 2018, 1 patient achieved partial response, 9 experienced stable disease, and 2 exhibited progressive disease. The objective response rate and disease control rate were 8.3% and 83%, respectively. CONCLUSIONS: In conclusion, apatinib combined with temozolomide was well tolerated and has demonstrated efficacy in advanced melanoma patients.
Introduction
Over the past decade, immunotherapeutic agents and targeted agents have made great progress in the treatment of melanoma, significantly prolonging overall survival of metastatic melanoma patients [1], [2], [3], [4]. However, in Asia, melanoma differs in subtype and gene variation in comparison with that of Caucasians. The efficacy of immune-checkpoint inhibitors has been relatively low in acral and mucosal melanoma patients, which are more common in China [5]. With a lower rate of BRAF mutation, less of the population benefits from BRAF or MEK inhibitors. Therefore, novel treatment strategies are demanded for patients after failure of conventional treatment.
Melanoma is a highly vascular tumor. Effective antiangiogenesis treatment options have been extensively searched for years. The vascular endothelial growth factor (VEGF) signaling pathway plays a crucial role in angiogenesis and tumor progression [6]. There have been some kinds of small molecular vascular endothelial growth factor receptor (VEGFR) inhibitors approved for treatment in different malignancies, such as sorafenib, sunitinib, and pazopanib [7]. Apatinib is a type of tyrosine kinase inhibitor that functions by inhibiting vascular proliferation by selectively binding to VEGFR-2, which is considered to be principally responsible for angiogenesis in tumors. Apart from approval for treatment of metastatic gastric cancer after failure of second-line chemotherapy in China, apatinib was found to be active against lung and breast cancers as well [8], [9].
Temozolomide is an orally administered alkylating agent that shows equivalent efficacy compared with dacarbazine when used in metastatic melanoma [10]. Because of ease of administration, tolerability, and predictable pharmacokinetics, temozolomide is an excellent candidate for inclusion in combination therapies for patients with advanced melanoma.
Chemotherapy combined with inhibition of the VEGF signaling pathway may lead to synergistic antitumor effect; thus, we undertook a phase I study of combining apatinib with temozolomide to investigate the safety profile and preliminary efficacy in patients with advanced melanoma after failure of conventional treatment including immunotherapy and targeted therapy.
Materials and Methods
Patient Selection
Patient enrollment criteria include age between 18 and 70 years old; Eastern Cooperative Oncology Group Performance status ≤2; life expectancy of at least 3 months; histologically confirmed advanced melanoma, not suitable for resection, with measurable disease as defined by Response Evaluation Criteria In Solid Tumors (RECIST), version 1.1; failure after conventional treatment, including chemotherapy, targeted therapy, or immunotherapy; acceptable hematologic, hepatic, and renal function; signed informed consent; and willingness and ability to comply with scheduled visits, treatment plans, response evaluation, and other study procedures.
Patients were excluded for any of the following conditions: poorly controlled hypertension (systolic pressure ≥ 140 mm Hg and/or diastolic pressure ≥ 90 mm Hg) despite standard medical management; grade 2 or above myocardial ischemia or myocardial infarction; uncontrolled arrhythmias (including QT interval for male ≥450 milliseconds and for female ≥470 milliseconds); previously received temozolomide; tumor invasion into important blood vessel or with a probability of invading important blood vessel leading to lethal hemorrhage; coagulant function abnormality (international normalized ratio > 1.5 or prothrombin time > upper limit of normal value + 4 seconds or activated partial thromboplastin time > 1.5 upper limit of normal value), with bleeding tendency or is treated with thrombolysis and anticoagulation; routine urine tests indicate that urine protein ≥ ++ or verify that the 24-hour urine protein quantitation ≥1.0 g; pregnant or lactating women, or female subjects of child-bearing potential who do not agree to or cannot use contraceptive measures; and evidence of significant medical illness that in the investigator's judgment will substantially increase the risk associated with the subject's participation in and completion of the study.
