Abstract
Purpose
This survey investigated patients’ and nurses’ preferences among four different autoinjectors used for subcutaneous delivery of medication for rheumatoid arthritis (RA).
Methods
In a multinational survey in five countries, 200 patients with RA and 100 nurses training patients on the use of autoinjectors participated in face-to-face interviews. Respondents were asked to rate the importance of eleven autoinjector attributes and to compare the autoinjectors for etanercept (Enbrel®, MyClic® autoinjector), adalimumab (Humira®, Humira pen), and an etanercept biosimilar (Benepali®, Molly® autoinjector) with a demonstration autoinjector for a new etanercept biosimilar – Erelzi® (SensoReady® autoinjector).
Results
Easy grip and ease of performing self-injection were the most important attributes identified by both groups. Overall, 79% of the patients rated the SensoReady autoinjector easier to use than their currently used injection device (86% of MyClic users, 84% of Humira pen users, and 63% of Molly users). In the patient survey, the SensoReady performed better than the other autoinjectors on the attributes visual feedback after completion of injection, easy to grip, and convenient shape. Nurses also rated the SensoReady easier to use than the MyClic (95%), Humira pen (97%), or Molly (91%). When asked which autoinjector they would recommend to a patient with RA who had not used an autoinjector before, 81% of patients and 90% of nurses selected the SensoReady.
Conclusion
Both patients and nurses perceived the SensoReady to be easier to use compared with other available injection devices. The main reasons for this preference were the buttonless injection, 360° viewing window for feedback (visual confirmation of dose injection), and convenient triangular shape making the injection device easy to grip. Patients and nurses were most likely to recommend the SensoReady autoinjector over other autoinjectors to patients with RA.
Keywords: SensoReady®, Erelzi® pen, easy grip, convenience, autoinjection-device test, etanercept biosimilar
Plain-language summary
This multinational survey was performed to help understand which attributes of autoinjectors used by patients suffering from rheumatoid arthritis (RA) are important, how patient satisfaction differs regarding their currently used injection device, and how current users rate a new autoinjector designed to meet the needs of patients with moderate–severe RA (the SensoReady® autoinjector available for Erelzi®, a new etanercept biosimilar). To confirm the results based on patient ratings, nurses training patients with RA on the use of autoinjectors were also included in the study. A total of 200 patients and 100 nurses were interviewed in five European countries. The results showed a clear preference for the SensoReady autoinjector over currently available autoinjectors regarding both rating of which injection device was easier to use (SensoReady pen vs autoinjectors available for Benepali®, Enbrel®, or Humira®) and which injection device the respondents would recommend for patients with moderate–severe RA. The main reasons for this preference were the buttonless injection, 360° viewing window for feedback (visual confirmation of dose injection), and convenient triangular shape making the new autoinjector easy to grip.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disorder in which the body’s immune system attacks the joints and creates inflammation of the synovium, resulting in swelling and pain in and around the joints. Globally, RA affects about 24.5 million people,1 with prevalence of 0.5%–1% and incidence of between five and 50 per 100,000 people developing the condition each year.2 Common treatments of RA include conventional disease-modifying antirheumatic drugs (cDMARDs), eg, methotrexate. cDMARDs aim for symptom improvement, reduction of functional disability, inflammation decrease, and improvement in overall quality of life.3,4 When patients with RA do not respond to cDMARDs, biological DMARDs (bDMARDs) or synthetic DMARDs that directly target components of the inflammatory cascade are increasingly being used.5,6 Currently, several bDMARDs are available, and those most often used are the subcutaneously administered TNF inhibitors etanercept and adalimumab.
Among patients with inadequate or no response to cDMARDs, bDMARDs have shown proven efficacy in reducing RA symptoms, slowing disease progression, and improving physical functions.3,6–8 In spite of these benefits, treatment costs limit broader use, particularly in lower-income European countries.9 The restricted access of patients to bDMARDs has improved after the introduction of biosimilars that are designed to match the reference medicine in terms of efficacy and safety.10 By 2017, two etanercept biosimilars had been approved and launched in Europe: Benepali® (manufactured by Samsung Bioepis, marketed by Biogen) and Erelzi® (manufactured and marketed by Sandoz).11 Introduction of biosimilars of adalimumab to the European market is expected in 2018.12
Different injection methods are available for bDMARDs that are administered subcutaneously: autoinjectors, pre-filled syringes, and vials with syringes. A number of studies have shown patient preference for autoinjectors (pens) over prefilled syringes or vials with syringes: autoinjectors were rated more convenient, easier to use, less painful, and less time-consuming to administer.13–15 Available autoinjectors for bDMARDs differ in several features, such as the length and diameter of the pen, pen shape, size of the window indicating that the full dose has been administered, and activation mechanism for the injection with or without pushing a button. As bDMARDs are frequently self-administered by patients (eg, in the case of etanercept once weekly), these features of the autoinjector are an important factor in patient satisfaction, and inconvenient administration may have an impact on treatment adherence.16,17
The results of two studies with patients with RA and nurses training patients on autoinjectors showed a preference for the etanercept (Benepali) autoinjector (Molly®; manufactured by Samsung Bioepis, Incheon, Republic of Korea marketed in Europe by Biogen, Zug, Switzerland) compared with the autoinjector of the reference etanercept (Enbrel®) MyClic® (Pfizer, New York, NY, USA).18,19 Important parameters for overall preference were “easy to operate the self-injection”, “buttonless autoinjector”, and “easy to grip”. A further study, where participants could perform injections into a pad simulating the skin, confirmed the preference for Molly over MyClic for both patients and nurses.20
The second etanercept biosimilar, Erelzi, has recently become available in Europe. The accompanying triangular, buttonless SensoReady (Sandoz, Holzkirchen, Germany) autoinjector is highly fitting for patients with RA who often have compromised dexterity. Head-to-head comparison studies are needed to determine whether this new model of autoinjector is associated with a higher level of patient satisfaction and/or is preferred by patients over other available injection devices. To this end, a survey was conducted among patients and nurses who perform autoinjector training.
