Abstract
Following injury, mesenchymal repair cells are activated to function as leader cells that modulate wound healing. These cells have the potential to differentiate to myofibroblasts, resulting in fibrosis and scarring. The signals underlying these differing pathways are complex and incompletely understood. The ex vivo mock cataract surgery cultures are an attractive model with which to address this question. With this model we study, concurrently, the mechanisms that control mesenchymal leader cell function in injury repair within their native microenvironment and the signals that induce this same cell population to acquire a myofibroblast phenotype when these cells encounter the environment of the adjacent tissue culture platform. Here we show that on injury, the cytoskeletal protein vimentin is released into the extracellular space, binds to the cell surface of the mesenchymal leader cells located at the wound edge in the native matrix environment, and supports wound closure. In profibrotic environments, the extracellular vimentin pool also links specifically to the mesenchymal leader cells and has an essential role in signaling their fate change to a myofibroblast. These findings suggest a novel role for extracellular, cell-surface–associated vimentin in mediating repair-cell function in wound repair and in transitioning these cells to a myofibroblast phenotype.
INTRODUCTION
In the normal repair process, mesenchymal cell populations restore tissue function in response to tissue insult or injury; however, their susceptibility to becoming myofibroblasts results in a response that promotes and sustains the fibrotic disease process. Fibrosis is a devastating progressive disease, its pathology characterized by the excessive production of extracellular matrix proteins like collagen I, with myofibroblasts being a major producer of this fibrosis-causing matrix. Fibrosis affects almost every organ of the body, causing irreparable damage wherever it occurs (Carver and Goldsmith, 2013). As fibrosis is an outcome of so many distinct disease states, it is considered a leading cause of death (Tsou et al., 2014).
Factors currently known to induce specific aspects of the fibrotic response to injury repair or pathogenesis include mechanotransduction-signaling events that involve integrin receptors, the actin cytoskeleton, TGFβ, and collagen I (Schwartz, 2010; Huang et al., 2012; Wong et al., 2012; Duscher et al., 2014; Hinz, 2015). However, it remains unclear how cells normally tasked with directing wound repair are signaled to alter their fate and differentiate to myofibroblasts, the cell type associated with fibrosis. Key features of myofibroblast differentiation include 1) high levels of expression of the intermediate filament protein vimentin, a mediator of cell migration and wound repair; 2) expression of α-smooth muscle actin (αSMA) and its organization into the stress fibers that mediate myofibroblast contractile function; and 3) production of extracellular matrix proteins such as collagen I that are secreted into the extracellular milieu. These myofibroblast properties create an aberrant microenvironment that leads to loss of tissue function.
Vimentin has emerged as a critical regulator of the wound-healing process in many distinct tissues and cell types. In studies of skin wound–repair, this protein has been demonstrated to function as a signaling integrator in tissue regeneration and healing (Cheng et al., 2016). Our own studies with an ex vivo, mock-cataract-surgery (MCS), epithelial wound–healing model have shown that vimentin is a defining characteristic of a resident mesenchymal “repair” cell population (Walker et al., 2010) that is located in niches among the cells of the lens equatorial epithelium. This mesenchymal cell population is activated on wounding to immediately migrate to and populate the leading edge of the injured epithelium (Walker et al., 2010; Menko et al., 2014a). Vimentin is required for the function of this mesenchymal leader cell population in directing the collective migration of the injured epithelium to close the wound (Walker et al., 2010; Menko et al., 2014a). Furthermore, we have found that when these cells are activated by wounding, they are also conferred with the ability to invade, a phenotype that is also dependent on vimentin (Bleaken et al., 2016). This finding is consistent with other studies showing that vimentin is required for an invasive-cell phenotype (Satelli and Li, 2011).
Here we investigate whether the properties of vimentin that confer leader cells with the ability to modulate wound repair and direct invasion also make these cells vulnerable to environmental factors that can alter their differentiation pathway to that of a myofibroblast. Other studies have linked vimentin intermediate filaments to the development of fibrosis. Mice lacking vimentin have exhibited both reduced scarring following corneal injury (Bargagna-Mohan et al., 2012) and a diminished fibrotic response to bleomycin-induced injury of the lung (dos Santos et al., 2015). However, the role of vimentin in inducing the fibrotic response and the specific features of vimentin that are associated with the fibrotic phenotype are not yet clear.
While long understood for their role as a highly stable, stress-resistant cytoskeleton, vimentin intermediate filaments are now appreciated as highly dynamic structures that exist in distinct assembly states and subcellular distributions that define their functions (Lowery et al., 2015; Herrmann and Aebi, 2016). The different states of vimentin organization range from nonfilamentous precursors to unit filaments to a highly organized cytoskeletal network, each with distinct cellular tasks (Herrmann et al., 2009; Lowery et al., 2015; Herrmann and Aebi, 2016). Soluble forms of vimentin are associated with signaling (Perlson et al., 2005), while an organized vimentin cytoskeletal network is critical to processes such as resisting stress and providing cell shape (Goldman et al., 2012). The assembly state of vimentin is regulated through posttranslational modifications (PTMs) that also modulate vimentin function. Two key PTMs linked to increased vimentin solubility are phosphorylation and citrullination (deimination). Some of the phosphorylation PTMs are known to trigger disassembly of intermediate filaments (Ando et al., 1989). Vimentin citrullination, which results from the conversion of an arginine to a citrulline by peptidyl arginine deiminase (PAD) enzymes, prevents vimentin assembly and promotes vimentin filament disassembly (Inagaki et al., 1989). In its soluble form, vimentin can function as an extracellular molecule that has been implicated in the repair process, as shown in a spinal cord injury model (Teshigawara et al., 2013; Shigyo et al., 2015; Shigyo and Tohda, 2016), and in modulating macrophage function in bacteria killing (Mor-Vaknin et al., 2003). In studies with our ex vivo MCS-wounded-explant cultures, a model that closely replicates the features of wound repair in vivo, we now investigate how a resident mesenchymal repair cell population that modulates repair of the injured lens epithelium can become misdirected and become an underlying cause of the aberrant wound-healing response of fibrosis. We show, for the first time, a role for extracellular vimentin in promoting the differentiation of these mesenchymal repair cells to the myofibroblast phenotype associated with fibrosis.
RESULTS
Resident repair cells of the lens direct wounded lens epithelial cells to migrate off their endogenous basement membrane and across an adjacent tissue culture substrate
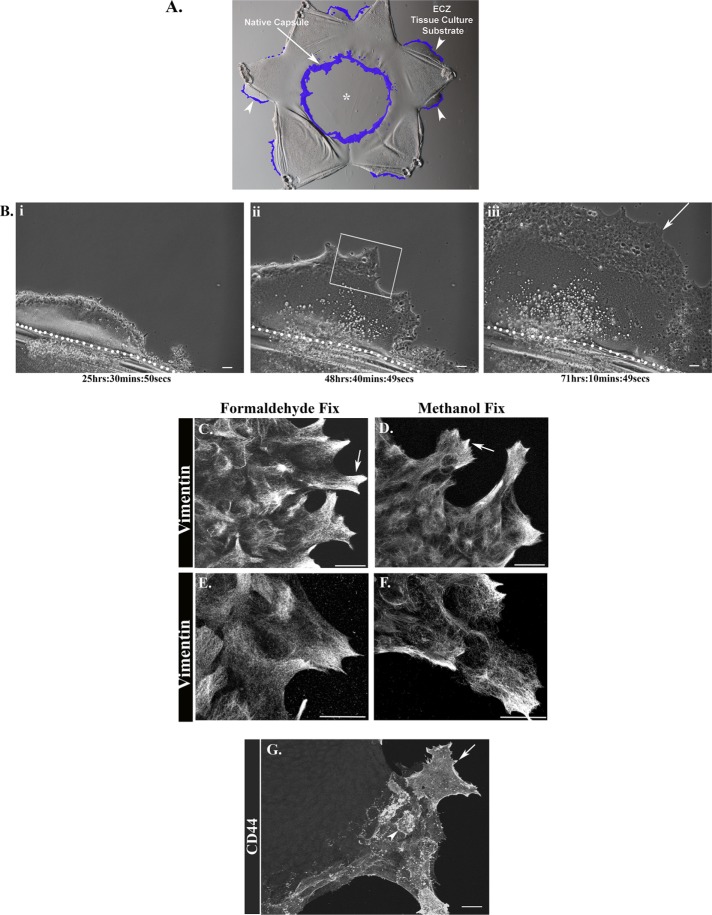
Our studies investigate how mesenchymal leader cells, whose normal role it is to direct regenerative repair of epithelia following wounding, can be induced to differentiate to myofibroblasts. They were conducted with the ex vivo MCS culture model we originally established to investigate mechanisms of wound repair (Walker et al., 2007, 2010, 2015; Menko et al., 2014a). This wound model has clinical relevance to fibrosis, as cataract surgery often elicits a fibrotic response, resulting in the disease known as posterior capsule opacification (PCO) (Wormstone et al., 2009; Wormstone and Eldred, 2016; Nibourg et al., 2015). We focus on the resident population of vimentin-rich, mesenchymal repair cells of the lens that, on MCS injury, are activated to migrate to and rapidly populate the wound edge of the lens epithelium (Figure 1A, D1 postinjury, repair cells at the MCS wound edge colored purple, arrow) (Walker et al., 2010). Here, functioning as classical leader cells, these cells direct regenerative repair of the injured epithelium, a task that is vimentin dependent (Menko et al., 2014a; Bleaken et al., 2016).
FIGURE 1:
Properties of leader cells activated on injury when they encounter a profibrotic microenvironment. (A) Ex vivo wound healing/fibrosis culture model, created by performing mock cataract surgery on isolated chick embryo lenses, which removes the lens fiber cell mass from the lens native basement membrane (BM) capsule (*). Mesenchymal repair cells (shaded purple) migrate to both the leading edge of the MCS wound (arrow) and to the cut edges created to flatten the lens capsule on the substrate (arrowhead). Repair cells at the cut edge lead the lens epithelium off the lens capsule onto and across the rigid tissue culture substrate, referred to the extracapsular zone (ECZ). (Bi–Biii) Representative still images from a time-lapse movie (Supplemental Video 1) taken over 3 d postinjury show the collective migration of the lens epithelial cells across the ECZ led by mesenchymal leader cells (arrow). The dotted white line in i, ii, and iii indicates the border of the native capsule and the ECZ. (C–F) Ex vivo MCS wounded-explant cultures were fixed in formaldehyde (C, E) or in methanol (D, F) 2 d postinjury, labeled for vimentin, and imaged by confocal microscopy (C, D) or superresolution confocal microscopy (E, F) in the ECZ, in a region similar to the boxed area in Bii. Vimentin was enriched in the lamellipodial protrusions extended by leader cells (C–F, arrows). When cultures were fixed with formaldehyde, both filamentous and diffuse populations of vimentin were observed at the tips of lamellipodia extensions (C, E, arrow). Methanol fixation conditions highlighted the vimentin cytoskeleton (D, F). (G) At 2 d postinjury the ex vivo explant cultures were labeled for CD44 and imaged by confocal microscopy in the ECZ. Mesenchymal leader cells were distinguished by their expression of the CD44 receptor (arrow), which was enhanced at cell borders (arrowhead). Magnification (Mag) bars = 20 µm. Images in C–G are presented as projections.
To create these cultures, the MCS-wounded lens is flattened on a tissue culture substrate by making cuts through the epithelium and its associated basement membrane, creating the star-shaped wounded-explant cultures. The cuts made to flatten the explants are additional injury sites that form the outside edge of the explant. These sites of injury are also rapidly populated by the wound-activated resident mesenchymal repair cells (Figure 1A, repair cells at the cut edge colored purple, arrowheads). These mesenchymal cells lead the lens epithelial cells from their original position on their native lens basement membrane capsule onto and across the adjacent, rigid tissue culture substrate. We refer to the region on the tissue culture plastic that the cells occupy postwounding as the extracapsular zone (ECZ). Formation of the ECZ in response to injury is best observed by time-lapse microscopy (Supplemental Video 1, representative still images shown in Figure 1, Bi–Biii). Live imaging revealed that the mesenchymal cells at the leading edge extend and retract lamellipodial protrusions along the substrate as they lead the forward movement of the wounded epithelium across the culture substrate. This behavior closely parallels the injury response we have observed for these resident repair cells previously, when they populate the leading edge of the MCS-wound and direct wound closure on the endogenous basement membrane substrate of the lens (Menko et al., 2014a; Bleaken et al., 2016).
