1. Introduction
Cancer is a disease of aging, and most cancer diagnoses and deaths worldwide occur in older adults. Although people aged 65 years and older represent less than 10% of the world’s population, they account for 48% of new cancer cases and 58% of cancer-related deaths globally.[1] In the United States, for example, the median age at the time of a cancer diagnosis is 66 years, and more than a quarter of individuals diagnosed with cancer are 75 years of age or older.[2] However, despite the high burden of cancer in older individuals, high-quality evidence regarding the optimal treatment strategies in older adults with cancer is scarce. Older patients are vastly underrepresented in both cooperative group clinical trials and in US Food and Drug Administration (FDA) registration trials,[3] leading to a situation in which most cancer treatments are developed on younger, healthier cohorts that fail to adequately represent real-world patient populations.
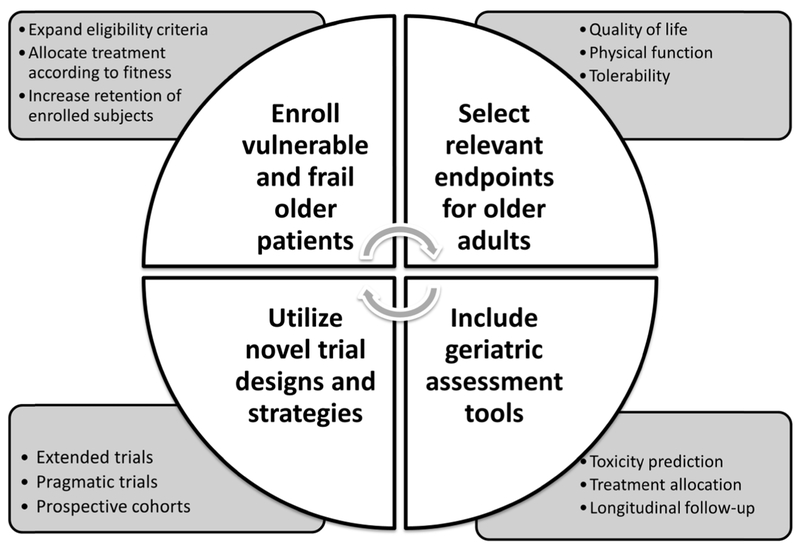
Several organizations, including the American Society of Clinical Oncology (ASCO),[3] the International Society of Geriatric Oncology (SIOG), and the European Organization for Research and Treatment of Cancer (EORTC), [4] have issued recommendations aimed at improving the evidence base for treating older patients with cancer by increasing their enrollment in clinical trials and improving trial design. These recommendations include the utilization of geriatric assessment tools in clinical trials; expanding the eligibility criteria for therapeutic studies; designing trials for vulnerable or frail individuals; selecting end-points which may be of particular relevance for older adults; and utilizing novel trial designs.
2. Including geriatric assessment tools in clinical trials
The geriatric assessment is a clinical evaluation of an older patient’s functional status, physical function, comorbidities, medication use, cognition, nutritional status, social support, and psychological state. Although the comprehensive geriatric assessment used in geriatric medicine may be time-consuming, shorter cancer-specific geriatric assessment tools have been proposed and studied in various tumor types and treatment settings.[5, 6] Some of these geriatric assessment tools can even be completed by self-report in a short period of time, and including them in therapeutic clinical trials has been shown to be feasible.[5, 6] Cancer-specific geriatric assessment tools are designed to provide a high volume of additional information by identifying impairments in patients who are categorized as fit by usual measures of performance status, clarifying patient priorities, detecting longitudinal changes in functional status, and predicting survival. [6] Furthermore, geriatric assessment-based tools, such as the Cancer and Aging Research Group (CARG) chemotherapy toxicity risk score and the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH), are superior to usual measures utilized in oncology for predicting chemotherapy toxicity, and could be used to stratify patients in therapeutic studies into different risk categories and treatment strategies.[6, 7, 8] An example of a randomized clinical trial (RCT) designed specifically for older patients that utilized longitudinal geriatric assessment tools is CALGB 49907, which compared two different types of adjuvant chemotherapy in older women with breast cancer.[9] Secondary analyses from this seminal RCT have made relevant contributions to our understanding of the trajectory of quality of life and cognition after chemotherapy, and have helped identify some of the long-term deleterious effects of cytotoxic treatment in older patients.[10, 11]
3. Expanding the eligibility criteria for clinical trials
Restrictive eligibility criteria in clinical trials limit their generalizability, make accrual difficult, and may lead to implications of clinical safety concerns where none may exist.[12] Some of the design barriers that limit the accrual of older adults into clinical trials include age limits, restrictive organ function criteria, exclusion of patients with comorbidities, and the utilization of generic measures of functional status such as the Karnofsky Performance Status (KPS) or Eastern Oncology Cooperative Group (ECOG) scales. Age limits preclude obtaining information pertaining to a large proportion of real-world cancer patients. Usual organ function criteria, such as serum creatinine, are not a good marker in older adults, and previous studies have shown that older patients with renal insufficiency who receive dose modifications are not at increased risk for complications compared with those with normal renal function who receive standard dosing.[13] Additionally, generic performance status measures such as KPS have been shown to be inferior for predicting treatment tolerance when compared with geriatric assessment-based tools.[6, 7]
4. Designing trials including vulnerable and frail older adults