The survey had four major objectives: to assess patients’ rating of importance of eleven autoinjector attributes; to confirm that patients who regularly use Molly are more satisfied with their injection device compared to patients who regularly use MyClic; to determine the extent of patient preference for SensoReady, the new etanercept autoinjector used for the administration of Erelzi, compared with other currently available autoinjectors of etanercept (Molly and MyClic) and adalimumab (Humira pen); and to confirm findings on patient satisfaction and preference through a separate survey for nurses who provide autoinjector training to patients with RA.
Methods
A patient survey and nurse survey were conducted for this study. All participants provided written informed consent. The patient survey included adult patients with moderate–severe RA currently using etanercept (Enbrel), biosimilar etanercept (Benepali), or adalimumab (Humira). For each bDMARD within each country, patients were recruited to meet the following quotas: sex (60% female, 40% male), level of education (≥33% primary or secondary school, ≥33% high school or university as highest level), and length of use of etanercept or adalimumab to investigate the possible effects of usage duration on satisfaction or preference (33% having used the autoinjector for 1–12 months, 67% having used it for >12 months). The patient survey was conducted by trained interviewers through 20-minute face-to-face interviews using a structured questionnaire.
The nurse survey included nurses who instruct patients with moderate–severe RA on the use of autoinjectors. Nurses were recruited if they had experience instructing patients on the use of etanercept autoinjectors (both MyClic and Molly) and the adalimumab autoinjector (Humira pen). The nurse survey was conducted by trained interviewers via 45-minute face-to-face interviews using a structured questionnaire.
The questionnaire for each survey was designed to assess the importance of autoinjector features, perceptions and satisfaction with the currently used autoinjectors, and the level of preference for SensoReady compared with the currently available autoinjectors for etanercept and adalimumab. Patients were asked to compare a demonstration autoinjector of SensoReady with their currently used auto-injector (MyClic, Molly, or Humira Pen), while nurses were asked to compare it with all three available autoinjectors.
Because this study involved a nonfunctioning demonstration autoinjector that did not pose any risk of harm, it did not require prior ethics approval. The study was designed and performed by Kantar Health in compliance with ISO 20252:2012, the international standard for market, opinion, and social research. As a member of several national and international market-research associations, including the European Pharmaceutical Marketing Research Association, European Society for Opinion and Market Research, British Healthcare Business Intelligence Association, and Arbeitskreis Deutscher Markt und Sozialforschungsinstitute EV, Kantar Health strictly adheres to the latest industry codes of conduct and guidelines in market research, including adverse-event reporting.
Questionnaires
Respondents were asked a series of questions, and all responses were documented. Each respondent was shown a demonstration SensoReady autoinjector and a handling leaflet. The demonstration materials did not contain a needle or fluid ingredients and were not labeled with any brand name. Although the demonstration autoinjector did not contain fluid, visual feedback was provided by a green indicator in the observation window, showing the progress of injection. After the trained interviewer had demonstrated how to use the autoinjector, respondents simulated a full injection process with it. In order to avoid bias, interviewers did not accompany the demonstration with any comments that might have directed attention to possible benefits of the autoinjector.
Five pilot interviews (three with patients, two with nurses) were conducted in Munich, Germany in an interviewing facility to verify that all questions were easily understood. Afterward, face-to-face interviews were conducted between June 2017 and October 2017 in five European countries (France, Germany, Italy, Spain, and the UK) in three to four cities per country.
The questionnaires contained four sections:
experience with injection devices: for patients, duration of current treatment and frequency of injections; for nurses, number of patients trained on each of the three available autoinjectors in the last 3 months and materials used for the training;
overall satisfaction and attribute satisfaction: participants were asked to rate their overall satisfaction with the currently used autoinjector (for nurses, all three autoinjectors) on a standard 5-point verbal scale (excellent, very good, good, average, poor), followed by open-ended questions on advantages and disadvantages of that autoinjector; participants were also asked to rate the importance of eleven prompted attributes of autoinjectors on a 10-point Likert scale (1, not important at all; 10, extremely important), and assessed the autoinjector they were currently using (MyClic, Molly, or Humira pen), while nurses did the assessment for all three autoinjectors;
new autoinjector: the SensoReady autoinjector was presented and assessed through open-ended questions on advantages and disadvantages and then rated on the same 5-point overall satisfaction scale, as well as for each of the eleven attributes;
autoinjector preference: patients were asked to place their current autoinjector next to the SensoReady pen and compare them on each of the attributes (whether the new autoinjector was better, the same, or worse than their current autoinjector). They were also asked which of the two autoinjectors they would recommend to a person with RA who had never used an autoinjector before, and which of the two autoinjectors would be easier to use themselves. Nurses were asked to rate how likely they were to recommend the SensoReady autoinjector for patients with RA if it were available for the patient’s current medication (5-point scale: definitely, probably, might or might not, probably not, definitely not). Nurses were also asked to rank the four injection devices from 1 to 4 (most, second-most, third-most, least likely to recommend) with reasons why. In a final paired-comparison task, the nurses compared the SensoReady autoinjector with each of the three currently used autoinjectors separately and selected the injection device that was easiest to use for the majority of patients with moderate–severe RA.
