Abstract
Ubiquitination and ubiquitin-like post-translational modifications (PTMs) regulate activity and stability of oncoproteins and tumor suppressors. This implicates PTMs as antineoplastic targets. One way to alter PTMs is to inhibit activity of deubiquitinases (DUBs) that remove ubiquitin or ubiquitin-like proteins from substrate proteins. Roles of DUBs in carcinogenesis are intensively studied yet, few inhibitors exist. Prior work provides a basis for the ubiquitin-specific protease 18 (USP18) as an antineoplastic target. USP18 is the major DUB that removes interferon stimulated gene 15 (ISG15) from conjugated proteins. Prior work discovered that engineered loss of USP18 increases ISGylation and in contrast to its gain decreases cancer growth by destabilizing growth-regulatory proteins. Loss of USP18 reduced cancer cell growth by triggering apoptosis. Genetic loss of USP18 repressed cancer formation in engineered murine lung cancer models. The translational relevance of USP18 was confirmed by finding its expression was deregulated in malignant versus normal tissues. Notably, the recent elucidation of the USP18 crystal structure offers a framework for developing an inhibitor to this DUB. This review summarizes strong evidence for USP18 as a previously unrecognized pharmacologic target in oncology.
Keywords: USP18, deubiquitinase, ISGylation, interferon signaling, antineoplastic target
Background
Growth regulatory proteins that drive carcinogenesis are altered by post-translational modifications (PTMs) (1). An improved understanding of how PTMs affect oncogenic, tumor suppressive and other critical tumorigenic pathways will uncover ways to counteract the consequences of those same pathways (2). PTMs that affect ubiquitination and related processes are under active study because they are deregulated in diverse cancers. One important outcome of regulated expression of ubiquitin-specific protease 18 (USP18) is to change the stability of key proteins that maintain or mediate the carcinogenesis process (3,4).
Ubiquitination is a tightly controlled process. It is regulated by the ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2) and ubiquitin ligase (E3) that collectively allow ubiquitin to conjugate to target proteins (5). Similarly, interferon-stimulated gene 15 (ISG15), the first ubiquitin-like protein discovered is activated by a three step enzymatic cascade that engages a specific E1-activating enzyme (UBE1L), E2 conjugating enzyme (typically UBCH8) and E3 ligase (commonly HERC5A) to complex ISG15 to protein substrates, as shown in Figure 1A (6). While it is known that poly-ubiquitination triggers protein degradation through the proteasome, mono-ubiquitination alters other cellular functions (7). In contrast, the functional consequences of ISGylation are less well understood. Mono-ISGylation elicits specialized functions including regulating activity of growth-regulatory proteins (6,8,9).
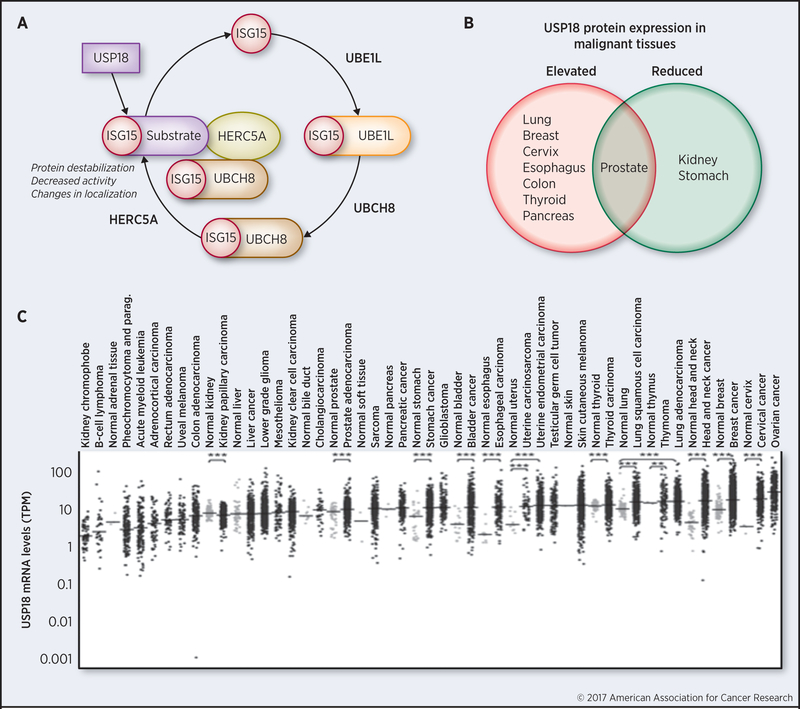
Figure 1.