As a result of strict eligibility criteria, older adults included in clinical trials are usually younger, fitter, with less comorbid conditions, and with better organ function than those seen in everyday clinical practice. A potential solution to this disparity is designing trials with no restrictions regarding functional status at the time of enrollment, in which treatment arms are adjudicated depending on the results of geriatric assessment tools. A notable example of this strategy is the randomized phase III ESOGIA RCT, which compared an age-based treatment allocation strategy with the use of geriatric assessment tools to select treatment options in older patients with advanced lung cancer.[14] Another example is the ongoing phase III ELAN-ONCOVAL study of older adults with head and neck cancer (NCT01884623), in which patients are allocated to various treatment arms depending on whether they are categorized as fit or unfit after undergoing a geriatric assessment. This type of trial design allows for the inclusion of all older patients, regardless of their functional status, and has the potential of answering important questions regarding the ideal therapeutic options for vulnerable or frail individuals.
5. Selecting relevant end points for older patients
Standard end points used in oncology trials, such as response rate (RR), progression-free survival (PFS), or overall survival (OS) may not be the most appropriate for older patients with cancer, for whom prolongation of life may not be the most important goal of treatment.[4] In older patients with life-threatening conditions, other outcomes such as quality of life, maintenance of functional status, and cognition may be as relevant or even more relevant than survival.[4] Thus, incorporating other end points such as the impact of treatment on quality of life and on geriatric-specific outcomes (such as preservation of independence over time) should be a priority in clinical trials including older adults. An example of a phase II trial studying geriatric-specific outcomes is the GERICO multicenter trial in metastatic breast cancer, in which a change in activities of daily living after chemotherapy was the primary end point.[15] Another novel strategy is the use of composite endpoints which take into account a combination of the tolerability and the efficacy of treatment.[4] An example of this is the MRC FOCUS2 RCT, which investigated reduced-dose chemotherapy options in older adults with advanced colorectal cancer.[16] In addition to usual outcomes, this trial included a composite endpoint called overall treatment utility (OTU), which was defined as a combination of clinical or radiological progression, major treatment toxicity and patient acceptability.[14]
6. Utilizing novel trial designs
Although RCT are still the gold standard of clinical trial design, conducting RCT for older adults is a costly and time intensive endeavor that requires stratifying by age groups or by specific geriatric characteristics.[4] Pragmatic clinical trials, which are conducted in the context of standard care and use broader eligibility criteria, represent an alternative trial design which could be used to enroll older and more vulnerable patients. In contrast with RCT, pragmatic trials test the effect of interventions in day-to-day practice settings, and allow for a more heterogeneous population to be included.[17] A recently published example is the GOG273 trial, which included patients age ≥ 70 with epithelial cancer of the ovary, peritoneum or fallopian tube, and allowed patients along with their treating physicians to choose between two regimens (combination therapy or single agent).[18] Other options for trial design in older patients include prospective cohort studies; embedded studies (utilizing geriatric assessment measures within larger parent trials); single arm phase II trials; and extended trials, in which a cohort of older patients is added to the superior treatment arm of a RCT.[3]
7. Expert Opinion
Historically, older adults have been greatly underrepresented in therapeutic clinical trials in oncology, meaning that treatment decisions for the majority of patients with cancer are based on data generated either in a younger population or in the fittest older adults. In order to fill this very relevant gap in knowledge, changes need to be made at every level of trial design (Figure 1). Restrictive eligibility criteria and age limits should be eliminated, and the inclusion of vulnerable and frail older adults must be encouraged. This can be partially achieved by enhancing physician education in order to eradicate unfounded perceptions, and by increasing the provision of resources and personnel needed to recruit and retain older patients in clinical trials.[19] The utilization of geriatric assessment tools can help identify unfit patients at greater risk of adverse events, which can then be allocated to different treatment strategies, such as reduced dosing or supportive care. Furthermore, study endpoints need to be tailored in order to match those outcomes that are most relevant to older patients, such as independence, physical function, cognition, and treatment tolerance. This can be achieved either by specifically designing randomized clinical trials for older patients with different fitness levels or by utilizing other study designs, such as pragmatic trials. Enhancing the evidence base for treating older adults with cancer by “geriatricizing” clinical trial design represents one of the top priorities of future research in oncology.