Video S1.
Collective movement of mesenchymal leader and epithelial follower cells across the tissue culture substrate (ECZ) in response to injury was followed by time-lapse imaging from D0-D3. The mesenchymal cells at the leading edge were easily distinguished morphologically from the lens epithelial follower cells.
We have shown previously that vimentin is a principal characteristic of the repair cells that direct wound closure at the MCS wound edge (Menko et al., 2014a). Here we examined whether vimentin was also a defining feature of the repair cells in their function as leader cells of the ECZ. Following immunolabeling for vimentin at D2 postinjury, the MCS-wounded-explant cultures were imaged in regions of the ECZ typical of the boxed area in Figure 1Biii. The leading-edge cells of the ECZ expressed high levels of vimentin, prominently localized to the lamellipodial protrusions that extend along their substrate surface (Figure 1, C and D, arrows). The organization of vimentin in these cells was examined in cultures fixed with either formaldehyde (Figure 1, C and E) or methanol (Figure 1, D and F), and the cells were imaged by both confocal (Figure 1, C and D) and superresolution confocal (Figure 1, E and F) microscopy. Methanol fixation, which extracts membrane lipids, highlighted the organized vimentin cytoskeleton in the leader cell population. Immunostaining of formaldehyde fixed cultures, permeabilized with Triton X-100 postfixation, revealed both a diffuse pattern of vimentin labeling and organized vimentin filaments. This suggests that formaldehyde fixation may enhance preservation of soluble vimentin populations.
We have shown previously that another defining feature of the vimentin-rich, leader cell population activated in response to MCS wounding was its expression of CD44 (Bleaken et al., 2016). While widely expressed, this cell-surface receptor for hyaluronic acid (HA) is characteristic of leukocytes and known for its role in cell adhesion and migration (McDonald and Kubes, 2015; Bano et al., 2016; Senbanjo and Chellaiah, 2017). Immunolabeling of the wounded-lens explant cultures with an antibody to the CD44 extracellular domain at D2 postinjury showed that, in the ECZ, CD44 is expressed exclusively by the repair cells at the leading edge (Figure 1G, arrow). CD44 is enriched at the plasma membrane of these leader cells, especially along their cell borders (arrowhead), and was not expressed by the lens epithelial cells that migrate behind the CD44+/vimentin+ leader cell population of the ECZ.
αSMA+ myofibroblasts appear at the leading edge of the ECZ, with their emergence linked to rigidity of the microenvironment
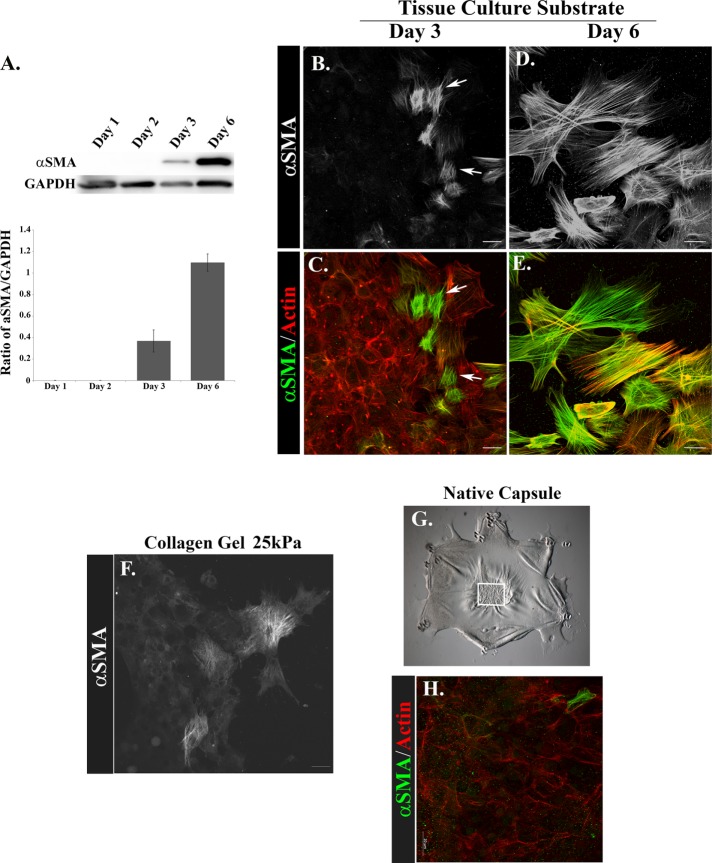
Substrate rigidity is a known factor in inducing cells that mediate regenerative repair to become myofibroblasts (Arora et al., 1999; Li et al., 2007; Liu et al., 2010; O’Connor et al., 2015). Reprogramming of cells to a myofibroblast phenotype is characterized by the induction of αSMA and its organization into stress fibers (Skalli et al., 1989; Schmitt-Graff et al., 1994). Western blot analysis of protein extracts from cells isolated from the ECZ of MCS-wounded-explant cultures from D1 to D6 postinjury showed that αSMA was first expressed by ECZ cells at culture D3 and increased greatly by D6 (Figure 2A). Since the ECZ is composed of both lens epithelial cells and the lens-resident, vimentin-rich repair cells that populate the wound edge postinjury, we performed immunofluorescence analysis to determine where in the ECZ the αSMA-expressing cells were localized. Confocal image analysis at culture D3 revealed that αSMA stress-fiber-positive myofibroblasts emerged exclusively at the leading edge of the ECZ (Figure 2, B and C), the area populated by the vimentin-rich leader cells (Figure 1, C and D). The presence of myofibroblasts at the leading edge of the ECZ had increased greatly by D6 postwounding (Figure 2, D and E), in part due to their proliferation (Supplemental Figure 1).
FIGURE 2:
Myofibroblasts emerge at the leading edge of the ECZ and their differentiation is influenced by the rigidity of the microenvironment. (A) Total cell lysates prepared from isolated ECZ regions at D1–D3, and D6 were immunoblotted for αSMA, with GAPDH as loading control. Results were quantified for three independent experiments and presented as a ratio of αSMA to GAPDH for each fraction. Results demonstrate that αSMA is first expressed on D3 and increases threefold by D6. (B–E, H) Ex vivo explant cultures were fixed D3 (B, C, H) and D6 (D, E) postinjury and labeled for αSMA (B–E, H). In C, E, and H αSMA is green and F-actin is red. Cultures were imaged by confocal microscopy in the ECZ (B–E) or on the native capsule (H). αSMA-positive myofibroblasts emerged at the leading edge of the ECZ at D3 (B, C, arrows) and by D6 myofibroblasts had expanded in number and formed extensive αSMA stress fibers (D, E). (F) To examine induction of myofibroblast differentiation in a physiological relevant profibrotic microenvironment, the ex vivo MCS-wounded explants were cultured for 3 d postinjury on 2.5 mg/ml collagen gels, corresponding to 25 kPa, and immunolabeled for αSMA. The result shows that leader cells are induced to acquire a myofibroblast phenotype in a microenvironment within the physiological range of fibrotic tissues. In contrast, few αSMA+ cells were observed on the native capsule at culture D3 (H), at which time wound healing was just completed (G, white box). Results show that the rigidity of the tissue culture substrate is a factor in the induction of myofibroblast differentiation. The results presented represent a minimum of three independent studies. Mag bar = 20 µm; images in B–E and G are presented as projections. Image in H is presented as single optical section.
We also examined whether a substrate in the physiological range of a profibrotic tissue (Wells, 2013) induces repair-modulating cells to alter their fate to differentiate into a myofibroblast phenotype. The ex vivo, MCS-wounded explants were placed on tissue culture dishes coated with collagen I gels of 2.5 mg/ml, corresponding to a Young’s modulus of 25 kPa to examine whether rigidity of the microenvironment is a factor in inducing cells involved in repair of the wounded-lens epithelium to alter their fate and differentiate to myofibroblasts. The stiffness of the collagen gel is in range of the rigidity of fibrotic tissues (Wells, 2013). Appearance of myofibroblasts in the ECZ of MCS-wounded explants cultured on the collagen I gel substrates was determined by confocal microscopy imaging following immunolabeling for αSMA. The results show that on collagen gels of 25 kPa, the leading-edge cells had differentiated to αSMA+ stress-fiber–containing myofibroblasts on D3 (Figure 2F).
The ex vivo MCS-injury model provides a unique opportunity to follow, concurrently, the function of resident repair cells in regulating wound healing in their native microenvironment and how these same repair-modulating cells may be induced to differentiate to myofibroblasts in the controlled environment of the ECZ. Therefore, we examined whether myofibroblasts also appeared on the native lens basement membrane capsule undergoing regenerative repair post-MCS (Figure 2, G and H). In contrast to the induction of myofibroblasts on the ECZ substrates, very few αSMA+ cells were induced to form on the native substrate of the explant where the mesenchymal leader cells effectively direct closure of the lens epithelial wound by culture D3.
Resident lens mesenchymal cells activated to modulate repair of the wounded lens epithelium are myofibroblast progenitors
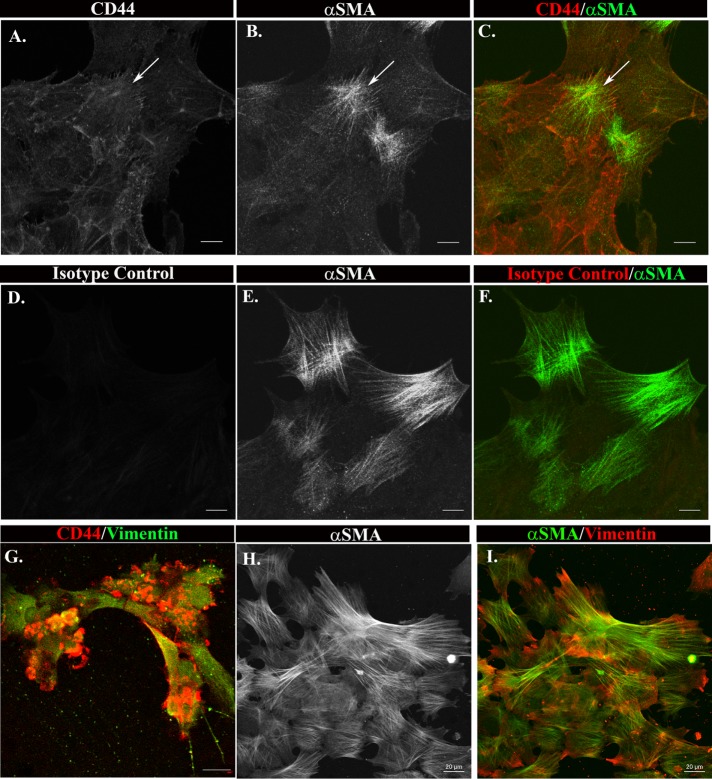
Cell fate–tracking studies were performed to examine whether the leader cells of the ECZ had the potential to become myofibroblasts. This approach took advantage of our ability to specifically tag the vimentin-rich repair cells at the leading edge of the ECZ with the extracellular domain CD44 antibody (Figure 1G). For these studies, the mesenchymal leader cells were tagged with the CD44 antibody by live labeling for 20 min at D1 postinjury. Then the cultures were grown through culture D3 when myofibroblasts first appear in the ECZ. To determine whether the cells labeled with CD44 at D1 had differentiated to myofibroblasts at D3, the cultures were fixed, the CD44-antibody-tagged cells labeled with a fluorescent-labeled secondary antibody, and the cells in the ECZ coimmunolabeled for αSMA. Confocal image analysis showed that all αSMA+ stress-fiber-expressing cells in the ECZ were positive for CD44 (Figure 3, A–C, arrow). As a control, the cultures were labeled with a nontargeting immunoglobulin G (IgG) of the same isotype as the CD44 antibody. The αSMA+ cells that emerge in these control cultures were not labeled by the nontargeting IgG (Figure 3, D–F), confirming the specificity of the CD44-tracking studies. These results demonstrate that the CD44+ leader cells, a vimentin-rich cell population, are progenitors of the αSMA+ myofibroblasts that emerge in the ECZ in response to wounding.