USP18 is an antineoplastic target.
A. USP18 activity once inhibited will increase ISGylation and decrease stability of critical growth-regulatory proteins that are destabilized by ISGylation. B. USP18 protein levels are elevated in most examined cancers (adapted from reference 13) when compared to histopathologically normal tissues (other than for prostate cancer where variable USP18 expression was detected). Reduced USP18 protein expression profiles were observed in kidney and stomach cancers as compared to corresponding histopathologically normal tissues. C. USP18 mRNA levels are significantly elevated in diverse cancers, as indicated by analysis of The Cancer Genome Atlas (**P < 0.01, ***P < 0.001, two-tailed t tests).
Ubiquitination and ISGylation modifications are removed by deubiquitinases (DUBs), as reviewed (10). Ubiquitin-specific proteases (USPs) are a class of DUBs that remove ubiquitin, ISG15 or other ubiquitin-related species from complexed proteins (10,11). About 100 DUBs are now known to exist and their precise biological roles are being elucidated (11). Individual DUBs likely confer specific actions (10,11). Likewise, if a particular DUB is pharmacologically affected, this should lead to desired outcomes on physiology or pharmacologic responses. Here, we describe the known activities of USP18, emphasizing its role as a candidate antineoplastic target. Recent work established that antagonism of USP18 expression conferred a prominent antitumorigenic response. Repression of USP18 protein reduced stability of critical growth-regulatory species and inhibited cancer cell growth and tumor formation by augmenting apoptotic and chemotherapeutic agent responses (12-15). Taken together, these and other findings described here implicate USP18 as a previously unrecognized antineoplastic target.
USP18 Functions
The DUB USP18 (originally known as UBP43), was first cloned from leukemia fusion protein expressing AML1-ETO mice and then independently identified by different groups using porcine and human models (16-18). ISG15 expression is markedly enhanced by treatment with type I interferon (IFN), all-trans-retinoic acid (RA), lipopolysaccharide or double-stranded RNA, indicating the diverse pharmacologic pathways that could be affected by USP18 inhibition (18-20). ISG15 expression is also elevated in tumor cells exposed to epigenetic agents. This triggers cytosolic sensing of double-stranded RNA, including endogenous retroviruses, which can alter type I IFN response (21,22). Protein sequence and biochemical analyses established that USP18 is a USP family member (16-18). USP18, like other ubiquitin-specific proteases, has DUB activity (16-18). An early role for USP18 in cancer biology came from evidence for its important role in hematopoiesis by modulating ubiquitin-dependent pathways and ubiquitination (see Supplemental Figure 1A), which regulate species that control myeloid cell differentiation (16-18).
Prior work revealed that USP18 predominately acts to remove ISG15 from substrate proteins (18). Initial studies that engineered and characterized USP18-deficient mice found that knockout of USP18 preferentially increased levels of intracellular ISG15-conjugates (18,23). Subsequent in vitro studies found that USP18 hydrolyzed ISG15 carboxyl-terminal extension proteins as compared to other ubiquitin-like proteins (18). This highlighted USP18 as an ISG15-specific protease that removes this PTM from exogenous and endogenous ISG15-complexed proteins (18), as displayed in Supplemental Figure 1B. These studies indicated that the previously reported in vitro deconjugase activity of USP18 for ubiquitin likely arose from the high enzyme to substrate ratios studied; ubiquitin is likely not a physiological substrate of USP18 (18).
It is important to elucidate the functional relationship between USP18 and ISGylation. This is because ISGylation can control stability, activity and even subcellular localization of critical growth-regulators (8,9,14). Recent work is beginning to uncover how ISG15 and USP18 affect the stability of target proteins. There is evidence that ISG15-conjugated proteins exist (such as p53) and are degraded via the 20S proteasome (8). Other work indicates that repression of USP18 changes the localization of target proteins and this in turn can lead to protein destabilization (14). Additional studies are needed to illuminate the functional and mechanistic consequences of ISGylation.