Study Design
This prospective, single-center and single-arm, phase I study was approved by Peking University Cancer Hospital Ethic Committee, focusing on combinative drugs dose escalation. The primary endpoint was safety [dose-limiting toxicity (DLT) and maximum tolerated dose (MTD)], and the secondary endpoint was objective response rate and disease control rate. We used the traditional 3 + 3 dose escalation design in this study. Three different dose levels of temozolomide and apatinib were planned involving three cases in each group. Grade 4 hematologic toxicity or grade 3 or 4 nonhematologic adverse event in the first 4-week period was defined as DLT. When all three patients had completed one treatment cycle at a dose level without developing DLT, a new cohort of patients was enrolled at the next dose level. When one of the three patients experienced a DLT, additional three patients were admitted to the same dose level. When two or more patients out of the six experienced a DLT in a cohort, the previous dose level was determined to be the MTD.
Patients would continue treatment after the first cycle unless experiencing intolerable toxicities, RECIST-defined disease progression, or consent withdrawal. During the first cycle, no procedure would be taken in prevention or treatment of toxicities. During the extended treatment period, dose reduction or medication was allowed if patients experienced severe adverse events.
Safety and Response Evaluation
Patients who completed at least one cycle of temozolomide and apatinib were assessed for safety using National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Medical records, physical examinations, and blood tests were performed at baseline and each follow-up time point throughout the trial. The incidences and severity of adverse events and concomitant medication of each cohort were recorded. Cumulative toxicities from extended treatment cycles were monitored as well.
Patients who received at least one cycle of therapy were evaluated for response using RECIST criteria, version 1.1. Computed tomography (CT) scans of chest, abdomen, and pelvis were performed at baseline and each cycle, as well as magnetic resonance imaging of brain when necessary. Objective response rate and disease control rate were observed. Patients with complete response, partial response, or stable disease for at least 4 weeks were considered to be clinically benefited. In addition, follow-up data of progression-free survival (PFS) and overall survival (OS) for each patient were obtained. The probability of PFS and OS was estimated by the Kaplan-Meier method using SPSS 22.0 software.
Results
Patient Characteristics
A total of 12 patients were enrolled in this trial between December 2016 and August 2017. Patient characteristics are listed in Table 1. The most common subtypes were acral melanoma and mucosal melanoma, including one BRAF-mutated patient. The most common metastatic sites were distant lymph nodes, lung, bone, and liver. All patients had previously received at least one line of systemic therapy; 75% received immunotherapy with nivolumab, pembrolizumab, or ipilimumab, while 75% received traditional chemotherapy. Other treatment regimens included BRAF and MEK inhibitor, oncolytic virotherapy and CDK4/6 inhibitor in the setting of clinical trial.
Table 1.
Patient Characteristics
| Characteristics | No. of Patients | % |
|---|---|---|
| Gender | ||
| Male | 4 | 33.3 |
| Female | 8 | 66.7 |
| Age, years | ||
| Median | 52 | |
| Range | 33–64 | |
| Primary site | ||
| Acral | 5 | 41.6 |
| Mucosal | 4 | 33.3 |
| Conjunctiva | 1 | 8.3 |
| Unknown primary | 2 | 16.7 |
| Metastatic sites | ||
| Distant lymph node only | 2 | 16.7 |
| Lung only | 2 | 16.7 |
| Liver | 3 | 25.0 |
| Bone | 4 | 33.3 |
| Other sites | 2 | 16.7 |
| BRAF mutation | 1 | 8.3 |
| Previous therapy | ||
| BRAF inhibitor | 1 | 8.3 |
| Immunotherapy | 9 | 75.0 |
| Chemotherapy | 9 | 75.0 |
| Containing DTIC | 2 | 16.7 |
| Not Containing DTIC | 7 | 58.3 |
DTIC, dacarbazine.
Dose Escalation
Twelve patients were enrolled and treated with various doses of temozolomide and apatinib according to the sequence of their enrollment. Three dose cohorts were tested as planned in the original protocol, namely, temozolomide 100 mg for day 1-5, apatinib 250 mg daily; temozolomide 200 mg for day 1-5, apatinib 250 mg daily; temozolomide 200 mg for day 1-5, apatinib 500 mg daily, every 4 weeks for a cycle. Because MTD had not been reached during the three levels, we subsequently added three patients to a higher dose level, in which patients were given temozolomide at a dose of 300 mg for day 1-5 and apatinib at a dose of 500 mg daily. The dose escalation was completed in August 2017. Drug dose of each cohort is listed in Table 2. At the time of the last follow-up (January 2018), two patients were still on treatment. The other 10 patients discontinued due to disease progression.
Table 2.