FIGURE 3:
CD44 positive leader cells are precursors of myofibroblasts. (A–F) ECZ cells were live labeled for 20 min at D1 postinjury with antibody to the cell surface antigen CD44 (A–C) or an isotype antibody control (D–F). (A–C) The fate of the antibody-tagged cells was followed for 2 more days until myofibroblasts first emerge, at which point the cultures were fixed and the CD44-tagged cells detected with fluorescent-tagged secondary antibody, the cells colabeled for αSMA and imaged by confocal microscopy. (D–F) Control study in which cells were labeled with an isotype control antibody and then treated under the same experimental conditions as in A–C. (G–I) Ex vivo MCS explants were placed cell side down in the top chamber of Matrigel transwell invasion chambers, and the properties of the cells that had invaded to the filter side facing the bottom chamber determined by immunofluorescence analysis. (G) At 2 d postinjury, the wound-activated CD44+/vimentin+ leader cells had invaded through the Matrigel and populated the bottom side of the filter, confirming the efficacy of this approach to isolate the repair cell population. (H, I) The cells on the bottom filter were cultured through D10 postinjury and the cells co-immunolabeled for vimentin and αSMA. The results show that the wound-activated repair cells that had invaded to the bottom filter were αSMA+, demonstrating that they had acquired a myofibroblast phenotype. Together these studies show that the CD44+/vimentin+ leader cell population activated on injury are myofibroblast progenitors. A–F, Mag bar = 10 µm. G–I, Mag bar = 20 µm. Images shown are single optical sections (A–F, H, and I) or projections (G).
As an alternative approach, we used a Boyden chamber invasion assay to examine the potential of vimentin-rich repair cells to differentiate to myofibroblasts. We have previously used this assay to reveal the invasive properties of the CD44+/vimentin+ mesenchymal subpopulation of the lens following their activation by MCS injury (Bleaken et al., 2016). For these studies, MCS-wounded-explant cultures are placed, cell-side down, on the Matrigel-coated filter of the top Boyden chamber immediately postinjury. Serum-free medium was placed in the top chamber and serum-containing medium in the bottom chamber. As shown in our previous study, at D2 postinjury the wound-activated CD44+/vimentin+ repair cells have invaded through Matrigel to the bottom filter surface of the Matrigel-coated transwell chamber (Figure 3G). The wounded-lens epithelial cells remain associated with their native basement membrane capsule in the top chamber (Bleaken et al., 2016). The vimentin-rich leader cells activated following injury that had invaded to the bottom of the Boyden chamber filter were fixed at culture D10 and immunolabeled for αSMA. Confocal image analysis showed that these cells expressed both vimentin and αSMA (Figure 3, H and I), evidence that the vimentin+/CD44+ repair cells of the lens can be targeted to become myofibroblasts.
Distinct organization of actin and vimentin when leader cells are in located in proregenerative and profibrotic microenvironments
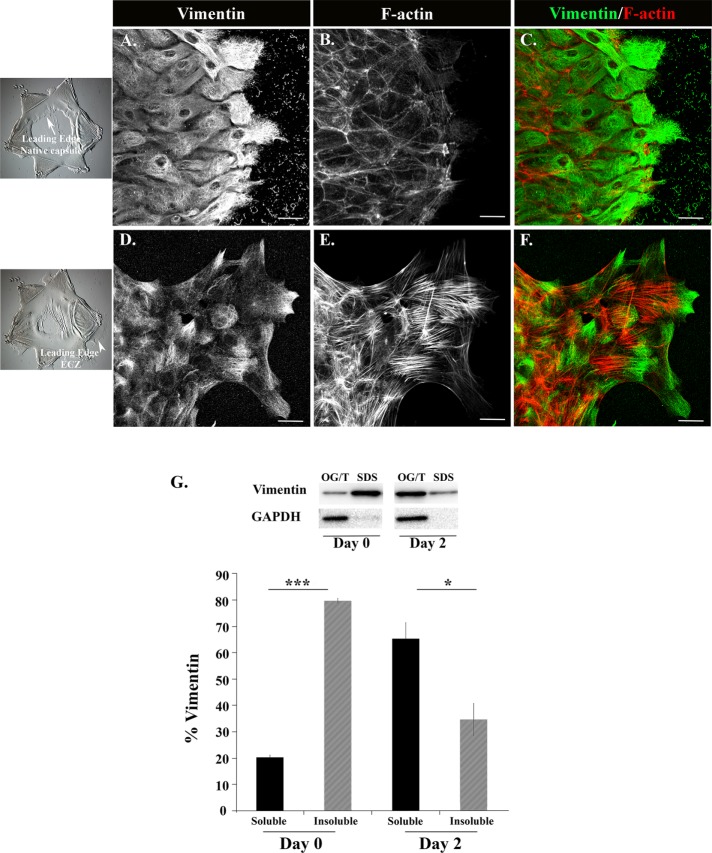
We found that rigidity is a factor in inducing wound-activated resident repair cells of the lens to become myofibroblasts (Figure 2). This suggests that the change in their differentiation state involves a mechanotransduction-signaling event. The cytoskeleton is a key factor in transmitting mechanotransduction signals. Therefore, we compared the organization of the actin and vimentin cytoskeletons in wound-activated repair cells at the leading edge of the MCS wound on the native lens basement membrane capsule to their organization in the leading-edge cells of the ECZ that forms on the tissue-culture substrate. The cultures were colabeled with antibody to vimentin and with fluorescent-tagged phalloidin that labels F-actin. Cytoskeletal organization was analyzed by confocal microscopy imaging (Figure 4, A–F). The vimentin-rich repair cells at the leading edge of the wound on the cells’ endogenous basement membrane capsule have a well-organized vimentin cytoskeleton (Figure 4, A and C, D1), but this leading-edge population is remarkably F-actin poor (Figure 4, B and C). In contrast, there is a significant population of vimentin that is unorganized in the repair cells at the leading edge of the ECZ, most highly localized to lamellipodial processes these cells extend along the substrate surface (Figure 4, D and F, D2). In contrast to the MCS-wound edge, the leading-edge cells in the ECZ are rich in actin stress fibers (Figure 4, E and F).
FIGURE 4:
Distinct organization of vimentin and actin filaments when leader cells are in proregenerative and profibrotic microenvironments. (A–F) Ex vivo MCS wounded-explant cultures were labeled for vimentin (A, C, D, F; green in C, F) and F-actin (B, C, E, F; red in C, F) on day 1 (A–C) and day 2 (D–F) postinjury, and imaged by confocal microscopy. Vimentin-rich leader cells at the MCS wound edge on the lens native basement membrane capsule exhibited a highly organized vimentin cytoskeleton (A, C) but were notably poor in F-actin (B, C). In contrast, in leader cells associated with the profibrotic environment of the ECZ there was a prominent population of vimentin with a diffuse labeling pattern that was highly enriched at the tips of lamellipodial extensions extended along the tissue culture substrate of the ECZ, in additional to a more classical vimentin cytoskeletal network (D, F). These cells contained abundant F-actin stress fibers (E, F). (G) Solubility of vimentin was determined at the time of wounding and in the ECZ cells on D2 postinjury. Cells were extracted sequentially in Triton X-100/octyl glucoside (Triton/OG) and SDS-containing solutions to separate the soluble vimentin populations, including unassembled vimentin and short vimentin filaments, from the highly insoluble vimentin cytoskeleton. Extracts were immunoblotted for vimentin and GAPDH and the percentage of total vimentin (G) in each fraction was quantified over three independent experiments. Results show that at the time of wounding vimentin was largely a detergent-insoluble cytoskeletal network, in contrast to its largely soluble state of organization in the ECZ at D2 postinjury, a finding consistent with the diffuse state of organization of vimentin in leader cells of the ECZ at this time (D, F). GAPDH fractionated with the Triton/OG soluble fraction. The histogram shows the percentage of vimentin ± SEM. The p values were generated by Student’s t test, *p < 0.05, ***p < 0.001. Mag bar = 20 µm. Images in A–F are presented as projections.
Increase in vimentin solubility postwounding precedes leader cell differentiation to myofibroblasts
The diffuse vimentin-labeling pattern in the lamellipodia of the highly migratory reparative cells at the leading edge of the ECZ indicated that the organization of this vimentin population was distinct from the more classical vimentin filament structures in these cells. While the vimentin cytoskeletal network present in most cell types is insoluble to Triton X-100 detergent extraction (Osborn and Weber, 1977; Blikstad and Lazarides, 1983; Gilbert and Fulton, 1985; Soellner et al., 1985), vimentin protein that is not organized into filaments, or present in the short filament structures that often appear as squiggles (Chou et al., 2007), is Triton X-100 soluble. Using a differential detergent extraction approach, we compared vimentin solubility in MCS-wounded explants at the time of injury to cells in ECZ at D2 postwounding (Figure 4G). Cells were extracted sequentially with a Triton X-100/octyl glucoside buffer (Brown and Rose, 1992) that isolates soluble forms of vimentin, including unassembled unit or short vimentin filaments, and a 4% SDS-containing buffer that extracts the highly insoluble vimentin cytoskeletal network. The extracted proteins were separated by gel electrophoresis and Western blotted for vimentin (Figure 4G). We immunoblotted for the Triton-soluble protein glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as control. The Western blot results were quantified and represented graphically as percentage of total vimentin that is soluble (Triton/OG) and insoluble (SDS) (Figure 4G). While at the time of wounding, vimentin was largely present as a detergent-insoluble cytoskeletal network, at D2 postinjury in the ECZ, vimentin had become highly soluble, with the majority of the vimentin protein fractionating together with GAPDH in the Triton X-100/OG extracts (Figure 4G). This high level of soluble vimentin was consistent with the diffuse labeling for vimentin observed in the leader cell population of the ECZ (see Figure 4D).
Posttranslational modification of vimentin following injury
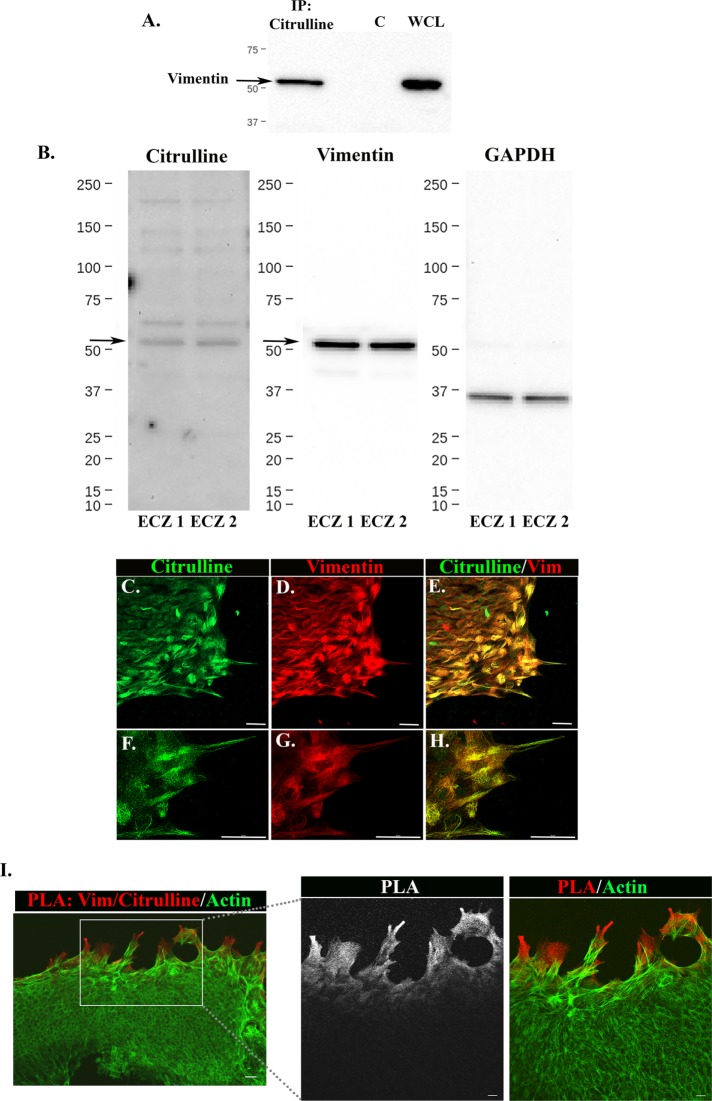
We investigated the possibility that posttranslational modifications (PTMs) of vimentin linked to vimentin solubility, including citrullination and phosphorylation (Ando et al., 1989; Inagaki et al., 1989; Sihag et al., 2007; Hyder et al., 2008; Snider and Omary, 2014; Gudmann et al., 2015), were associated with the repair-modulating leader cell population activated by MCS injury. The presence of citrullinated proteins in MCS-wounded-explant cultures was examined using an immunoprecipitation approach. At 2 d postinjury, protein extracts were immunoprecipitated with antibody to citrulline and the immunoprecipitated, citrullinated proteins immunoblotted for vimentin (Figure 5A). The results showed that vimentin is present in these citrulline immunprecipitates, indicating that vimentin is citrullinated in the MCS-wounded-explant cultures. Next, we extracted the proteins from the ECZ region at D2 postinjury, electrophoretically separated the proteins, immunoblotted the ECZ extracts for the citrulline PTM, and reprobed for vimentin (Figure 5B). This study demonstrated the citrullinated protein of greatest abundance migrated at 55 kDa and was labeled with antibody to vimentin; this supports the conclusion that vimentin is citrullinated in cells that form the ECZ postwounding.