Statistical analysis
The analysis included all patients and nurses who met the screening criteria and completed the survey. Data analysis was performed by Kantar Health. Descriptive statistics were used to summarize responses to individual questions. Subgroup comparisons (eg, differences between patient subgroups and differences between patients and nurses) were analyzed with χ2-tests for nominal data or t-tests for two independent (or dependent) samples to compare means of a normally distributed, interval-dependent variable for two independent (or dependent) groups. Binomial tests were performed for questions with binary-answer categories. Correlations between importance ratings of autoinjector features were analyzed with Pearson correlation coefficients.
Results
Study participants
A total of 200 patients participated in the survey (France 36, Germany 45, Italy 45, Spain 27, UK 47). Patients were recruited from different sources, including existing panels (37%), physicians (34%), nurses (10%), patient associations (12%), and social patient networks (6%). Age, sex, and education level were balanced across the three patient subgroups (users of MyClic 70, users of Molly 54, users of Humira pen 76; Table 1). Due to the longer availability of Enbrel (since June 2000) and Humira (since September 2003), the average usage duration of these injection devices was significantly higher than for Molly (first European market entry of Benepali was in April 2016).11 As in the previous study conducted in 2016 with patients receiving etanercept,19 it was more difficult to identify etanercept-autoinjector users in Spain; therefore, significantly fewer etanercept users were recruited from Spain.
Table 1.
Demographic characteristics of patient sample (adults with moderate–severe RA) with breakdown by currently used autoinjector (n=200)
| Currently used autoinjector
|
||||
|---|---|---|---|---|
| Molly® for etanercept (Benepali), n=54 | MyClic® for etanercept (Enbrel), n=70 | Humira® pen for adalimumab (Humira), n=76 | ||
| Sex | ||||
| Female | 64% | 61% | 64% | 66% |
| Male | 36% | 39% | 36% | 34% |
| Age | ||||
| Average | 52 years | 55 years | 51 years | 50 years |
| ≤30 years | 2% | 0 | 1% | 4% |
| 31–40 years | 12% | 6% | 17% | 11% |
| 41–50 years | 29% | 24% | 29% | 32% |
| 51–60 years | 36% | 41% | 33% | 34% |
| 61–69 years | 23% | 30% | 20% | 20% |
| Highest level of education | ||||
| Primary school | 2% | 2% | 0 | 3% |
| Secondary school | 29% | 31% | 27% | 28% |
| High school | 36% | 39% | 33% | 36% |
| University | 35% | 28% | 40% | 34% |
| Duration of use | ||||
| Average | 27 months | 7 months | 32 monthsa | 37 monthsa |
| ≤12 months | 47% | 89%b,c | 34% | 29% |
| 13–36 months | 34% | 11% | 47%a | 38%a |
| >36 months | 19% | 0 | 19%a | 33%a |
| Country | ||||
| France | 18% | 11% | 21% | 20% |
| Germany | 23% | 28% | 21% | 20% |
| Italy | 23% | 28% | 21% | 20% |
| Spain | 14% | 6% | 11% | 21%a,b |
| UK | 24% | 28% | 24% | 20% |
Notes:
Significantly higher than Benepali (P<0.01);
significantly higher than Enbrel (P<0.01);
significantly higher than Humira (P<0.01). Two-tailed significance tests.
Abbreviation: RA, rheumatoid arthritis.
A total of 100 nurses (20 in each country) were recruited from existing panels and by contacting hospitals and private practices. The average working experience of instructing patients with RA on the use of autoinjectors was 9 years. In the 3 months prior to study entry, the average patient numbers that the nurses had trained on autoinjector use was eleven for MyClic, five for Molly, and eleven for the Humira pen. Additionally, 88% of the nurses performed injections with autoinjectors, eg, during instruction on using the autoinjector or on patients who were not able to perform the injection themselves.
Importance of autoinjector attributes
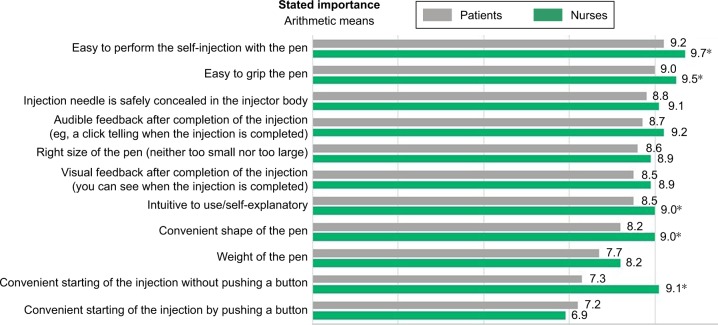
Of the eleven attributes of autoinjectors, patients rated “Easy to perform the self-injection with the pen” as the most important attribute (average 9.2 on the 10-point scale), followed by “Easy to grip” (9.0), and “Injection needle is safely concealed in the body” (8.8). “Weight of the pen” and “Starting the injection without pushing a button” were rated as least important (Figure 1). Two attributes significantly differed on their importance in subgroups of patients using different autoinjectors. Users of Molly rated the attributes “Convenient starting of the injection without pushing a button” and “Weight of the pen” as significantly more important than users of MyClic and the Humira pen (P<0.01 for both attributes; data not shown).
Figure 1.
Patient and nurse ratings of importance of attributes of an autoinjector.
Notes: *Nurse rating was significantly higher than that of patients (P<0.01). Arithmetic means on a scale of 1–10 (1, not important at all; 10, extremely important).
The two most important attributes based on nurses’ ratings were the same as for the patients: “Easy to perform the self-injection with the pen” (average 9.7) and “Easy to grip the pen” (9.5); however, five attributes were rated as significantly more important by the nurses than by the patients, eg, “Starting the injection without pushing a button” and “Shape of the pen” (P<0.01 for each comparison; Figure 1). Notably, nurses rated the feature “Convenient starting of the injection without pushing a button” to be significantly more important than “Convenient starting of the injection by pushing a button” (9.1 vs 6.9; P<0.01).