Dose Cohorts
| Cohort | Temozolomide Dose | Apatinib Dose |
|---|---|---|
| 1 | 100 mg QD day 1-5 | 250 mg QD |
| 2 | 200 mg QD day 1-5 | 250 mg QD |
| 3 | 200 mg QD day 1-5 | 500 mg QD |
| 4 | 300 mg QD day 1-5 | 500 mg QD |
Safety
All 12 patients were available for safety evaluation. No DLT had been observed in the first 4 weeks, and the MTD was not reached. The incidence of all treatment-related toxicities for each cohort is listed in Table 3. The most frequently occurring adverse events were hypertension (33.3%), hand-foot syndrome (33.3%), proteinuria (25.0%), nausea (25.0%), neutropenia (25.0%), and fatigue (41.6%), while hyperbilirubinemia (8.3%), diarrhea (8.3%), and thrombocytopenia (8.3%) were observed occasionally. All treatment-related adverse events were mild and manageable. Hypertension was controlled by antihypertensive agents. Hand-foot syndrome was relieved by application of moisturizers. None of the three patients with proteinuria developed nephrotic syndrome due to apatinib treatment.
Table 3.
Incidence of Treatment-Related Toxicities for Each Dose Cohort
| Adverse Events | No. of Patients (All Grades) |
||||
|---|---|---|---|---|---|
| Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | Total (%) | |
| Hypertension | 1 | 1 | 1 | 1 | 4 (33.3) |
| HFS | 1 | 2 | 0 | 1 | 4 (33.3) |
| Proteinuria | 0 | 2 | 0 | 1 | 3 (25.0) |
| Nausea | 1 | 1 | 0 | 1 | 3 (25.0) |
| Neutropenia | 0 | 1 | 1 | 1 | 3 (25.0) |
| Fatigue | 1 | 1 | 2 | 1 | 5 (41.6) |
| Hyperbilirubinemia | 1 | 0 | 0 | 0 | 1 (8.3) |
| Diarrhea | 0 | 1 | 0 | 0 | 1 (8.3) |
| Thrombocytopenia | 0 | 0 | 0 | 1 | 1 (8.3) |
HFS, hand-foot syndrome.
During the extended treatment cycles, three patients required a dose reduction of apatinib because of hypertension, from 500 mg to 250 mg, and received two kinds of medication, including two patients in cohort 3 and one patient in cohort 4. Additionally, one patient at dose level 4 experienced grade 4 thrombocytopenia after four cycles of treatment. With infusion of platelets and support of recombinant human interleukin-11 her platelet count recovered, and temozolomide dosage was reduced during the next treatment cycle. Given that toxicities were cumulative over time, dose escalation was not further attempted.
Tumor Response
All 12 patients were available for response evaluation. One patient achieved partial response in cohort 2 and was still on treatment after 7 months (Figure 1). Nine patients achieved stable disease. Among these patients, one had BRAF V600E mutation and progressed after BRAF and MEK inhibitors; two patients had previously received dacarbazine-combined chemotherapy. The objective response rate was 8.3% and disease control rate was 83%, respectively. Response evaluation for each cohort is shown in Table 4.
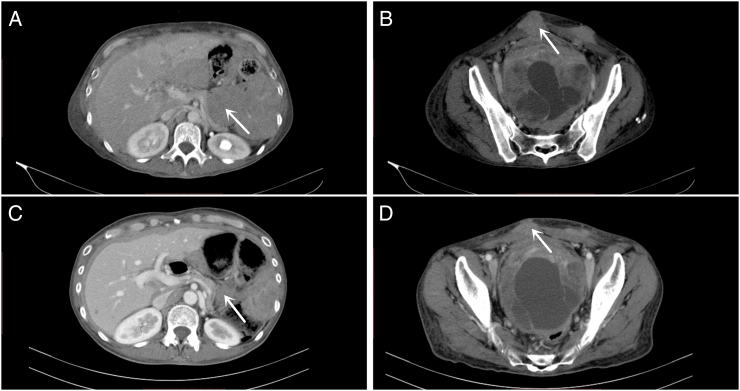
Figure 1.
The CT scan image of a patient with partial response. A 54-year-old female, originated from gastroesophageal junction metastatic to abdominal cavity and subcutaneous tissue, pretreated with nivolumab + ipilimumab for 4 months and CDK4/6 inhibitor (clinical trial) for 6 months. (A) Abdominal mass at baseline CT scan; (B) subcutaneous metastasis at baseline CT scan; (C) abdominal mass after one treatment cycle; (D) subcutaneous metastasis after one treatment cycle.