FIGURE 5:
Citrullinated vimentin is detected postinjury and associated with repair-modulating leader cells. (A) Proteins from D2 MCS-wounded-explant cultures were extracted, immunoprecipitated with a citrulline antibody, and immunoblotted for vimentin. The results showed the presence of vimentin in these citrulline immunoprecipitates. Whole cell lysates (WCL) of MCS-wounded explants were immunoblotted for vimentin as a control. (B) Lysates of cells in the ECZ regions at D2 postwounding were immunoblotted for citrulline and reprobed for vimentin. Results shown for two independent studies (ECZ1 and ECZ2) demonstrate that vimentin comigrates with the principal citrullinated band at 55 kDa (arrow), supporting the conclusion that vimentin is citrullinated in cells of the ECZ. GAPDH is shown as a loading control. (C–H) MCS-wounded explant cultures were coimmunolabeled at D2 postinjury with antibodies to citrulline (C, E, F, H) and vimentin (D, E, G, H). The cultures were imaged in the ECZ by confocal microscopy (C–E), and at the leading edge of the same ECZ by superresolution confocal microscopy (F–H). While citrullinated proteins localized throughout the ECZ, they were enriched in leading edge cells, including many regions where citrulline colocalized with vimentin. (I) Proximity ligation assay (PLA) was performed in the ECZ at 2 d postinjury with antibodies to vimentin and citrulline and then the cultures were labeled for F-actin with fluorescent-conjugated phalloidin. A positive PLA signal (red) was detected in the lamellipodia tips of the leading-edge cells of the ECZ, shown at two levels of magnification. Mag bars, C–H, and low-magnification image in I = 20 µm. In high-magnification images in I the Mag bar = 10 µm; images are presented as projections.
Next, cell-based studies were performed to examine the localization of citrullinated proteins in ECZ region at D2 postwounding. The cultures were coimmunolabeled for citrulline and vimentin (Figure 5, C–H). While there are citrullinated proteins throughout the ECZ, they are enriched in the cells at the leading edge (Figure 5, C–E). Superresolution confocal imaging of the protrusions extended along the substrate by the leader cells of the ECZ showed coincident immunolabeling of citrulline with a subset of the vimentin protein in these cells (Figure 5, F–H). To further examine vimentin citrullination in the lamellipodial protrusions of the leader cells in the ECZ we performed a proximity ligation assay (PLA) with antibodies to vimentin and the citrulline PTM and postlabeled the cells for F-actin. The results showed a strong vimentin/citrulline PLA signal specific to the cells at the leading edge of the ECZ (Figure 5I).
Another PTM associated with vimentin solubility is its phosphorylation at sites such as Ser38. Immunolabeling for pSer38-vimentin at D2 post-MCS wounding showed that pSer38-vimentin+ cells were associated with cells remaining on the wounded explant but that there were very few pSer38-vimentin+ cells at the leading edge of the ECZ (Supplemental Figure 2). As there are multiple potential sites for vimentin phosphorylation, phosphorylation PTM cannot be ruled out as a factor in regulating vimentin organization and function in the leader cells of the ECZ.
On injury extracelluar vimentin is released into the extracellular space and associated with the surface of wound-activated leader cells and their underlying matrix substrate
Although best known as a component of the intermediate filament cytoskeleton, vimentin has also been shown to function as an extracellular protein. In this form, vimentin binds to the cell surface at distinct sites, including specific cell-surface receptors, where it initiates a cellular response (Garg et al., 2006; Shigyo et al., 2015). Among the roles identified for extracellular vimentin are the regulation of 1) immune cell function (Mor-Vaknin et al., 2003), 2) viral/bacterial entry into cells (Koudelka et al., 2009; Chi et al., 2010; Das et al., 2011; Liang et al., 2011; Du et al., 2014; Mak and Bruggemann, 2016; Yu et al., 2016), and 3) tumor progression (van Beijnum et al., 2006; Satelli and Li, 2011). In studies with our clinically relevant MCS-injury model, we examined whether extracellular vimentin was also a factor in the tissue response to wounding. We began with an immunoblot analysis of conditioned media collected from the MCS-wounded-explant cultures at D1 postinjury, which showed that on injury vimentin was released into the culture media during the repair process (Figure 6A). Medium alone does not contain vimentin. Since in a previous study we found no evidence of cell death following MCS (Walker et al., 2010), we considered this an unlikely source of the vimentin released into the medium. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay analysis performed at D1 postinjury showed that very few cells were TUNEL positive in the MCS-wounded-explant cultures (Supplemental Figure 3), supporting this conclusion. These findings imply that the release of vimentin into the media is an active process of the wounding-healing response.
FIGURE 6:
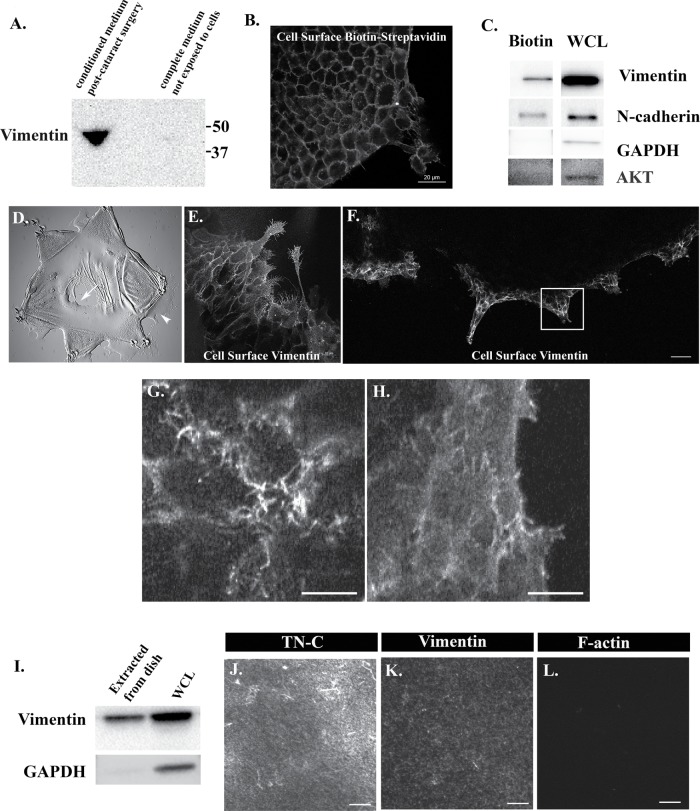
Extracellular vimentin is released in response to injury, associated with the cell surface of leader cells and localized along the ECM substrate beneath the cells. (A) Conditioned media was collected from ex vivo MCS cultures at 1 d postinjury and immunoblotted for vimentin. The results show that an extracellular form of vimentin is released in the media. No vimentin is present in culture media prior to exposure to the cells. (B) Cell surface proteins in the ECZ were biotinylated at culture D2 and fixed and the biotin label was tagged with fluorescent-conjugated streptavidin. The results showed that biotin labeling is restricted to the cell surface of cells in the ECZ. (C) Cell surface proteins in the ECZ were biotinylated at culture D2, extracted, and immunoblotted for vimentin, N-cadherin (positive control), and GAPDH and Akt (negative controls). The results demonstrated that extracellular vimentin is associated with the surface of cells in the ECZ. (D–H) Paraformaldehyde fixed but not permeabilized ex vivo wounded cultures were immunolabeled for vimentin to examine cell type specificity for extracellular-surface–associated vimentin at both the MCS wound edge (D, arrow) and the leading edge of the ECZ (D, arrowhead). Cell-surface vimentin localized exclusively to leader cells of both the native BM capsule (E) and in the ECZ (F). Boxed region in F is shown at higher magnification along apicolateral (G) and basal (H) aspects of the cells. While extracellular vimentin was most prominent on cellular protrusions at the apicolateral surface of the leader cells in the ECZ, the extracellular vimentin pool localized all along the cells’ basal surfaces of these cells where they contact the extracellular matrix and to basal membrane extensions between cells. (I) To determine whether basal extracellular vimentin also was associated with the substrate beneath the cells, cell-free extracts of substrate-associated proteins were examined by Western blot. Extracts of isolated ex vivo explants were included as a control (WCL). Results show that extracellular vimentin is associated with the proteins on the substrate. Immunoblotting for GAPDH confirmed that extracellular lysates were largely cell free. Results are representative of three independent experiments. (J–L) To directly view the substrate-linked extracellular vimentin, extracellular proteins in the ECZ were cross-linked with the membrane-impermeable BS3 cross-linker, cells extracted with RIPA, and the cross-linked proteins fixed and labeled for tenascin-C (TN-C; J), vimentin (K), and F-actin (L). The results show that extracellular vimentin was associated with the extracellular matrix substrate organized beneath the leader cells of the ECZ (J, K). No staining for F-actin was detected in the cross-linked/extracted cultures (L) confirming effective removal of the cells. Mag bars B, E = 20 µm; F = 50 µm; G, H, J–L = 10 µm. Images in B, E, F, G, and H are presented as single optical sections. Images in J–L are presented as projections.
The impact of extracellular vimentin on cell behavior following wounding is expected to involve its association with the cell surface. To determine whether vimentin is on the surface of cells, we labeled the cells within the ECZ with a cell-impermeable biotinylation reagent at D2 postwounding. Labeling with fluorescent-conjugated streptavidin, which directly binds to biotin, confirmed that the biotin label in this study was limited to the cell surface (Figure 6B). Following biotinylation, the ECZ cells were extracted and their biotin-tagged surface proteins isolated on an avidin column. The isolated, biotin-tagged proteins were immunoblotted for vimentin (Figure 6C), for the surface receptor N-cadherin (positive control), and for the cytoplasmic proteins GAPDH and Akt (negative controls) (Figure 6C). The results showed that extracellular vimentin was linked to the surface of cells in the ECZ. Cell-surface-linked vimentin may associate directly with the cell surface as it is released from the cells or become associated with the cell surface after it is released to the medium.
To determine whether there was cell-type specificity of vimentin cell-surface association, MCS-wounded-explant cultures were fixed with paraformaldehyde and not permeabilized prior to immunolabeling for vimentin. We examined both the MCS wound edge on the native basement membrane capsule (Figure 6E) and the leading edge of the cells migrating across the ECZ (Figure 6F) by confocal imaging for the presence of cell-surface–associated vimentin. The results show that extracellular vimentin was bound exclusively to the wound-activated repair cells at the leading edge of both the MCS wound and the ECZ. Z-stacks collected at high magnification in the ECZ revealed that extracellular vimentin was widely distributed all along these cells’ basal surfaces, with higher levels at the filopodia/lamellipodial processes extended at the cells’ periphery (Figure 6H). At these cells’ apical domains, extracellular vimentin was mostly located to the surface of the filopodia/lamellipodial processes extended between the cells (Figure 6G).