As the attribute “Easy to perform the self-injection” may be interpreted in various ways, the correlation of this attribute with other more descriptive attributes was calculated (Table 2). For patients, “Easy to perform the injection” closely correlated with “Easy to grip the pen” (r=0.54, P<0.01), “Intuitive use” (r=0.46, P<0.01), and “Visual feedback after completion of the injection” (r=0.42, P<0.01). For nurses, the correlation between “Easy to perform the injection” and “Easy to grip” was lower (r=0.25, P<0.05), and the highest correlation was observed between “Easy to perform the injection” and “Intuitive to use/self-explanatory” (r=0.42, P<0.01). Furthermore, “Easy to perform the self-injection” showed only a weak and nonsignificant correlation with “Visual feedback after completion of the injection” for nurses (r=0.18) compared with patients.
Table 2.
Correlations between importance of ease of performance of self-injection with pen and importance of other autoinjector features
| Patients n=200 | Nurses n=100 | |
|---|---|---|
|
| ||
| Pearson’s r | ||
| Easy to grip the pen | 0.54a | 0.25b |
| Intuitive to use/self-explanatory | 0.46a | 0.42a |
| Visual feedback after completion of injection (one can see when injection is completed) | 0.42a | 0.18 |
| Right size of the pen (neither too small nor too large) | 0.41a | 0.22b |
| Audible feedback after completion of the injection (eg, a click telling when injection completed) | 0.33a | 0.27a |
| Injection needle safely concealed in injector body | 0.29a | 0.10 |
| Convenient starting of injection without pushing a button | 0.22a | −0.03 |
| Weight of pen | 0.19a | −0.01 |
| Convenient starting of injection by pushing a button | 0.19a | 0.18 |
| Convenient shape of pen | 0.18b | 0.07 |
Notes:
P<0.01 (two-tailed);
P<0.05 (two-tailed).
Satisfaction with currently available autoinjectors
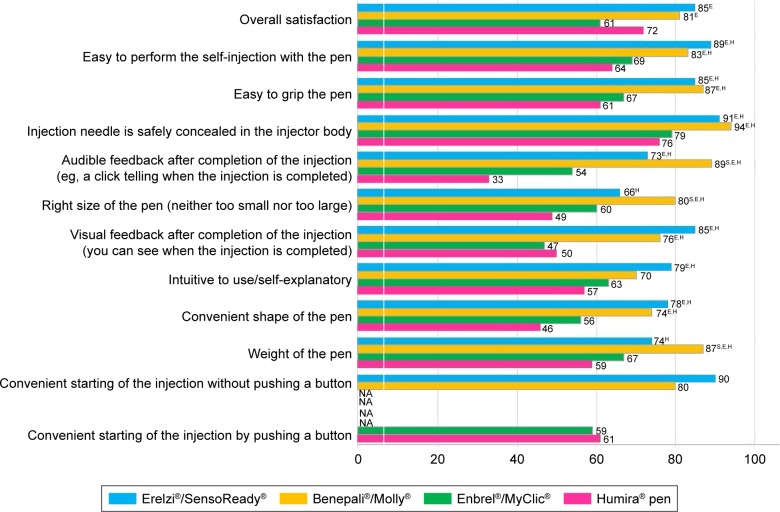
Figure 2 shows the percentage of patients rating the currently available autoinjectors and the new SensoReady pen “excellent” or “very good”. Only one patient rated his autoinjector “poor” (a patient using MyClic), and only two patients using MyClic and two patients using the Humira pen rated their autoinjectors “moderate”. To confirm the superiority of Molly documented in a previous study,19 the hypothesis of whether satisfaction with their current autoinjector was higher among patients using Molly than among patients using MyClic was tested. The difference between both user groups was statistically significant for overall satisfaction (Molly users 81%, MyClic users 61%, P<0.01) and all attributes, except “Intuitive to use”.
Figure 2.
Patient satisfaction with the four autoinjectors.
Notes: Satisfaction shown as percentage of patients rating each injection device excellent or very good (based on a verbal 5-point scale: excellent, very good, good, moderate, poor). Esignificantly higher than MyClic (P<0.01; shape of pen, P<0.05); Hsignificantly higher than Humira pen (P<0.01); Ssignificantly higher than SensoReady (P<0.05). Molly and SensoReady compared with MyClic and Humira pen using one-tailed significance tests. Other comparisons performed using two-tailed tests.
Abbreviation: NA, not applicable.
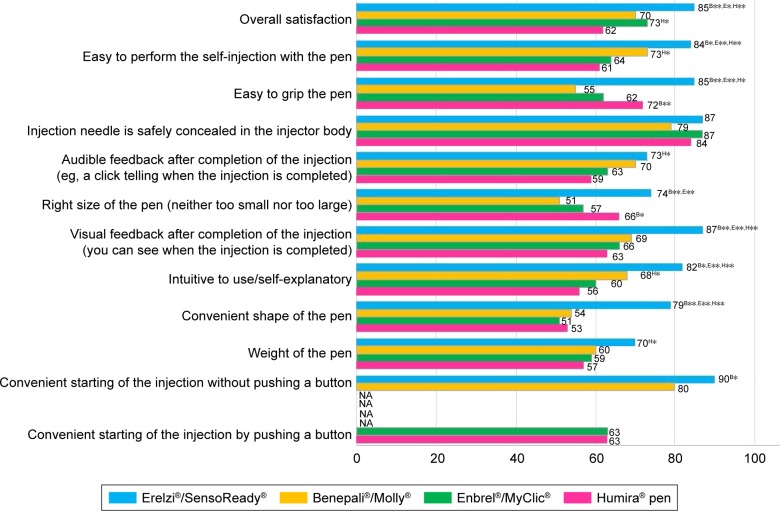
The nurses’ preference for Molly compared with other injection devices observed in the previous study17 was not confirmed by our results (Figure 3). Except “Convenient starting of the injection without pushing a button”, which applied only to the Molly injection device, this autoinjector did not perform better than MyClic on any other attribute. Furthermore, Molly performed worse than MyClic and the Humira pen on the attributes “Easy to grip the pen” and “Right size of the pen”.