Table 4.
Response Evaluation for Each Dose Cohort
| Cohort | CR | Response (n = 12) |
DCR (%) CR + PR + SD |
||
|---|---|---|---|---|---|
| PR | SD | PD | |||
| 1 | 0 | 0 | 2 | 1 | 2 (66.7) |
| 2 | 0 | 1 | 2 | 0 | 3 (100) |
| 3 | 0 | 0 | 3 | 0 | 3 (100) |
| 4 | 0 | 0 | 2 | 1 | 2 (66.7) |
| Total | 0 | 1 | 9 | 2 | 10 (83.3) |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; DCR, disease control rate.
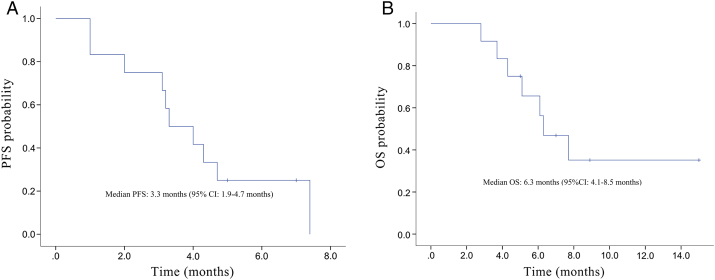
The median follow-up time for the entire group of patients was 3 months (range: 3-15 months). Median progression-free time was 3.3 months (95% CI 1.9-4.7 months), and median overall survival time was 6.3 months (95% CI 4.1-8.5 months), as is shown in Figure 2.
Figure 2.
Kaplan-Meier curves for (A) PFS and (B) OS.
Discussion
Based on this phase I study, we infer that temozolomide and apatinib can be safely combined at a dose of temozolomide 300 mg on day 1-5 and apatinib 500 mg daily every 4 weeks. As DLTs were not observed in this study, this combination dosage was considered the recommended phase II dose.
The safety profile of apatinib was similar to that of other VEGFR inhibitors [11], [12]. From the prior clinical trials, we already know that apatinib inhibits VEGF receptors on arterial endothelial cells, peritubular capillary cells, skin cells, and bone marrow progenitor cells, which may cause systemic hypertension, proteinuria, hand-foot syndrome, as well as bone marrow suppression [13]. Temozolomide was well tolerated and produced a transient myelosuppression. In addition, the most common nonhematologic toxicities of temozolomide were mild to moderate nausea and vomiting [10]. In this clinical trial, the most common adverse events were hypertension, hand-foot syndrome, proteinuria, nausea, neutropenia, and fatigue, while the less common toxicities included hyperbilirubinemia, diarrhea, and thrombocytopenia. These toxicities from the combination of temozolomide and apatinib were mild and manageable. No unexpected and severe toxicity was observed.
During the extended course of treatment, some cumulative toxicities were documented, including three grade 3 hypertension events and one grade 4 thrombocytopenia event. With dose reduction and antihypertension agents, hypertension was controlled. The platelet count also recovered quickly with supportive care. Although temozolomide and apatinib dosage was reduced during the next treatment cycle, the patient continued to experience clinical benefit (stable disease).
Generally speaking, the efficacy conclusions of phase I studies are limited. However, it is noteworthy that 83% patients experienced clinical benefit for a median PFS of 3.3 months, especially after previous heavy treatment with checkpoint inhibitors and specific targeted therapy of BRAF or CDK4/6 inhibitors. Additionally, more than 70% of patients in this study had acral or mucosal primaries with poor prognosis. Although immunomodulatory agents have revolutionized the treatment of metastatic cutaneous melanoma in recent years, they are not well described in melanomas arising from acral and mucosal surfaces [14]. The use of targeted therapies aimed at BRAF mutation is also limited as the proportion of BRAF mutation is relatively low in these melanoma subtypes. Traditional cytotoxic chemotherapy yields poor outcome in terms of either response rate or overall survival. However, in our study, the combination of VEGFR inhibitor and temozolomide showed promising antitumor activity, which may promote further studies for phase II clinical trial.