Using a biochemical approach, we investigated if vimentin released in response to wounding was associated with matrix substrate underlying the leader cells in the ECZ. For this study, the wounded explants were detached from the tissue culture substrate and the ECZ cells removed by gentle scraping at D2 postinjury, leaving behind proteins linked to the substrate surface of the ECZ. These substrate-associated proteins were extracted and immunoblotted for vimentin, which was shown to be a component of this protein fraction (Figure 6I). Immunoblotting for GAPDH confirmed that these substrate-associated extracellular lysates were largely cell free. In parallel, extracts from the wounded explants that were detached from the dish were immunoblotted for vimentin and GAPDH. In a different approach, the cells in the ECZ were exposed to the membrane-impermeable cross-linker bis (sulfosuccinimidyl) suberate (BS3) at D2 postinjury to link cell-surface–associated proteins to proteins in their ECM microenvironment. After radioimmunoprecipitation assay (RIPA) buffer extraction of cellular proteins the extracellular proteins were coimmunolabeled for vimentin and the ECM protein tenascin-C that forms a provisional matrix on the underlying substrate of ECZ cells migrating in response to wounding. Tenascin-C has been implicated in the induction of fibrosis (Kasprzycka et al., 2015; Bhattacharyya et al., 2016; Fu et al., 2017). Confocal imaging showed codistribution of tenascin-C and vimentin on the substrate surface in an area underneath the leader cells of the ECZ (Figure 6, J and K). No F-actin was detected in these cross-linking/extraction studies (Figure 6L).
Extracellular vimentin signals leader cell behavior in response to wounding and the differentiation of these cells to a myofibroblast phenotype
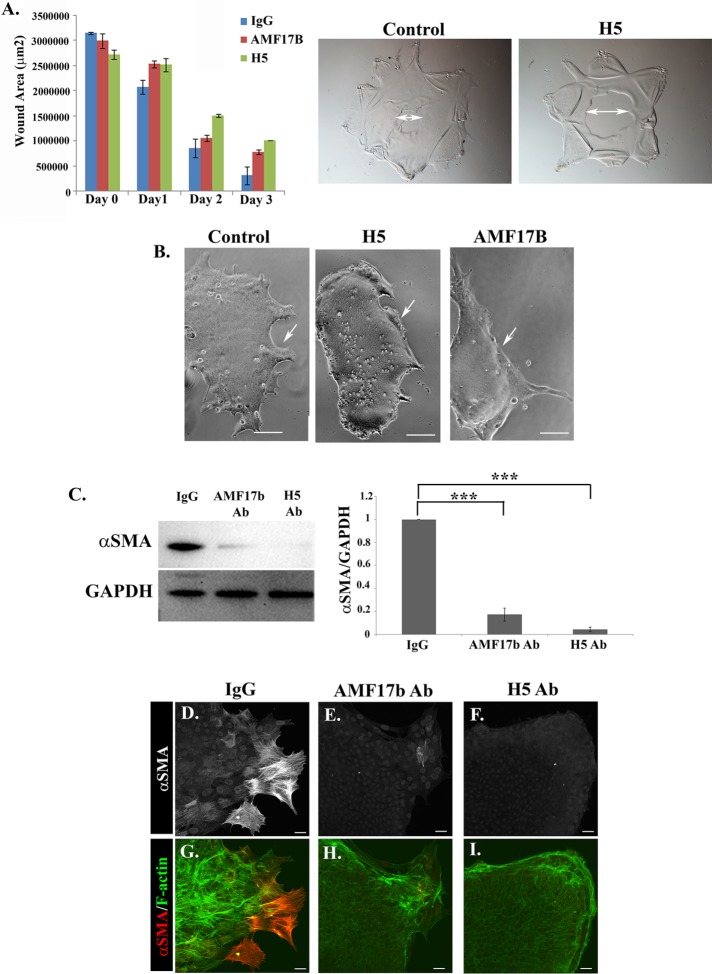
Our results showed that a soluble, extracellular, vimentin pool was released by the cells into the culture medium during the repair process, so we examined whether the timing of myofibroblast emergence could be enhanced by the addition of soluble vimentin into the explant culture medium. Those results showed that increasing the concentration of vimentin over that produced by the cells did not alter the timing of myofibroblast emergence or significantly alter the degree of myofibroblast induction in this clinically relevant wound-repair culture model (Supplemental Figure 4). Therefore, we examined the functional roles of extracellular vimentin produced by the cells in response to MCS wounding in both wound repair and the induction of fibrosis. For these studies, the wounded cultures were grown in the presence of monoclonal antibodies to vimentin. To analyze effects on wound closure and emergence of myofibroblasts, we used two distinct vimentin antibodies, AMF17B and H5, with IgG of the same isotype as control. In our previous studies, we have shown that wound closure on the native substrate of the basement membrane capsule is typically complete by D3 post-MCS (Menko et al., 2014a,b). For these studies, antibodies to vimentin were added to the explant cultures at the time of wounding (D0), the cultures imaged daily through culture D3, and open-wound area quantified. Wound repair in the presence of the vimentin antibodies slowed wound closure (Figure 7A), with dynamics and phenotype similar to what we observed previously with small interfering RNA vimentin knockdown (Menko et al., 2014a).
FIGURE 7:
Extracellular vimentin mediates leader cell behavior associated with wound healing and their transition to myofibroblasts. To examine the role of extracellular vimentin in both wound repair and fibrosis, the MCS-wounded-explant cultures were grown in the presence of two distinct antibodies to vimentin, AMF7b, and H5 or in the presence of a nontargeting IgG of the same isotype as control. (A) For the wound-healing studies, the MCS explants were treated with the vimentin antibodies from the time of wounding (D0) through D3, by which time the wound is typically healed. Open wound area was quantified each day and the results presented graphically. Representative phase-contrast images are shown at culture D3 (double arrow indicates open wound area). The presence of either vimentin antibody during wound-repair slowed wound closure. (B–I) Isolated ECZ regions were exposed to the vimentin antibodies (AMF17b and H5) or IgG isotype control for 2 d beginning at D1 postinjury to determine the effect of blocking extracellular vimentin function on myofibroblast emergence. (B) Phase-contrast imaging showed that lamellipodia extension at the leading-edge of the ECZ was impaired by treatment with either the AMF17B or the H5 vimentin antibodies (see arrows). (C) Total cell lysates prepared from ECZ regions exposed to AMF17B, H5, or isotype control antibodies were immunoblotted for αSMA and GAPDH, the results quantified and presented as a ratio of αSMA to GAPDH. This study showed that exposure to either of the vimentin antibodies inhibited emergence of αSMA+ myofibroblasts. (D–I) ECZ cells were treated with either the vimentin antibody AMF17b, the vimentin antibody H5, or an isotype-specific IgG beginning at D1 postinjury and fixed at culture D3. The cultures were immunolabeled for αSMA (D–I, red in G–I) and colabeled for F-actin (G–I, green). Emergence of αSMA+ myofibroblasts at the leading edge of the ECZ was inhibited by blocking extracellular vimentin function. p values generated by Student’s t test ***p < 0.001. Mag bar B = 10 µm and D–I = 20 µm. Images in D–I are presented as projection images.
To investigate whether extracellular vimentin regulates leader cell function in the ECZ and signals differentiation of leader cells to myofibroblasts, we exposed the cells in the ECZ to antibodies to vimentin from D1 postinjury, after removing the original lens explants from the culture dish, and examined them for effects on leader cell behavior and emergence of myofibroblasts at D3 in culture. Both vimentin antibodies suppressed the extension of lamellipodial processes by the leader cells and interfered with the movement of leader cells across the ECZ (Figure 7B). The H5 monoclonal antibody (mAb) had a more potent effect on lamellipodia extension than AMF17B. Biochemical analysis revealed that blocking the extracellular vimentin signal inhibited expression of the myofibroblast protein αSMA by cells in the ECZ, with H5 being slightly more efficacious at blocking αSMA expression than AMF17B (Figure 7C). Immunolabeling confirmed that these vimentin antibodies suppressed the differentiation of leader cells to αSMA+ myofibroblasts (Figure 7, E and F and H and I). The isotype IgG control had no effect on either cell migration or the development of fibrosis (Figure 7, D and G). We also treated the ex vivo MCS cultures with low doses of Withaferin A (WFA), which targets vimentin-soluble pools to inhibit vimentin function (Bargagna-Mohan et al., 2013) but can also have off-target effects (Vanden Berghe et al., 2012). We found that WFA, added to the media at D1 postinjury, decreased soluble pools of vimentin, altered the motility of the leader suppressing the extension of lamellipodial protrusions along the substrate surface, and blocked emergence of αSMA+ myofibroblasts in a dose-dependent manner (Supplemental Figure 5). Together, these studies show that the extracellular population of vimentin has the potential to regulate leader cell function in the repair process and to signal their differentiation to myofibroblasts.
DISCUSSION
Fate of mesenchymal repair cells is determined by their microenvironment
In studies of wound repair, there is growing evidence that mesenchymal cell populations activated to direct regenerative repair become the progenitors of the myofibroblasts that underlie the aberrant repair processes of fibrosis and scarring. We have discovered that the cytoskeletal protein vimentin, which is essential to the normal function of these repair-modulating cells in tissue regeneration, can tip the balance from regenerative to fibrotic repair when these cells encounter a profibrotic microenvironment. Our studies examined this dichotomous role for repair cells in the clinically relevant ex vivo MCS wound-repair/fibrosis culture model. In their native microenvironment of the lens basement membrane capsule, these vimentin-rich mesenchymal repair cells rapidly migrate to the leading edge of the MCS wound where they direct lens epithelial tissue repair in a vimentin-dependent manner. When these same repair cells move to the outside edges of the MCS explant cut to flatten the lens capsule on the substrate, they lead lens-epithelial cells off the lens capsule onto and across the culture dish, where these vimentin-rich leader cells are induced to differentiate to αSMA+ myofibroblasts, the cell type associated with fibrotic disease. This same change in cell phenotype was induced when these repair cells encounter a collagen gel microenvironment whose stiffness is in the physiological range of fibrotic tissues.
Cell-surface–associated vimentin is a factor in determining repair cell fate and function
In our wound healing/fibrosis model, using vimentin antibodies that blocked the function of extracellular vimentin, we found that vimentin plays a role in promoting fibrosis beyond its classically described structural functions. Our studies show for the first time that an extracellular, cell-surface–associated vimentin population released in response to injury is linked specifically to the wound-activated mesenchymal repair cells that localize to the leading edge of the MCS wound. This extracellular vimentin pool is required for the function of these mesenchymal leader cells in directing the regenerative repair process in the cells’ native basement membrane microenvironment (modeled in Figure 8). Our data suggest that the fate of these cells is altered to that of a myofibroblast when they associate with a profibrotic ECM microenvironment. This interaction has the potential to signal a change in cell fate to a myofibroblast and alter the function of these repair cells from proregenerative to agents that promote fibrotic disease. It is possible that this extracellular vimentin population functions by binding to a putative receptor for extracellular vimentin located on the cell surface of the repair cells.
FIGURE 8:
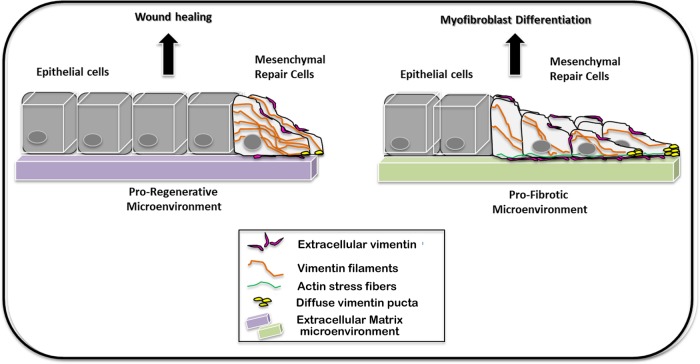
Model depicting differences in vimentin and actin cytoskeleton organization in mesenchymal leader cells in proregenerative and profibrotic environments. The mesenchymal leader cells that locate to the wound edge in a proregenerative environment organize an extensive vimentin intermediate filament cytoskeleton that extends into the cells’ lamellipodial processes. These repair-modulating cells are remarkably poor in actin stress fibers. The cytoskeletal organization is altered when these mesenchymal leader cells migrate into a profibrotic environment. Here, these cells organize extensive actin stress fibers and vimentin is most striking for its high expression in the cells’ lamellipodia where it is present in a diffusely organized form. On injury, vimentin is also released to the extracellular space and becomes linked to the cell surface of the mesenchymal repair cells at the leading edge of both environments where it plays a role in both wound repair and altering these cells’ fate to myofibroblasts.