Figure 3.
Nurse satisfaction with the four autoinjectors.
Notes: Satisfaction shown as percentage of nurses rating each injection device excellent or very good (based on a verbal 5-point scale: excellent, very good, good, moderate, poor). B*Significantly higher than Molly (P<0.05); B**significantly higher than Molly (P<0.01); E*significantly higher than MyClic (P<0.05); E**significantly higher than MyClic (P<0.01); H*significantly higher than Humira pen (P<0.05); H**significantly higher than Humira pen (P<0.01). Molly and SensoReady compared with MyClic and Humira pen using one-tailed significance tests. Other comparisons performed using two-tailed tests.
Abbreviation: NA, not applicable.
Compared with patients, nurses rated Molly significantly worse on the attributes “Easy to grip the pen” (nurses, 55% excellent/very good; patients, 87% excellent/very good), “Right size of the pen/neither too small nor too large” (nurses, 51%; patients, 80%), “weight of the pen” (nurses, 60%; patients, 87%) and “audible feedback after completion the injection” (nurses, 70%; patients, 89%; P<0.01 for all).
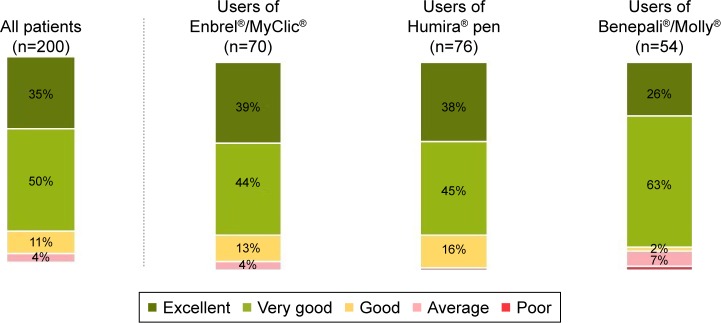
Rating of SensoReady autoinjector
Figure 4 shows the overall rating of SensoReady on the 5-point satisfaction scale. Across all patients (n=200), 85% rated the new injection device presented during the interview as “excellent” or “very good”. No significant differences across the three patient subgroups were observed. Compared with other injection devices, SensoReady was perceived to be significantly better than MyClic and the Humira pen on all attributes, except “Right size of the pen” and “Weight of the pen” (better than Humira pen only; Figure 2). SensoReady was rated similar to Molly for all attributes, except “Audible feedback after completion of the injection”, “Right size of the pen”, and “Weight of the pen”, where Molly performed better. In the nurses’ ratings, SensoReady performed significantly better than all other autoinjectors on five attributes: “Convenient shape of the pen”, “Visual feedback after completing the injection”, “Intuitive use/self-explanatory”, “Easy to grip”, and “Easy to perform the self-injection with the pen” (Figure 3).
Figure 4.
Overall rating of SensoReady after presentation and simulation of use of injection device.
Direct comparison of SensoReady autoinjector with other injection devices
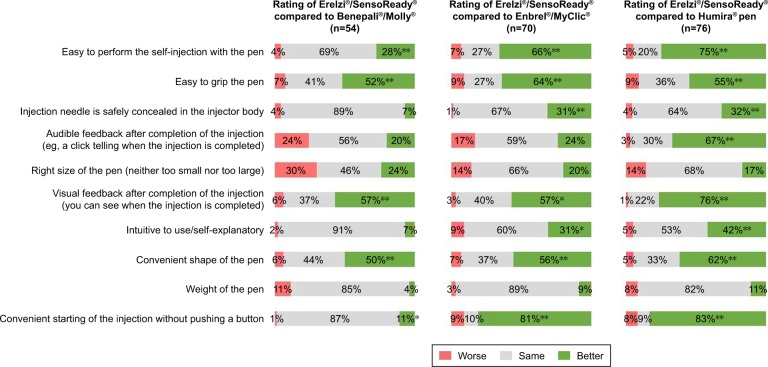
The results of patients comparing SensoReady with their currently used autoinjector are displayed in Figure 5. The strongest differentiating attribute of SensoReady was “Convenient starting of the injection without pushing a button”. SensoReady was rated better than MyClic or the Humira pen by 81% and 83% of patients, respectively.
Figure 5.
Direct comparison of SensoReady with the autoinjector currently used by the patients.
Notes: *SensoReady selected significantly more often than the comparator (P<0.05; one-tailed binomial tests); **SensoReady selected significantly more often than the comparator (P<0.001).
Compared with the other three autoinjectors, Senso-Ready received a higher rating on the following attributes: “Easy to perform the self-injection”, “Visual feedback after completion of the injection”, “Easy to grip”, and “Convenient shape”. SensoReady was rated to be the same as the other three autoinjectors by >50% of patients for the attributes “Weight”, “Injection needle is safely concealed in the injector body”, and “Intuitive use”.