USP18 studies found it was the enzymatic activity of USP18 that conferred its biological effects (16-18). Not long after USP18 was linked to regulating ISG15-conjugation, its catalytically inactive form was shown to play a role in regulating IFN signaling (24,25,26). As an example of this, USP18 was able to regulate JAK-STAT signaling by blocking interactions between the IFNAR2 receptor and JAK1 (24), as depicted in Supplemental Figure 1C. It is notable that repressing USP18 activity, enhanced response to IFN treatment (24). Notably, USP18 modulated dendritic cell development via its inhibitory effect on type I IFN signaling (27). These cells play a critical role in linking innate and adaptive immune responses (27).
USP18 Antineoplastic Activity
USP18 regulates ISGylation and IFN signaling (18,24). Beyond their roles in immunity, these species are also linked to control of tumorigenesis (4,28). Consistent with this view, USP18 mRNA and protein (Figure 1B) are highly expressed in most cancer types examined as compared to corresponding normal tissues (13). At the same time, a decline in USP18 expression is associated with a favorable cancer-specific survival in some contexts (29). Together, these findings indicate that USP18 inhibition can exert beneficial antineoplastic effects.
Ubiquitin is a PTM that covalently binds to specific proteins. This in turn alters the function and stability of complexed proteins. Given this, it is not surprising that ubiquitination is involved in controlling carcinogenesis (3). Several USPs are known to promote tumorigenesis by affecting stabilities of key oncoproteins and tumor suppressors (30,31). As examples, USP5 negatively regulates the tumor suppressor p53, USP28 stabilizes Myc and USP9X promotes β-catenin oncogenic signaling (31). Enhanced USP18 expression occurs in diverse cancers (13), as displayed in Figure 1C. Augmented USP18 expression promotes tumorigenesis by increasing stability of critical ISG15-conjugated proteins that promote carcinogenesis. Examples of this include PML-RARα, cyclin D1 and KRAS proteins (12-14).
The dominant-negative translocation product PML/RARα was identified as a target of ISGylation in acute promyelocytic leukemia (APL) (32). Subsequently, engineered repression of USP18 was shown to destabilize this oncoprotein as well as to reduce proliferation and increase apoptosis in APL and lung cancer cell lines (12,13). In marked contrast, introduction into cancer cells of an enzymatically-inactive USP18 species did not confer the same effects as wild-type USP18 (13). This implied that enzymatically active USP18 conferred antineoplastic effects (13). An inverse relationship was found to exist between the E1 protein UBE1L and expression of the cell cycle regulator cyclin D1 (33). Subsequent investigations determined that engineered loss of USP18 expression in lung cancer cells conferred cyclin D1 protein destabilization (13). As a result, this augmented apoptosis and reduced lung cancer cell line growth in vitro while inhibiting lung cancer formation in syngeneic mice (13). Intriguingly, engineered loss of USP18 expression also enhanced response of lung cancer cells to chemotherapeutic agents (13). This finding has implications for combination cancer therapy with an agent that can antagonize USP18 activity. Of note, engineered loss of USP18 expression reduced growth of lung cancer cell lines that were driven by activated KRAS expression (14).
Prior work found that KRAS and ISGylation share a regulatory feedback loop (34). Other studies established that loss of USP18 expression mislocalized KRAS from the plasma membrane and reduced stability of this oncoprotein due to the consequences of ISGylation (14). It is notable that when mice engineered with loss of USP18 were crossed with engineered mice with lung cancers arising from activated KRAS expression, this caused a statistically significant decline in lung cancer formation in the compound mice having germ line loss of USP18 (14). Together, these observations underscore a critical role for USP18 in controlling carcinogenesis.