Corrleation between vimentin solubility and citrullination following injury
A high percentage of the vimentin pool in the cells of the ECZ following wounding was Triton/OG detergent soluble, contrasting the highly detergent-insoluble vimentin cytoskeletal network of the lens before wounding. Detergent insolubility is a feature of this intermediate filament protein typical of most cell and tissue types (Osborn and Weber, 1977; Blikstad and Lazarides, 1983; Gilbert and Fulton, 1985; Soellner et al., 1985). It is likely that the soluble pool of vimentin in the ECZ includes both cytoplasmic and extracellular forms of this protein. Our data suggest that maintaining a large, soluble pool of vimentin in response to the MCS wound is important to the mechanism by which the mesenchymal leader cells function in directing regenerative repair.
Vimentin solubility is typically associated with PTMs of this protein, with both citrullination and phosphorylation believed to be primary PTMs that lead to and/or maintain vimentin protein-soluble states (Ando et al., 1989; Inagaki et al., 1989; Sihag et al., 2007; Hyder et al., 2008; Snider and Omary, 2014). Our studies show that vimentin citrullination was a feature of the mesenchymal leader cells located at the leading edge of the cells migrating across the ECZ. This finding is interesting in light of evidence that suggests citrullinated forms of vimentin play a role in fibrosis, including a report that patients with fibrosis express PAD enzymes that catalyze vimentin citrullination (Abdeen and Olusi, 2010; Gudmann et al., 2015) and the finding that patients with fibrosis produce antibodies to citrullinated vimentin (Abdeen et al., 2011; Vassiliadis et al., 2012, 2013).
Dynamic differences in vimentin and actin cytoskeletal organization in proregenerative and profibrotic environments
The lamellipodial protrusions that the mesenchymal leader cells extend along the rigid substrate of the ECZ were rich in vimentin, with a high level of the vimentin labeling of these cells appearing diffuse. This immunolabeling pattern likely represents a concentration of soluble forms of vimentin, which could include unassembled vimentin protein, and unit or short vimentin, likely including vimentin that is released to their cell surface. A distinguishing feature of the mesenchymal repair cells is the assembly of actin stress fibers in the more rigid ECZ microenvironment, but not when these cells populate the MCS wound edge on their native basement membrane. It has been shown that the presence of an organized vimentin intermediate filament cytoskeletal network can negatively regulate Rho activity and block both actin stress fiber formation and myosin contractility (Jiu et al., 2017). This suggests that there is a dynamic, coordinated regulation of cytoskeletal elements in these cells and indicates that the predominance of soluble vimentin forms may be necessary to establish the contractile phenotype of αSMA+ myofibroblasts.
On injury vimentin is released into the extracellular space
Our discoveries that extracellular vimentin is released in these wound-repair cultures in response to injury, that it associates with the cell-surface of repair-modulating cells, and that it mediates the differentiation of the leader/repair cells to myofibroblasts are important new findings. The mechanism of vimentin release is not yet clear. While vimentin lacks a classic secretory signal sequence, it is secreted by macrophages in response to both pro- and anti-inflammatory cytokines (Mor-Vaknin et al., 2003). The release in exosomes is another potential source for the extracellular vimentin pool (Chen et al., 2016). Our own studies of the invasive potential of these wound-activated repair cells have shown that when these cells migrate through three-dimensional Matrigel, they are surrounded by vimentin concentrated in puncta that could represent vimentin-containing vesicles (Bleaken et al., 2016).
Potential mechanisms of extracellular vimentin function
While extracellular vimentin has been localized to the cell surface of different cell types, including macrophages (Mor-Vaknin et al., 2003), endothelial cells (Xu et al., 2004; Zou et al., 2006; Koudelka et al., 2009; Glaser-Gabay et al., 2011), neutrophils (Moisan and Girard, 2006), astrocytes (Teshigawara et al., 2013), and circulating tumor cells (Satelli et al., 2014, 2016, 2017), its extracellular function has been poorly understood. In the mesenchymal repair cells that populate the leading edge of the ECZ of our MCS-wounded cultures, extracellular vimentin was most highly localized to the cells’ basal surfaces (see model, Figure 8). Vimentin could mediate signaling binding to cell-surface receptors, transmitting a mechanotransduction signal that leads to the change in cell fate associated with fibrosis. In support of this hypothesis, studies have reported that vimentin can act as a ligand for cell-surface receptors, such as CD44 (Pall et al., 2011) and IGF-1R (Garg et al., 2006; Shigyo et al., 2015). While future studies will be aimed at elucidating the mechanisms by which extracellular vimentin signals myofibroblast differentiation, our current studies show that the microenvironment is a critical driver of the function of the extracellular pool of vimentin. In wound repair, the microenvironment determines whether vimentin associated with the surface of the mesenchymal cells activated on wounding promotes their role in regenerative repair or their transition to myofibroblasts associated with fibrosis.
MATERIALS AND METHODS
Ex vivo MCS explant cultures
Ex vivo MCS explant cultures were prepared as previously described (Walker et al., 2007, 2010, 2015; Menko et al., 2014a,b). Cells were cultured in complete media (Media 199) (Invitrogen, Carlsbad, CA) that included 1% penicillin/streptomycin (Mediatech-Cellgro, Manassas, VA), and 1% l-glutamine (Mediatech-Cellgro) with 10% fetal calf serum (Invitrogen). The mock cataract surgery is performed after lenses are removed from E15 chick embryos (Poultry Futures, Lititz, PA) to ex vivo culture. This microsurgical procedure removes the fiber cells that comprise the mass of lens tissue (Walker et al., 2007, 2015), leaving the basement membrane that surrounds the lens in vivo as a capsular bag structure containing only the wounded lens epithelial cells and a subpopulation of vimentin-rich mesenchymal repair cells (Walker et al., 2010; Menko et al., 2014a). The lens epithelium and subpopulation of mesenchymal repair cells associated with the lens capsule are placed on tissue plastic cell side up. By making cuts in the anterior aspects of the lens capsule, we flatten the postcataract surgery lens capsular bag with its lens epithelial and mesenchymal repair cells still attached. The resultant star-shaped, flattened explants are placed on the culture dish, cell side up (Figure 1A). The wounded lens epithelial cells are located at the points of the star, surrounding the denuded lens capsule in the center of the explants where the fiber cells had been attached (Figure 1A, asterisk).
Since differentiation to myofibroblasts is promoted in a rigid extracellular environment (Tomasek et al., 2002; Hinz, 2007; Carver and Goldsmith, 2013), we studied myofibroblast emergence in cells that migrate from the outside cut edges of the ex vivo MCS-wounded-explant cultures onto and across the rigid microenvironment of the tissue culture plastic (Figure 1, A and B). The lens epithelial cells are led off the lens capsule by vimentin-rich mesenchymal cells that had rapidly migrated to the outside cut edge of the capsule in response to the cut wound. We refer to the region just beyond the lens capsule as the ECZ, the cells of which provide an ideal reductionist model with which to study how cells critical to regulating injury repair can be induced to change their fate and become the myofibroblasts that underlie fibrosis.
For collagen gel substrate studies, MCS-wounded-explant cultures were placed directly on tissue culture dishes coated with 1 ml of 2.5 mg/ml rat tail collagen I gels, according to the manufacturer’s instructions (Millipore, Billerica, MA). For Boyden Chamber invasion studies, MCS-wounded-explant cultures were placed cell side down on Matrigel-coated membrane filters in Matrigel-Transwell assays (BD Biosciences, San Jose, CA, and Corning, Corning, NY). Serum-free media was placed in the upper chamber and serum-containing medium to the lower chamber. The underside of the filters containing invaded cells was processed for immunostaining. Phase images were acquired with a Nikon Eclipse Ti microscope using imaging software (NIS Elements; Nikon). Time-lapse video was acquired using a Nikon Eclipse Ti microscope driven by image analysis software (NIS Elements). For time-lapse studies the culture dishes were contained in a Tokai Hit stage-top incubator. Vimentin function was blocked with vimentin monoclonal antibodies, H5 (10 µg/ml) or AMF17b (20 µg/ml) (mAbs; Developmental Studies Hybridoma Bank, Bank Iowa City, IA), with mouse IgG isotype antibody (20 µg/ml) from Jackson Immunoresearch Laboratories (West Grove, PA) as control. In supporting studies vimentin was blocked with the 0.5–1 μM Withaferin A (Tocris, Ellisville, MO) with the vehicle dimethyl sulfoxide (DMSO; Sigma Aldrich, St. Louis, MO) as control. Fresh antibody or inhibitor was added each day. To determine the effect of exogenous vimentin on myofibroblast differentiation, recombinant vimentin (catalogue no. 2105-VI-100 from R&D Systems, Minneapolis, MN) was added to ex vivo cultures on day 1. For proliferation analysis, cells were pulsed with EdU (Click-iT EdU Imaging Kits; Invitrogen, Carlsbad, CA) for 2 h and analyzed according to the manufacturer’s instructions. TUNEL assay (catalogue no. 12156792910) from Sigma Aldrich (St. Louis, MO) was performed according to the manufacturer’s instructions.
Sequential detergent extraction
As we have described previously (Leonard et al., 2011; Menko et al., 2014a), for sequential extraction studies samples were extracted with Triton X-100/octyl glucoside (Triton/OG) extraction buffer (44.4 mM n-octyl β-d-glucopyranoside, 1% Triton X-100, 100 mM NaCl, 1 mM MgCl2, 5 mM EDTA, 10 mM imidazole, pH 7.4). The Triton/OG-insoluble proteins were solubilized in 2× sample buffer (125 mM Tris-HCl, 4% SDS, 20% glycerol, 2% β-mercaptoethanol, 0.5% bromophenol blue). To determine the relative distribution of Triton/OG soluble and insoluble proteins the Triton/OG soluble fractions were loaded at equal protein concentration and the SDS fractions were loaded at volumes equal to the respective Triton/OG soluble fractions.
Western blot analysis
Samples were extracted in Triton/OG except for studies of α-SMA expression for which the cells were extracted in RIPA buffer (5 mM EDTA, 150 mM NaCl, 1% NP40, 1% sodium deoxycholate, 1% SDS 20% solution, 50 mM Tris-HCl, pH 7.4) containing protease/phosphatase inhibitor cocktail (Cell Signaling, Danvers, MA). All protein concentrations were determined by a BCA protein assay (BCA Assay; Pierce, Rockford, IL). All proteins were separated on Tris-glycine gels (Novex, San Diego, CA), under reducing conditions followed by electrophoretically transferring to membranes (Immobilon-P; Millipore), and subsequently immunoblotted as described previously (Walker and Menko, 1999). Enhanced chemiluminescence (ECL) and ECL Plus Western blotting substrate (Pierce; ThermoFisher, Waltham, MA) were used for detection. Antibodies used for Western blotting (see Table 1) included vimentin (AMF17b mAb; Developmental Studies Hybridoma Bank, Iowa City, IA), vimentin polyclonal antibody (a generous gift from Paul FitzGerald, University of California, Davis, CA), GAPDH (catalogue no. sc-25778) from Santa Cruz Biotechnology (Santa Cruz, CA), α-smooth muscle actin (catalogue no. ab5694) from Abcam (Cambridge, United Kingdom), N-cadherin (catalogue no. 610921) from BD Biosciences (San Jose, CA) and Akt (catalogue number 9272) from Cell Signaling (Danvers, MA). Densitometry analysis was performed using AlphaView Software (Protein Simple; San Jose, CA). α-SMA expression in the time course is presented as a ratio to GAPDH. For the WFA and vimentin antibody studies results were normalized to control and presented as a ratio to GAPDH. Sequential detergent extractions are shown as the percentage of total vimentin in each fraction. Histograms are shown ± SEM, with p values generated by Student’s t test.