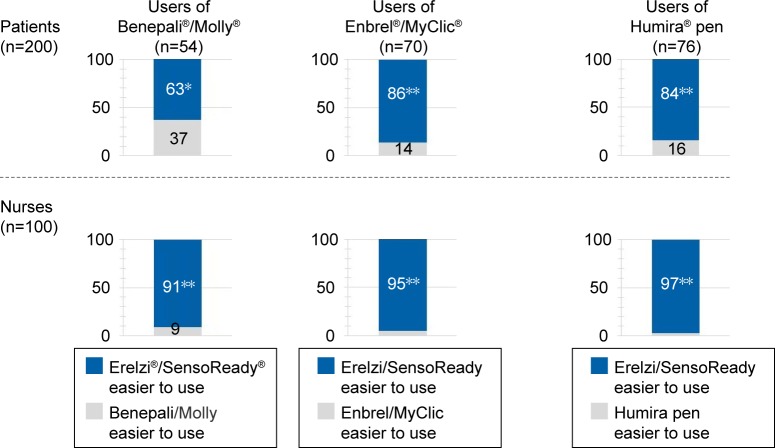
When patients were asked in a forced-choice task to select the injection device that was easier to use, SensoReady was selected by 63% of patients using Molly, 86% using Enbrel/MyClic, and 84% using the Humira pen (Figure 6; P<0.05 for all). The most frequently mentioned reasons for preferring SensoReady were “No button to start the injection” (33%), “Good visual feedback/large window to see that the injection is completed” (23%), “Easy to grip” (19%), and “Triangular shape” (18%).
Figure 6.
Forced-choice selection of autoinjector perceived to be easier to use.
Notes: Pairwise comparisons between SensoReady autoinjector and autoinjectors currently used by patients and nurses. *SensoReady selected significantly more often than comparator (P<0.05; one-tailed binomial tests); **SensoReady selected significantly more often than comparator (P<0.001).
The superiority of SensoReady was confirmed by nurses (Figure 6). In the pairwise forced-choice task, SensoReady was selected as easier to use for the majority of patients with moderate–severe RA than Molly, MyClic, or the Humira pen by 91%, 95%, and 97% of nurses, respectively. The most frequently mentioned reasons for selecting SensoReady were “Triangular shape” (41%), “No button to start the injection” (32%), “Good visual feedback/large window to see that the injection is completed” (29%), and “Easy to grip” (21%).
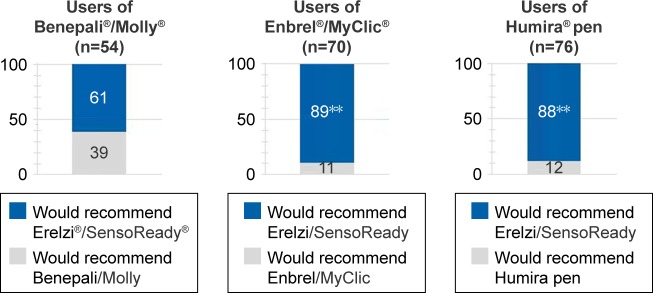
Recommendation of autoinjectors to patients with RA
When asked which autoinjector they would recommend to a patient with RA who had not used an autoinjector before, 81% of all patients selected SensoReady (61% of patients using Molly, 89% using MyClic, and 88% =using the Humira pen; Figure 7, P<0.001 for all). Similarly, 90% of nurses selected SensoReady as the injection device they would most likely recommend to patients with RA. The main reasons for preferring SensoReady were “Easy/easier to use” (69%), “Triangular shape/easy to grip” (41%), and “Larger window/better visual feedback” (32%). Molly was ranked first by 4% of the nurses (rank 2, 60%; rank 3, 14%; rank 4/least likely, 22%). MyClic was ranked first by 4% of the nurses (rank 2, 16%; rank 3, 50%; rank 4, 31%). The Humira pen was ranked first by 3% of the nurses (rank 2, 18%; rank 3, 34%; rank 4, 45%).
Figure 7.
Forced-choice selection of autoinjector to recommend to other patients.
Notes: Pairwise comparisons between SensoReady and autoinjectors currently used by patients. **SensoReady selected significantly more often than comparator (P<0.001).
Data availability
Data sets generated during the current study are available from the corresponding author on reasonable request.
Discussion
In addition to proven efficacy and safety, convenient and safe delivery of self-administered medication is a general consideration for the treatment of patients with RA. This multinational survey analyzed patient and nurse preference for the four currently available autoinjectors. The results confirmed the findings of previous studies18,19 that ease of performing self-injection, a safely concealed needle preventing accidental injuries, and audible and visual feedback indicating that the dose has been completely injected belong to the most important features of an autoinjector (Figure 1). Further important attributes were easy grip, size, and convenient shape. These attributes are considered very important, because of the compromised dexterity associated with RA, where restricted ability to grip the injection device may have a negative impact on the ease of performing the self-injection. The possibility of starting the injection without pushing a button is also relevant for patients with RA. Patients using a buttonless autoinjector rated this attribute more highly than patients using an auto-injector with a necessity to push a button, suggesting that preference for this feature is experience-dependent.
“Easy to perform the self-injection” and “Easy to grip the pen” were the two most important features for patients and nurses; however, features like shape, easy grip, “Starting the injection without pushing a button”, “Easy to perform” and “Intuitive use” were more important for nurses than for patients. These differences may reflect differences in perspective between nurses and patients. While assessing an injection device, nurses are more likely to refer to the range of dexterity that they encounter in the clinic. Interestingly, patients’ importance ratings showed a close correlation between “Easy to perform the self-injection”, “Easy to grip the pen”, and “Visual feedback after completion of the injection”, whereas in the nurses’ assessment “Easy to perform the self-injection” correlated only weakly with these attributes. These results may indicate that patients have a more concrete and sensorimotoric understanding of “Easy to perform the self-injection” than nurses.