USP18 exerts functions that are independent of its ISG15 deconjugase activity. For example, USP18 is a positive regulator of the epidermal growth factor receptor (EGFR) by negatively regulating transcription of microRNA-7 levels that in turn reduces expression of EGFR mRNA in several cancer cell lines (15). Also, in the kidney USP18 is a transcriptional target of WT1 that plays a critical role in Wilms’ tumor biology (35). Lack of USP18 expression promotes a tumor-suppressive microenvironment by upregulating IFN-λ (36). This leads to recruitment of Th1 subtype CD4+ T cells in mammary epithelial cells (36). USP18 deficiency causes resistance to oncogenic transformation by BCR-ABL via type 1 IFN-signaling (37). These observations are consistent with the hypothesis that targeting USP18 for repression elicits substantial antitumor actions. It is known that USP18 is a major regulator of the interferon signaling network that affects inflammatory and apoptotic responses (38,39). USP18 positively regulates the oncogenic NF-kB signaling pathway by disrupting ISGylation of key NF-kB proteins (40). Some of the major USP18 targets are summarized in Supplemental Table 1.
USP18 has diverse functions. For instance, USP18 is important for antitumor immunity and for inhibition of tumorigenesis in melanoma (41). USP18 expression in B16 melanoma tumor cells was shown to reduce tumor cell-mediated inhibition of T cell proliferation and suppress PD-1 expression (41). In human leiomyosarcoma cases, reduced USP18 expression is associated with an unfavorable clinical outcome and mice engineered as null for Usp18 develop these sarcomas (42). It was speculated that the IFN-hypersensitive microenvironment found in USP18 null mice deregulated proliferation of vascular smooth muscle cells, initiating leiomyosarcoma formation (42). Recent work found that PTEN is an ISGylation target that is destabilized after engineered loss of USP18 expression (43). These observations are not unexpected since DUBs exert both oncogenic and tumor suppressor actions (30,31). Yet, in most studied settings the balance between these functions exerts a net antitumor effect once USP18 actions are antagonized. Even so, the precise consequence of USP18 repression must be determined in each cancer context (31).
Different cellular and tumor contexts determine whether a DUB has a net oncogenic or tumor suppressive effect (30,31). DUBs can exert different functional effects based on substrate abundance as well as subcellular localization, cell type and physiological states (30,31). Each cancer type may respond differently to USP18 loss. To elucidate definitively whether oncogenic or tumor-suppressive functions of USP18 predominate will require the development and in depth characterization of USP18 inhibitors. An inhibitor against USP18 is hypothesized as clinically useful in cancers where USP18 primarily functions as an oncoprotein.
Clinical-Translational Advances
Based on recent findings, USP18 is proposed as a previously unrecognized antineoplastic target regulating key proteins in cancer biology including PML/RARα, cyclin D1, KRAS, and PTEN (13,14,43). Although USP18 can regulate many different ISGylated substrates (12-15,41-43) it typically functions as a tumor-promoting enzyme. Antagonizing USP18 activity or reducing its expression inhibits actions of critical oncoproteins by destabilizing their respective protein expression (12-15). This activity has added benefits for cancer therapy or prevention since multiple oncoproteins are simultaneously affected. Because USP18 is the ISG15-specific DUB (18), its inhibition will preferentially enhance ISGylation. Other DUBs would likely not compensate for this loss. Development of USP18 inhibitors is a worthy objective because of their anticipated activity against diverse cancers. At the same time, since USP18 is an IFN and retinoid-regulated species, regulation of USP18 levels would affect response to these and other antineoplastic agents (13). Prior work revealed that USP18 knockdown followed by RA, cisplatin or IFN treatments increased growth-inhibitory and pro-apoptotic effects of each agent, thus providing a rationale for combination therapy (13,44). Both in vitro and clinically-predictive genetically-engineered mouse models exist to interrogate actions of such an optimal USP18 inhibitor.
Relating activity of different DUBs with specific pathways in cancer would identify biomarkers useful in diagnosis or in assessing clinical prognosis (45). As an example of this, the oncogenic DUB OTUD1 serves as a biomarker in thyroid cancer because it is prominently upregulated in this malignancy (45). Loss of the tumor suppressive DUB USP44 is a surrogate marker for ovarian cancer given that it is silenced by an oncogenic transcription factor upregulated in this cancer (45). It needs to be learnt if the DUB USP18 serves as a clinically-relevant biomarker in specific cancer contexts. Unlike some DUBs that are deregulated in specific cancers (45), USP18 expression is often augmented in many cancers (13). This indicates that its inhibition would likely confer a therapeutic effect over a broad spectrum of cancers.