TABLE 1:
Antibody sources, product numbers, and dilutions used in these studies.
| Antibody name | Source | Product number | Dilution factor |
|---|---|---|---|
| Alpha smooth muscle actin | Millipore Sigma | CBL171 | 1/200 (IF) |
| Alpha smooth muscle actin | Abcam | Ab5694 | 1/100 (IF); 1/1000 (WB) |
| Akt | Cell Signaling | 9272 | 1/1000 (WB) |
| CD44 | Developmental Studies Hybridoma Bank (DSHB) | 1D10 | 1/10 (IF) |
| Citrulline | Abcam | Ab6464 | 1/100 (IF) |
| Citrulline | Millipore Sigma | MABN328 | 1/200 (IF); 1/500 (WB) |
| GAPDH | Santa Cruz | Sc-25778 | 1/1000 (WB) |
| N-cadherin | BD Transduction Lab | 610921 | 1/2000 (WB) |
| Tenascin | Millipore Sigma | AB19013 | 1/200 (IF) |
| Vimentin | DSHB | AMF17B | 1/10 (IF); 1/100 (WB) |
| Vimentin | DSHB | H5 | 1/10 (IF) |
| Vimentin | Abcam | Ab92547 | 1/2500 (WB) |
| Vimentin (phosphoS38) | Abcam | Ab52942 | 1/100 (IF) |
| Vimentin | Gift, Paul FitzGerald, University of California, Davis | 1/100 (IF) |
Cell-surface biotinylation
For biotinylation of cell-surface proteins we used the Cell Surface Protein Isolation Kit (Pierce; ThermoFisher, Waltham, MA) according to manufacturer’s directions. Briefly, cells were incubated with the membrane-impermeable Sulfo-NHS-SS-Biotin reagent. After the reaction was stopped, cells were gently scraped, lysed, and transferred to a NeutroAvidin agarose column for isolation of biotin-labeled cell-surface proteins. Finally, biotin labeled cell-surface proteins were eluted from the column using DTT solution and SDS–PAGE buffer and analyzed by Western blot analysis. Biotin labeled cultures were also fixed and labeled with a fluorescent-conjugated streptavidin (catalogue no. 21832) from Invitrogen (Carlsbad, CA) to confirm that biotin labeled cell surfaces.
Immunofluorescence
Immunostaining of epithelial explants was performed as previously described (Walker et al., 2007, 2010). Briefly, prior to immunostaining, explants were fixed in 3.7% formaldehyde in phosphate-buffered saline (PBS) and permeabilized in 0.25% Triton X-100 (Sigma-Aldrich) in PBS. For immunostaining of nonpermeabilized cells to detect only surface proteins cultures were fixed with either 1 or 2% methanol-free paraformaldehyde, as indicated. The following primary antibodies were used for the immunofluorescence studies (see Table 1): vimentin (H5 and AMF17b mAb; Developmental Studies Hybridoma Bank, Iowa City, IA), vimentin polyclonal antibody (a generous gift from Paul FitzGerald), vimentin polyclonal antibody (catalogue no. ab92547) from Abcam (Cambridge, MA), CD44 (1D10 mAb, Developmental Studies Hybridoma Bank, Iowa City, IA), tenascin-C (catalogue no. AB19013) from Millipore (Burlington, MA), pSerine 38 vimentin rabbit (catalogue no. ab115150), αSMA (catalogue no. ab5694), and citrulline antibody (catalogue no. ab6464) from Abcam (Cambridge, MA), and a citrulline antibody F95 (catalogue no. MABN328) from MilliporeSigma (Burlington, MA). Cells were incubated with primary antiserum followed by rhodamine-conjugated (Jackson ImmunoResearch Laboratories and Millipore), fluorescein-conjugated (Jackson ImmunoResearch Laboratories, Westgrove, PA), or Alexa Fluor 488–conjugated (ThermoFisher, Waltham, MA), secondary antibodies. As indicated, cells were counterstained with Alexa Fluor 488–conjugated phalloidin, Alexa Fluor 633 or Alexa Fluor 647 phalloidin (ThermoFisher, Waltham, MA) to label filamentous actin (F-actin), and/or the nuclear stain TO-PRO 3 (Invitrogen, Carlsbad, CA). To track the fate of CD44 leader cells to become myofibroblasts, cultures were live labeled with CD44 antibody for 20 min. Antibody was removed and media was replaced for 2 d subsequent to fixation and processing for immunostaining. Immunostained samples were examined by confocal microscopy (LSM 510, LSM 800 with Super Resolution Airyscan, Zeiss, Thornwood, NY). Single images or Z-stacks were collected and analyzed; the data presented represent single optical planes or maximal intensity projection images.
Proximal ligation assay
To determine whether vimentin was citrullinated, we performed proximal ligation assay (PLA) according to manufactures guidelines (Duolink; Sigma-Aldrich). Briefly, fixed cultures were incubated with antibody to citrulline (catalogue no. ab6464) from Abcam and vimentin antibody H5 from Developmental Studies Hybridoma Bank simultaneously, followed by PLA affinity probes for rabbit and mouse (Duolink; Sigma-Aldrich) that binds to the target antibodies. Cells were then incubated with Detection Reagents Red (Duolink; Sigma-Aldrich), which includes a ligation solution and a connecter oligonucleotide that hybridizes to the probes that allows amplification of DNA to produce a signal to be detected by fluorescence imaging.
Cross-link extraction
To examine whether vimentin associated with the extracellular matrix deposited beneath the cells on the substrate, we performed cross-link extractions as previously described (Enomoto et al., 1993). Cells were then incubated with the cell-impermeable cross-linker BS3 (ThermoFisher, Waltham, MA) in PBS with phenylmethylsulfonyl fluoride (PMSF) protease inhibitor (Sigma-Aldrich). The reaction was quenched with (50 mM Tris-HCl), and the cells were extracted with RIPA buffer containing protease/phosphatase inhibitor cocktail (Cell Signaling, Danvers, MA) and the substrate-associated proteins fixed with 3.7% formaldehyde in PBS.
Supplementary Material
Acknowledgments
We thank Liping Zhang and Heather Paulson for their excellent technical assistance. The monoclonal antibodies AMF17b (vimentin) developed by Alice B. Fulton, H5 (vimentin) developed by J. R. Sanes, and 1D10 (CD44) developed by W. M. Halfter, were all obtained from the Developmental Studies Hybridoma Bank, created by the National Institute of Child Health and Human Development and maintained by the Department of Biology, University of Iowa. Vimentin polyclonal antibody was a generous gift from Paul FitzGerald (University of California, Davis, Davis, CA). This work was supported by National Institutes of Health Grant EY021784 to A.S.M.
Abbreviations used:
- αSMA
α-smooth muscle actin
- ECM
extracellular matrix
- ECZ
extracapsular zone
- PBS
phosphate-buffered saline
- PTM
posttranslational modification
- TN-C
tenascin-C.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E17-06-0364) on May 2, 2018.
REFERENCES
- Abdeen S, Olusi SO, George S. (2011). Serum anti-modified citrullinated vimentin antibody concentration is associated with liver fibrosis in patients with chronic hepatitis. Hepat Med , 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdeen SM, Olusi SO. (2010). Peptidyl arginine deiminase: a novel immunohistochemical marker for liver fibrosis in patients with chronic hepatitis. Acta Histochem , 592–603. [DOI] [PubMed] [Google Scholar]
- Ando S, Tanabe K, Gonda Y, Sato C, Inagaki M. (1989). Domain- and sequence-specific phosphorylation of vimentin induces disassembly of the filament structure. Biochemistry , 2974–2979. [DOI] [PubMed] [Google Scholar]
- Arora PD, Narani N, McCulloch CA. (1999). The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts. Am J Pathol , 871–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bano F, Banerji S, Howarth M, Jackson DG, Richter RP. (2016). A single molecule assay to probe monovalent and multivalent bonds between hyaluronan and its key leukocyte receptor CD44 under force. Sci Rep , 34176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargagna-Mohan P, Deokule SP, Thompson K, Wizeman J, Srinivasan C, Vooturi S, Kompella UB, Mohan R. (2013). Withaferin A effectively targets soluble vimentin in the glaucoma filtration surgical model of fibrosis. PLoS One , e63881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargagna-Mohan P, Paranthan RR, Hamza A, Zhan CG, Lee DM, Kim KB, Lau DL, Srinivasan C, Nakayama K, Nakayama KI, et al (2012). Corneal antifibrotic switch identified in genetic and pharmacological deficiency of vimentin. J Biol Chem , 989–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Wang W, Morales-Nebreda L, Feng G, Wu M, Zhou X, Lafyatis R, Lee J, Hinchcliff M, Feghali-Bostwick C, et al (2016). Tenascin-C drives persistence of organ fibrosis. Nat Commun , 11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleaken BM, Menko AS, Walker JL. (2016). Cells activated for wound repair have the potential to direct collective invasion of an epithelium. Mol Biol Cell , 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blikstad I, Lazarides E. (1983). Vimentin filaments are assembled from a soluble precursor in avian erythroid cells. J Cell Biol , 1803–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Rose JK. (1992). Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell , 533–544. [DOI] [PubMed] [Google Scholar]
- Carver W, Goldsmith EC. (2013). Regulation of tissue fibrosis by the biomechanical environment. Biomed Res Int , 101979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Yang L, Cui Y, Zhou Y, Yin X, Guo J, Zhang G, Wang T, He QY. (2016). Cytoskeleton-centric protein transportation by exosomes transforms tumor-favorable macrophages. Oncotarget , 67387–67402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F, Shen Y, Mohanasundaram P, Lindstrom M, Ivaska J, Ny T, Eriksson JE. (2016). Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-beta-Slug signaling. Proc Natl Acad Sci USA , E4320–E4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi F, Jong TD, Wang L, Ouyang Y, Wu C, Li W, Huang SH. (2010). Vimentin-mediated signalling is required for IbeA+ E. coli K1 invasion of human brain microvascular endothelial cells. Biochem J , 79–90. [DOI] [PubMed] [Google Scholar]
- Chou YH, Flitney FW, Chang L, Mendez M, Grin B, Goldman RD. (2007). The motility and dynamic properties of intermediate filaments and their constituent proteins. Exp Cell Res , 2236–2243. [DOI] [PubMed] [Google Scholar]
- Das S, Ravi V, Desai A. (2011). Japanese encephalitis virus interacts with vimentin to facilitate its entry into porcine kidney cell line. Virus Res , 404–408. [DOI] [PubMed] [Google Scholar]
- dos Santos G, Rogel MR, Baker MA, Troken JR, Urich D, Morales-Nebreda L, Sennello JA, Kutuzov MA, Sitikov A, Davis JM, et al (2015). Vimentin regulates activation of the NLRP3 inflammasome. Nat Commun , 6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du N, Cong H, Tian H, Zhang H, Zhang W, Song L, Tien P. (2014). Cell surface vimentin is an attachment receptor for enterovirus 71. J Virol , 5816–5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duscher D, Maan ZN, Wong VW, Rennert RC, Januszyk M, Rodrigues M, Hu M, Whitmore AJ, Whittam AJ, Longaker MT, Gurtner GC. (2014). Mechanotransduction and fibrosis. J Biomech , 1997–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto MI, Boettiger D, Menko AS. (1993). Alpha 5 integrin is a critical component of adhesion plaques in myogenesis. Dev Biol , 180–197. [DOI] [PubMed] [Google Scholar]
- Fu H, Tian Y, Zhou L, Zhou D, Tan RJ, Stolz DB, Liu Y. (2017). Tenascin-C is a major component of the fibrogenic niche in kidney fibrosis. J Am Soc Nephrol , 785–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg A, Barnes PF, Porgador A, Roy S, Wu S, Nanda JS, Griffith DE, Girard WM, Rawal N, Shetty S, Vankayalapati R. (2006). Vimentin expressed on Mycobacterium tuberculosis-infected human monocytes is involved in binding to the NKp46 receptor. J Immunol , 6192–6198. [DOI] [PubMed] [Google Scholar]
- Gilbert M, Fulton AB. (1985). The specificity and stability of the triton-extracted cytoskeletal framework of gerbil fibroma cells. J Cell Sci , 335–345. [DOI] [PubMed] [Google Scholar]
- Glaser-Gabay L, Raiter A, Battler A, Hardy B. (2011). Endothelial cell surface vimentin binding peptide induces angiogenesis under hypoxic/ischemic conditions. Microvasc Res , 221–226. [DOI] [PubMed] [Google Scholar]