Figure 1.
Considerations for the design of clinical trials in older adults with cancer.
Acknowledgments
Funding Details: Funded in part through the Memorial Sloan Kettering Cancer Center Support Grant P30 CA008748. E Soto-Perez-de-Celis is supported by a 2016 Long Term International Fellowship from the Conquer Cancer Foundation.
Funding
E. Soto-Perez-de-Celis is supported by a 2016 Long Term International Fellowship of the Conquer Cancer Foundation. E. Lichtman is supported by a Center Support Grant from the National Cancer Institute (P30 CA008748) to Memorial Sloan Kettering Cancer Center.
Footnotes
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Ferlay J SI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancerbase No. 11 [internet]. 2013. [Google Scholar]
- 2.Howlader N NA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER cancer statistics review, 1975-2012. Bethesda, MD: National Cancer Institute [Google Scholar]
- 3.Hurria A, Levit LA, Dale W, et al. Improving the evidence base for treating older adults with cancer: American Society of Clinical Oncology Statement. J Clin Oncol 2015;33:3826–3833. [DOI] [PubMed] [Google Scholar]; •• Recommendations and action items for improving clinical research in older patients with cancer from the American Society of Clinical Oncology.
- 4.Wildiers H, Mauer M, Pallis A, et al. End points and trial design in geriatric oncology research: A joint European Organisation for Research and Treatment of Cancer--Alliance for Clinical Trials in Oncology--International Society of Geriatric Oncology position article. J Clin Oncol 2013;31:3711–3718. [DOI] [PubMed] [Google Scholar]; •• International recommendations for clinical trial designs and endpoints in older adults with cancer, including examples of successfully completed studies.
- 5.Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol 2011;29:1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamaker ME, Wildes Rostoft T. Time to Stop Saying Geriatric Assessment Is Too Time Consuming. J Clin Oncol. 2017. June 19:JCO2017728170. doi: 10.1200/JCO.2017.72.8170. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]; • Recent commentary summarizing the existing evidence regarding the utilization of the geriatric assessment in oncology.
- 7.Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol 2016;34:2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2012; 13: 3377–86. [DOI] [PubMed] [Google Scholar]
- 9.Muss HB, Berry DA, Cirrincione CT, et al. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med 2009;360:2055–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Seminal randomized clinical trial comparing two adjuvant chemotherapy strategies in older adults with breast cancer.
- 10.Kornblith AB, Lan L, Archer L, et al. Quality of life of older patients with early-stage breast cancer receiving adjuvant chemotherapy: A companion study to Cancer and Leukemia Group B 49907. J Clin Oncol 2011;29:1022–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedman RA, Pitcher B, Keating NL, et al. Cognitive function in older women with breast cancer treated with standard chemotherapy and capecitabine on Cancer and Leukemia Group B 49907. Breast Cancer Res Treat 2013;139:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim ES, Bernstein D, Hilsenbeck SG, et al. Modernizing eligibility criteria for molecularly driven trials. J Clin Oncol 2015;33:2815–2820. [DOI] [PubMed] [Google Scholar]
- 13.Lichtman SM, Cirrincione CT, Hurria A, et al. Effect of pretreatment renal function on treatment and clinical outcomes in the adjuvant treatment of older women with breast cancer: Alliance A171201, an ancillary study of CALGB/CTSU 49907. J Clin Oncol 2016;34:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corre R, Greillier L, Le Caer H, et al. Use of a comprehensive geriatric assessment for the management of elderly patients with advanced non-small-cell lung cancer: The phase III randomized ESOGIA-GFPC-GECP 08–02 study. J Clin Oncol 2016;34:1476–1483. [DOI] [PubMed] [Google Scholar]; • The first phase III randomized clinical trial utilizing geriatric assessment characteristics to allocate treatment in older adults with cancer.
- 15.Brain EGC, Mertens C, Girre V, et al. Impact of liposomal doxorubicin-based adjuvant chemotherapy on autonomy in women over 70 with hormone-receptor-negative breast carcinoma: A French Geriatric Oncology Group (GERICO) phase III multicentre trial. Crit Rev Oncol Hematol 2011;80:160–170. [DOI] [PubMed] [Google Scholar]
- 16.Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): An open-label, randomised factorial trial. The Lancet 2011;377:1749–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nipp RD, Yao NA, Lowenstein LM, et al. Pragmatic study designs for older adults with cancer: Report from the U13 Conference. J Geriatr Oncol 2016;7:234–241. [DOI] [PubMed] [Google Scholar]
- 18.von Gruenigen VE, Huang HQ, Beumer JH, et al. Chemotherapy completion in elderly women with ovarian, primary peritoneal or fallopian tube cancer - An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2017;144:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23:3112–3124. [DOI] [PubMed] [Google Scholar]