Linking DUBs to specific growth-regulatory pathways in cancer would help highlight those that are potential antineoplastic targets. There are currently few specific or nonspecific DUB inhibitors (45,46). Non-specific inhibitors target multiple DUBs simultaneously to elicit diverse cellular response whereas specific inhibitors selectively target a single or limited number of DUBs. Specific inhibitors have proven effective in reversing oncogenic functions of DUBs (46). Several of these are small molecule inhibitors and representative ones are displayed in Table 1. For instance, the non-specific DUB inhibitors Velcade and Kyprolis are FDA-approved for the treatment of multiple myeloma (45). Because these inhibitors are non-specific proteasome inhibitors they do cause clinical toxicities (45). Other inhibitors are needed to be developed with increased specificity towards specific DUBs in order to increase selectivity and reduce clinical toxicity (45).
Table 1.
Representative inhibitors against specific deubiquitinases (DUB).
DUBs that have an inhibitor already available are displayed along with their individual roles in cancer biology. Known inhibitors against these DUBs and their activities are presented emphasizing how they act in reversing effects of these respective DUBs. Representative inhibitors are displayed that disrupt functions of specific DUBs. Non-selective DUB inhibitors are not displayed. This Table was modified from reference 46.
| Deubiquitinase | Function | Inhibitor | Inhibitor Function |
|---|---|---|---|
| USP7 |
Increases Mdm2 levels and decreases p53 stability |
P022077, P5091 and HBX 19818 |
Induces apoptosis and inhibits tumor growth |
| USP14 |
Inhibits proteasome activity role in ubiquitin recycling |
IU1 |
Enhances proteasome function and increases proteasomal degradation |
| UCHL1 |
Overexpressed in cancer and an early event in transformation |
LDN-57444 and LDN91946 |
Increases polyubiquitination and induces apoptosis |
| USP1/UAF1 |
Roles in DNA repair and DNA damage response |
GW7647 and Pimozide |
Inhibits proliferation and acts cooperatively with cisplatin |
Recently, the crystal structure of murine USP18 alone and in complex with ISG15 was solved (47). The C-terminal domain of ISG15 was found as essential for USP18 activity (47). Having the crystal structure in hand will enable development of the next generation of inhibitors that would block USP18 activities. This insight, when coupled with prior evidence showing antineoplastic effects of USP18 inhibition (12-14), makes pharmacologic targeting of USP18 a tractable strategy.
Future studies need to elucidate ways to selectively inhibit DUBs (45). One way to accomplish this is by targeting regulatory or protein interaction domains to avoid cross-reactivity with other DUBs (45). Many of the known specific DUB inhibitors are being validated for specificity and off-target effects before the pursuit of clinical trials (45,46). Some drugs that antagonize a single DUB, such as USP14 inhibitors, show encouraging preclinical findings and are being examined in clinical trials to determine whether they exert beneficial therapeutic effects (48,49). Further understanding of how DUBs like USP18 regulate stability of specific oncoproteins or tumor suppressor proteins will help unravel antineoplastic opportunities (45,46).
In summary, the DUB USP18 is an attractive antineoplastic target. The basis for this includes: (1) it was shown to control the expression of key proteins involved in cancer formation and progression, (2) it is the only known ISG15-specific DUB and antagonism of this species will not be compensated for by other DUBs, and (3) elucidation of the USP18 crystal structure enables development of selective USP18 inhibitors that could be explored for efficacy within different cancer contexts. Thus, inhibitors against USP18 are needed because they have promise for cancer treatment and prevention.
Supplementary Material
Acknowledgements
We thank all members of the Dmitrovsky laboratory for their helpful consultation.