The second objective of this survey was to confirm the previous findings of higher patient satisfaction with Molly compared with MyClic in patients with RA.18–20 Our results confirmed that patients using Molly showed higher satisfaction with their injection device than patients using MyClic, both overall and by individual attributes. Interestingly, the higher satisfaction with Molly was not confirmed for nurses, who rated Molly lower than MyClic or the Humira pen on the size and ease of grip. The difference between patients’ and nurses’ preference may stem from Molly being smaller than the other autoinjectors. It is conceivable that nurses may consider a smaller autoinjector less suitable for the whole patient population, whereas individual patient preferences may vary to a stronger degree. In the sample of this study, the patients using Molly may have represented a subgroup of the patient population that had a specific preference for a smaller autoinjector.
Time of use of injection device may contribute to the level of patient satisfaction, eg, Molly was characterized by a high level of patient satisfaction and a low average time of use due to its rather recent availability in market (7 months, with 89% of patients using it between 1 and 12 months; Table 1). However, when patient satisfaction was compared in subsets of patients who had used their autoinjector for 1–12 months (46 patients using MyClic or Humira pen) or longer (100 patients using MyClic or Humira pen), no significant differences were found in overall satisfaction or satisfaction with individual attributes.
The third objective of this study was to establish the level of preference for the SensoReady autoinjector over other available autoinjectors. It was hypothesized that SensoReady would be preferred over other autoinjectors because of a more convenient design (Table 3), characterized by the following features:
triangular with rounded angles, making the injection device easier to grip;
larger window, providing better visual feedback from all sides when the injection is completed;
larger size and diameter compared with Molly, implying that SensoReady would fit better into the fist of patients with RA who may have compromised dexterity.
Table 3.
Features of autoinjectors investigated in this survey
| SensoReady® for etanercept (Erelzi®) | Molly® for etanercept (Benepali®) | MyClic® for etanercept (Enbrel®) | Humira® pen for adalimumab | |
|---|---|---|---|---|
| Length | 166 mm | 142 mm | 158 mm | 187 mm |
| Diameter | 20 mm | 17 mm | 18 mm | 20 mm |
| Weight (empty without active ingredient) | 42 g | 26 g | 27 g | 34 g |
| Shape | Triangular with rounded angles | Round | Round | Round |
| Caps to be removed before injection | One (at needle) | One (at needle) | One (at needle) | Two (one at needle + one at release button) |
| Starting the injection | Without button | Without button | With button | With button |
| First click indicating start of injection | ✓ | ✓ | ✓ | ✓ |
| Second click indicating end of injection | ✓ | ✓ | ✓ | No |
| Size of window for visual feedback (indicator that full dose had been injected) |
One window (360°) 32×20 mm |
Two windows 32×5 mm |
Two windows 32×5 mm |
Two windows 26×5 mm |
As shown in Figure 6, both patients and nurses showed a significant preference for SensoReady in a forced-choice selection. The preference for the SensoReady was confirmed internally when the majority of respondents selected it as the injection device they would most likely recommend to patients with RA.
The respondents often mentioned in an open-ended question the distinguishing design features of the SensoReady auto-injector as reasons for preferring this autoinjector. The specific features were buttonless release of the injection (compared with MyClic and Humira pen), better visual feedback due to a larger window, ease of grip, and convenient triangular shape.
A paired comparison showed that SensoReady was selected over other autoinjectors based on “Visual feedback after completion of the injection”, easy grip, and convenient shape (Figure 5). This preference was in accordance with the rating of importance of various autoinjector attributes, and thus easy grip was rated the second-most important attribute by both patients and nurses.
SensoReady and Molly received similar ratings for the buttonless start, and more than 80% of users of MyClic and the Humira pen rated the buttonless injection start with SensoReady higher than starting the injection by pushing a button with their currently used injection device. These results agree with the results of previous studies, which showed nurses’ and patients’ preference for a buttonless injection device.19,20 Although “visual feedback/larger window” attribute only weakly correlated with “Easy to perform the self-injection” in nurses’ ratings regarding importance (Table 2), this feature of SensoReady provides clear feedback that the injection has been completed and thus may also contribute to overall preference. Interestingly, SensoReady was not rated superiorly to Molly in patient satisfaction rating based on the 5-point scale. This may be because of the high patient satisfaction rating for Molly that was established before SensoReady was presented to respondents (the so-called ceiling effect).
Limitations
One of the limitations of this study was a possible bias of patient preference toward the new autoinjector. To prevent bias, the interviewers demonstrated the new autoinjector without comment that may have emphasized possible benefits, and the questionnaire was designed and phrased in a neutral away, eg, respondents always had the choice of rating the demonstration autoinjector as better, the same, or worse than the currently used autoinjector. Of note, patient preference for Molly over MyClic observed in this study closely mirrors the results of a previous study that showed superior ratings for Molly, which was new at the time.19 Nevertheless, future studies with active users of SensoReady should be conducted to confirm the superior ratings we observed for the demonstration autoinjector of SensoReady vs other autoinjectors.
Another limitation of this study is the possible impact of individual patient needs and autoinjector preferences when patients using different autoinjectors are compared. Future studies on patient preferences for injection devices should investigate patient bias for specific attributes that are present in the patient’s currently used autoinjector. For example, the higher importance of a buttonless injection may be determined by positive prior experience. Conversely, high importance of this attribute may influence the patients’ choice of biosimilar etanercept (Benepali; Molly) vs reference medicine (Enbrel; MyClic), with the assumption that the patient can influence the choice.
Our results indicate that patient preference for various attributes may influence overall patient satisfaction, suggesting that a broad range of autoinjectors should be made available to patients with RA who may show individual preference for specific attributes of autoinjectors and may have their dexterity affected to a varying degree. Based on the findings of this and previous surveys, the preferred injection device would be a buttonless autoinjector with surface, shape, and size providing easy injection, with a large window, and with audible feedback that the injection has been completed. The positive perception of SensoReady with regard to these features explains the preference for this autoinjector expressed by patients and nurses.