- Goldman RD, Cleland MM, Murthy SN, Mahammad S, Kuczmarski ER. (2012). Inroads into the structure and function of intermediate filament networks. J Struct Biol , 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmann NS, Hansen NU, Jensen AC, Karsdal MA, Siebuhr AS. (2015). Biological relevance of citrullinations: diagnostic, prognostic and therapeutic options. Autoimmunity , 73–79. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Aebi U. (2016). Intermediate filaments: structure and assembly. Cold Spring Harb Perspect Biol , a018242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann H, Strelkov SV, Burkhard P, Aebi U. (2009). Intermediate filaments: primary determinants of cell architecture and plasticity. J Clin Invest , 1772–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B. (2007). Formation and function of the myofibroblast during tissue repair. J Invest Dermatol , 526–537. [DOI] [PubMed] [Google Scholar]
- Hinz B. (2015). The extracellular matrix and transforming growth factor-beta1: tale of a strained relationship. Matrix Biol , 54–65. [DOI] [PubMed] [Google Scholar]
- Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. (2012). Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol , 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder CL, Pallari HM, Kochin V, Eriksson JE. (2008). Providing cellular signposts–post-translational modifications of intermediate filaments. FEBS Lett , 2140–2148. [DOI] [PubMed] [Google Scholar]
- Inagaki M, Takahara H, Nishi Y, Sugawara K, Sato C. (1989). Ca2+-dependent deimination-induced disassembly of intermediate filaments involves specific modification of the amino-terminal head domain. J Biol Chem , 18119–18127. [PubMed] [Google Scholar]
- Jiu Y, Peranen J, Schaible N, Cheng F, Eriksson JE, Krishnan R, Lappalainen P. (2017). Vimentin intermediate filaments control actin stress fiber assembly through GEF-H1 and RhoA. J Cell Sci , 892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprzycka M, Hammarstrom C, Haraldsen G. (2015). Tenascins in fibrotic disorders-from bench to bedside. Cell Adh Migr , 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koudelka KJ, Destito G, Plummer EM, Trauger SA, Siuzdak G, Manchester M. (2009). Endothelial targeting of cowpea mosaic virus (CPMV) via surface vimentin. PLoS Pathog , e1000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard M, Zhang L, Zhai N, Cader A, Chan Y, Nowak RB, Fowler VM, Menko AS. (2011). Modulation of N-cadherin junctions and their role as epicenters of differentiation-specific actin regulation in the developing lens. Dev Biol , 363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. (2007). Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology , 1246–1256. [DOI] [PubMed] [Google Scholar]
- Liang JJ, Yu CY, Liao CL, Lin YL. (2011). Vimentin binding is critical for infection by the virulent strain of Japanese encephalitis virus. Cell Microbiol , 1358–1370. [DOI] [PubMed] [Google Scholar]
- Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. (2010). Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol , 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery J, Kuczmarski ER, Herrmann H, Goldman RD. (2015). Intermediate filaments play a pivotal role in regulating cell architecture and function. J Biol Chem , 17145–17153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak TN, Bruggemann H. (2016). Vimentin in bacterial infections. Cells , 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B, Kubes P. (2015). Interactions between CD44 and hyaluronan in leukocyte trafficking. Front Immunol , 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menko AS, Bleaken BM, Libowitz AA, Zhang L, Stepp MA, Walker JL. (2014a). A central role for vimentin in regulating repair function during healing of the lens epithelium. Mol Biol Cell , 776–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menko AS, Bleaken BM, Walker JL. (2014b). Regional-specific alterations in cell-cell junctions, cytoskeletal networks and myosin-mediated mechanical cues coordinate collectivity of movement of epithelial cells in response to injury. Exp Cell Res , 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisan E, Girard D. (2006). Cell surface expression of intermediate filament proteins vimentin and lamin B1 in human neutrophil spontaneous apoptosis. J Leukoc Biol. , 489–498. [DOI] [PubMed] [Google Scholar]
- Mor-Vaknin N, Punturieri A, Sitwala K, Markovitz DM. (2003). Vimentin is secreted by activated macrophages. Nat Cell Biol , 59–63. [DOI] [PubMed] [Google Scholar]
- Nibourg LM, Gelens E, Kuijer R, Hooymans JM, van Kooten TG, Koopmans SA. (2015). Prevention of posterior capsular opacification. Exp Eye Res , 100–115. [DOI] [PubMed] [Google Scholar]
- O’Connor JW, Riley PN, Nalluri SM, Ashar PK, Gomez EW. (2015). Matrix rigidity mediates TGFbeta1-induced epithelial-myofibroblast transition by controlling cytoskeletal organization and MRTF-A localization. J Cell Physiol , 1829–1839. [DOI] [PubMed] [Google Scholar]
- Osborn M, Weber K. (1977). The detertent-resistant cytoskeleton of tissue culture cells includes the nucleus and the microfilament bundles. Exp Cell Res , 339–349. [DOI] [PubMed] [Google Scholar]
- Pall T, Pink A, Kasak L, Turkina M, Anderson W, Valkna A, Kogerman P. (2011). Soluble CD44 interacts with intermediate filament protein vimentin on endothelial cell surface. PLoS One , e29305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M. (2005). Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron , 715–726. [DOI] [PubMed] [Google Scholar]
- Satelli A, Batth I, Brownlee Z, Mitra A, Zhou S, Noh H, Rojas CR, Li H, Meng QH, Li S. (2017). EMT circulating tumor cells detected by cell-surface vimentin are associated with prostate cancer progression. Oncotarget , 49329–49337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satelli A, Batth IS, Brownlee Z, Rojas C, Meng QH, Kopetz S, Li S. (2016). Potential role of nuclear PD-L1 expression in cell-surface vimentin positive circulating tumor cells as a prognostic marker in cancer patients. Sci Rep , 28910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satelli A, Li S. (2011). Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci , 3033–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satelli A, Mitra A, Cutrera JJ, Devarie M, Xia X, Ingram DR, Dibra D, Somaiah N, Torres KE, Ravi V, et al (2014). Universal marker and detection tool for human sarcoma circulating tumor cells. Cancer Res , 1645–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt-Graff A, Desmouliere A, Gabbiani G. (1994). Heterogeneity of myofibroblast phenotypic features: an example of fibroblastic cell plasticity. Virchows Arch , 3–24. [DOI] [PubMed] [Google Scholar]
- Schwartz MA. (2010). Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol , a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senbanjo LT, Chellaiah MA. (2017). CD44: a multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front Cell Dev Biol , 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigyo M, Kuboyama T, Sawai Y, Tada-Umezaki M, Tohda C. (2015). Extracellular vimentin interacts with insulin-like growth factor 1 receptor to promote axonal growth. Sci Rep , 12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigyo M, Tohda C. (2016). Extracellular vimentin is a novel axonal growth facilitator for functional recovery in spinal cord-injured mice. Sci Rep , 28293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihag RK, Inagaki M, Yamaguchi T, Shea TB, Pant HC. (2007). Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp Cell Res , 2098–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli O, Schurch W, Seemayer T, Lagace R, Montandon D, Pittet B, Gabbiani G. (1989). Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest , 275–285. [PubMed] [Google Scholar]
- Snider NT, Omary MB. (2014). Post-translational modifications of intermediate filament proteins: mechanisms and functions. Nat Rev Mol Cell Biol , 163–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soellner P, Quinlan RA, Franke WW. (1985). Identification of a distinct soluble subunit of an intermediate filament protein: tetrameric vimentin from living cells. Proc Natl Acad Sci USA , 7929–7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshigawara K, Kuboyama T, Shigyo M, Nagata A, Sugimoto K, Matsuya Y, Tohda C. (2013). A novel compound, denosomin, ameliorates spinal cord injury via axonal growth associated with astrocyte-secreted vimentin. Br J Pharmacol , 903–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. (2002). Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol , 349–363. [DOI] [PubMed] [Google Scholar]
- Tsou PS, Haak AJ, Khanna D, Neubig RR. (2014). Cellular mechanisms of tissue fibrosis. 8. Current and future drug targets in fibrosis: focus on Rho GTPase-regulated gene transcription. Am J Physiol Cell Physiol , C2–C13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beijnum JR, Dings RP, van der Linden E, Zwaans BM, Ramaekers FC, Mayo KH, Griffioen AW. (2006). Gene expression of tumor angiogenesis dissected: specific targeting of colon cancer angiogenic vasculature. Blood , 2339–2348. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe W, Sabbe L, Kaileh M, Haegeman G, Heyninck K. (2012). Molecular insight in the multifunctional activities of Withaferin A. Biochem Pharmacol , 1282–1291. [DOI] [PubMed] [Google Scholar]
- Vassiliadis E, Oliveira CP, Alvares-da-Silva MR, Zhang C, Carrilho FJ, Stefano JT, Rabelo F, Pereira L, Kappel CR, Henriksen K, et al (2012). Circulating levels of citrullinated and MMP-degraded vimentin (VICM) in liver fibrosis related pathology. Am J Transl Res , 403–414. [PMC free article] [PubMed] [Google Scholar]
- Vassiliadis E, Veidal SS, Kristiansen MN, Hansen C, Jorgensen M, Leeming DJ, Karsdal M. (2013). Peptidyl arginine deiminase inhibitor effect on hepatic fibrogenesis in a CCl4 pre-clinical model of liver fibrosis. Am J Transl Res , 465–469. [PMC free article] [PubMed] [Google Scholar]
- Walker JL, Bleaken BM, Wolff IM, Menko AS. (2015). Establishment of a clinically relevant ex vivo mock cataract surgery model for investigating epithelial wound repair in a native microenvironment. J Vis Exp 2015, e52886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JL, Menko AS. (1999). alpha6 Integrin is regulated with lens cell differentiation by linkage to the cytoskeleton and isoform switching. Dev Biol , 497–511. [DOI] [PubMed] [Google Scholar]
- Walker JL, Wolff IM, Zhang L, Menko AS. (2007). Activation of SRC kinases signals induction of posterior capsule opacification. Invest Ophthalmol Vis Sci , 2214–2223. [DOI] [PubMed] [Google Scholar]
- Walker JL, Zhai N, Zhang L, Bleaken BM, Wolff I, Gerhart J, George-Weinstein M, Menko AS. (2010). Unique precursors for the mesenchymal cells involved in injury response and fibrosis. Proc Natl Acad Sci USA , 13730–13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells RG. (2013). Tissue mechanics and fibrosis. Biochim Biophys Acta , 884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong VW, Longaker MT, Gurtner GC. (2012). Soft tissue mechanotransduction in wound healing and fibrosis. Semin Cell Dev Biol , 981–986. [DOI] [PubMed] [Google Scholar]
- Wormstone IM, Eldred JA. (2016). Experimental models for posterior capsule opacification research. Exp Eye Res , 2–12. [DOI] [PubMed] [Google Scholar]
- Wormstone IM, Wang L, Liu CS. (2009). Posterior capsule opacification. Exp Eye Res , 257–269. [DOI] [PubMed] [Google Scholar]
- Xu B, deWaal RM, Mor-Vaknin N, Hibbard C, Markovitz DM, Kahn ML. (2004). The endothelial cell-specific antibody PAL-E identifies a secreted form of vimentin in the blood vasculature. Mol Cell Biol , 9198–9206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YT, Chien SC, Chen IY, Lai CT, Tsay YG, Chang SC, Chang MF. (2016). Surface vimentin is critical for the cell entry of SARS-CoV. J Biomed Sci , 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, He L, Huang SH. (2006). Identification of a surface protein on human brain microvascular endothelial cells as vimentin interacting with Escherichia coli invasion protein IbeA. Biochem Biophys Res Commun , 625–630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.