Antiangiogenesis is a potential strategy in the treatment of melanoma. Bevacizumab, recombinant human endostatin, as well as some other small tyrosine kinase inhibitors in combination with chemotherapy have already been tested in clinical trials through the years and showed promising outcomes [15], [16], [17]. In this study, we chose one potent and selective VEGFR inhibitor in combination with chemotherapy and demonstrated promising antitumor activity. Two patients had been pretreated with dacarbazine and still had stable disease, which may indicate that most of the benefit came from apatinib. Further investigation is required for a detailed understanding of the mechanism regarding the effect of apatinib in treatment of metastatic melanoma. As apatinib can block VEGFR-2 activation, we can analyze the expression of VEGFR-2 to determine whether it is correlated with tumor response in our future work.
This proposed regimen comprised of two orally taken agents was well tolerated. The combination was also found with evidence of antitumor activity. For patients after failure of previous conventional treatment, regardless of BRAF status, the combination of temozolomide and apatinib regimen could serve as an alternative treatment option.
Conclusions
Apatinib combined with temozolomide was well tolerated and has demonstrated efficacy in advanced melanoma patients.
Footnotes
Funding: This work was supported by grants from CSCO-Hengrui Oncology Research Fund (Y-HR2015-065).
References
- 1.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu WJ, Gangadhar TC. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 5.Chi Z, Li S, Sheng X, Si L, Cui C, Han M, Guo J. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: a study of 522 consecutive cases. BMC Cancer. 2011;11:85. doi: 10.1186/1471-2407-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellamy WT, Richter L, Frutiger Y, Grogan TM. Expression of vascular endothelial growth factor and its receptors in hematopoietic malignancies. Cancer Res. 1999;59:728–733. [PubMed] [Google Scholar]
- 7.Glade-Bender J, Kandel JJ, Yamashiro DJ. VEGF blocking therapy in the treatment of cancer. Expert Opin Biol Ther. 2003;3:263–276. doi: 10.1517/14712598.3.2.263. [DOI] [PubMed] [Google Scholar]
- 8.Hu X, Zhang J, Xu B, Jiang Z, Ragaz J, Tong Z, Zhang Q, Wang X, Feng J, Pang D. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer. 2014;135:1961–1969. doi: 10.1002/ijc.28829. [DOI] [PubMed] [Google Scholar]
- 9.Li F, Zhu T, Cao B, Wang J, Liang L. Apatinib enhances antitumour activity of EGFR-TKIs in non-small cell lung cancer with EGFR-TKI resistance. Eur J Cancer. 2017;84:184–192. doi: 10.1016/j.ejca.2017.07.037. [DOI] [PubMed] [Google Scholar]
- 10.Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, Gore M, Aamdal S, Cebon J, Coates A. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 11.Di Lorenzo G, Porta C, Bellmunt J, Sternberg C, Kirkali Z, Staehler M, Joniau S, Montorsi F, Buonerba C. Toxicities of targeted therapy and their management in kidney cancer. Eur Urol. 2011;59:526–540. doi: 10.1016/j.eururo.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, Sun G, Yang Y, Wang L, Xu N. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31:3219–3225. doi: 10.1200/JCO.2013.48.8585. [DOI] [PubMed] [Google Scholar]
- 14.Shoushtari AN, Munhoz RR, Kuk D, Ott PA, Johnson DB, Tsai KK, Rapisuwon S, Eroglu Z, Sullivan RJ, Luke JJ. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer-Am Cancer Soc. 2016;122:3354–3362. doi: 10.1002/cncr.30259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott DF, Sosman JA, Gonzalez R, Hodi FS, Linette GP, Richards J, Jakub JW, Beeram M, Tarantolo S, Agarwala S. Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 Study Group. J Clin Oncol. 2008;26:2178–2185. doi: 10.1200/JCO.2007.14.8288. [DOI] [PubMed] [Google Scholar]
- 16.Kim KB, Sosman JA, Fruehauf JP, Linette GP, Markovic SN, McDermott DF, Weber JS, Nguyen H, Cheverton P, Chen D. BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J Clin Oncol. 2012;30:34–41. doi: 10.1200/JCO.2011.34.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui C, Mao L, Chi Z, Si L, Sheng X, Kong Y, Li S, Lian B, Gu K, Tao M. A phase II, randomized, double-blind, placebo-controlled multicenter trial of Endostar in patients with metastatic melanoma. Mol Ther. 2013;21:1456–1463. doi: 10.1038/mt.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]