Conclusion
The results of this survey conducted in five European countries suggest that nurses and patients with RA consider SensoReady easier to use than Molly, MyClic, and Humira autoinjector. Patients and nurses were most likely to recommend SensoReady over other autoinjectors to patients with RA. Main reasons for this preference were the buttonless injection, the large window offering a 360° view for the visual feedback that the full dose had been injected, and the triangular shape, which makes the autoinjector easy to grip.
Footnotes
Disclosure
Bernd Tischer is an employee of Kantar Health GmbH and consults for Biogen, Pfizer, and AbbVie. Andrea Mehl is an employee of Sandoz International GmbH. The authors report no other conflicts of interest in this work.
References
- 1.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Disease and Injury Incidence and Prevalence, Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D, Mcinnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 3.Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370(9602):1861–1874. doi: 10.1016/S0140-6736(07)60784-3. [DOI] [PubMed] [Google Scholar]
- 4.van der Kooij SM, de Vries-Bouwstra JK, Goekoop-Ruiterman YP, et al. Patient-reported outcomes in a randomized trial comparing four different treatment strategies in recent-onset rheumatoid arthritis. Arthritis Rheum. 2009;61(1):4–12. doi: 10.1002/art.24367. [DOI] [PubMed] [Google Scholar]
- 5.Avci AB, Feist E, Burmester GR. Biologicals in rheumatoid arthritis: current and future. RMD Open. 2015;1(1):e000127. doi: 10.1136/rmdopen-2015-000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtis JR, Singh JA. Use of biologics in rheumatoid arthritis: current and emerging paradigms of care. Clin Ther. 2011;33(6):679–707. doi: 10.1016/j.clinthera.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strand V, Singh JA. Improved health-related quality of life with effective disease-modifying antirheumatic drugs: evidence from randomized controlled trials. Am J Manag Care. 2007;13(Suppl 9):S237–S251. [PubMed] [Google Scholar]
- 8.Singh JA, Christensen R, Wells GA, et al. A network meta-analysis of randomized controlled trials of biologics for rheumatoid arthritis: a Cochrane overview. CMAJ. 2009;181(11):787–796. doi: 10.1503/cmaj.091391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Putrik P, Ramiro S, Kvien TK, et al. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis. 2014;73(1):198–206. doi: 10.1136/annrheumdis-2012-202603. [DOI] [PubMed] [Google Scholar]
- 10.Schiestl M, Zabransky M, Sörgel F. Ten years of biosimilars in Europe: development and evolution of the regulatory pathways. Drug Des Devel Ther. 2017;11:1509–1515. doi: 10.2147/DDDT.S130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Generics and Biosimilars Initiative Biosimilars of etanercept. 2017. [Accessed July 1, 2018]. Available from: http://gabionline.net/Biosimilars/General/Biosimilars-of-etanercept.
- 12.Generics and Biosimilars Initiative Biosimilars of adalimumab. 2018. [Accessed July 1, 2018]. Available from: http://gabionline.net/Biosimilars/General/Biosimilars-of-adalimumab.
- 13.Kivitz A, Cohen S, Dowd JE, et al. Clinical assessment of pain, tolerability, and preference of an autoinjection pen versus a prefilled syringe for patient self-administration of the fully human, monoclonal antibody adalimumab: the TOUCH trial. Clin Ther. 2006;28(10):1619–1629. doi: 10.1016/j.clinthera.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Demary W, Schwenke H, Rockwitz K, et al. Subcutaneously administered methotrexate for rheumatoid arthritis, by prefilled syringes versus prefilled pens: patient preference and comparison of the self-injection experience. Patient Prefer Adherence. 2014;8:1061–1071. doi: 10.2147/PPA.S64111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borrás-Blasco J, Gracia-Pérez A, Rosique-Robles JD, Casterá MD, Abad FJ. Acceptability of switching adalimumab from a prefilled syringe to an autoinjection pen. Expert Opin Biol Ther. 2010;10(3):301–307. doi: 10.1517/14712590903530633. [DOI] [PubMed] [Google Scholar]
- 16.Kivitz A, Segurado OG. Humira pen: a novel autoinjection device for subcutaneous injection of the fully human monoclonal antibody adalimumab. Expert Rev Med Devices. 2007;4(2):109–116. doi: 10.1586/17434440.4.2.109. [DOI] [PubMed] [Google Scholar]
- 17.van den Bemt BJ, Zwikker HE, van den Ende CH. Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert Rev Clin Immunol. 2012;8(4):337–351. doi: 10.1586/eci.12.23. [DOI] [PubMed] [Google Scholar]
- 18.Thakur K, Biberger A, Handrich A, Rezk MF. Perceptions and preferences of two etanercept autoinjectors for rheumatoid arthritis: a new European Union-approved etanercept biosimilar (Benepali) versus etanercept (Enbrel) – findings from a nurse survey in Europe. Rheumatol Ther. 2016;3(1):77–89. doi: 10.1007/s40744-016-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thakur K, Biberger A, Handrich A, Rezk MF. Patient perceptions and preferences of two etanercept autoinjectors for rheumatoid arthritis: findings from a patient survey in Europe. Rheumatol Ther. 2016;3(2):245–256. doi: 10.1007/s40744-016-0048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egeth M, Soosaar J, Nash P, et al. Patient and healthcare professionals preference for Brenzys vs. Enbrel autoinjector for rheumatoid arthritis: a randomized crossover simulated-use study. Adv Ther. 2017;34(5):1157–1172. doi: 10.1007/s12325-017-0523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sets generated during the current study are available from the corresponding author on reasonable request.