Grant Support
This work was supported by National Institutes of Health (NIH) and National Cancer Institute (NCI) grants R01-CA087546 (E. Dmitrovsky, S.J. Freemantle, LM Mustachio, and X. Liu) and R01-CA190722 (E. Dmitrovsky, S.J. Freemantle, and M. Kawakami), a Samuel Waxman Cancer Research Foundation Award (E. Dmitrovsky and M. Kawakami), a UT-STARs award (E. Dmitrovsky) and an American Cancer Society Clinical Research Professorship (E. Dmitrovsky).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest exist.
References
- 1.Krueger KE, Srivastava S. Posttranslational protein modifications: current implications for cancer detection, prevention, and therapeutics. Mol Cell Proteomics 2006;5:1799–1810. [DOI] [PubMed] [Google Scholar]
- 2.Konstantinopoulos PA, Karamouszis MV, Papavassiliou AG. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat Rev Drug Discov 2007;6:541–555. [DOI] [PubMed] [Google Scholar]
- 3.Shen M, Schmitt S, Buac D, Dou QP. Targeting the ubiquitin-proteasome system for cancer therapy. Expert Opin Ther Targets 2013;17:1091–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desai SD. ISG15: A double edged sword in cancer. Oncoimmunology 2015;4:e1052935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell 1994;79:13–21. [DOI] [PubMed] [Google Scholar]
- 6.Zhang D, Zhang DE. Interferon-stimulated gene 15 and the protein ISGylation system. J Interferon Cytokine Res 2011;31:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadowski M and Sarcevic B. Mechanisms of mono- and poly-ubiquitination: Ubiquitination specificity depends on compatibility between the E2 catalytic core and amino acid residues proximal to the lysine. Cell Div 2010;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang YF, Wee S, Gunaratne J, Lane DP, Bulavin DV. Isg15 controls p53 stability and functions. Cell Cycle 2014;13:2200–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malakhova OA, Zhang DE. ISG15 inhibits Nedd4 ubiquitin E3 activity and enhances the innate antiviral response. J Biol Chem 2008;283:8783–8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komander D, Clague MJ, Urbé S. Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol 2009;10:550–563. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Dixit VM. Drugging the undruggables: exploring the ubiquitin system for drug development. Cell Res 2016;26:484–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Y, Dolinko AV, Chinyengetere F, Stanton B, Bomberger JM, Demidenko E, et al. Blockade of the ubiquitin protease UBP43 destabilizes the transcription factor PML/RARα and inhibits growth of acute promyelocytic leukemia. Cancer Res 2010;70:9875–9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y, Chinyengetere F, Dolinko AV, Lopez-Aguiar A, Lu Y, Galimberti F, et al. Evidence for the ubiquitin protease UBP43 as an antineoplastic target. Mol Cancer Ther 2012;11:1968–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mustachio LM, Lu Y, Tafe LJ, Memoli V, Rodriguez-Canales J, Mino B, et al. Loss of the deubiquitinase USP18 mislocalizes and destabilizes KRAS in lung cancer. Mol Cancer Res 2017;15:905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duex JE, Comeau L, Sorkin A, Purow B, Kefas B. Usp18 regulates epidermal growth factor (EGF) receptor expression and cancer cell survival via microRNA-7. J Biol Chem 2011. ;286:25377–25386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu LQ, Ilaria R Jr, Kingsley PD, Iwama A, van Etten RA, Palis J, et al. A novel ubiquitin-specific protease UBP43 cloned from leukemia fusion protein AML1-ETO-expressing mice, functions in hematopoietic cell differentiation. Mol Cell Biol 1999;19:3029–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwer H, Liu LQ, Zhou L, Little MT, Pan Z, Hetherington CJ, et al. Cloning and characterization of a novel human ubiquitin-specific protease, a homologue of murine UBP43 (USP18). Genomics 2000;65:44–52. [DOI] [PubMed] [Google Scholar]
- 18.Malakhov MP, Malakhova OA, Kim KI, Ritchie KJ, Zhang DE. UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J Biol Chem 2002;277:9976–9981. [DOI] [PubMed] [Google Scholar]
- 19.Zou W, Kim JH, Handidu A, Li X, Kim KI, Yan M, et al. Microarray analysis reveals that type I interferon strongly increases the expression of immune-response related genes in Ubp43 (Usp18) deficient macrophages. Biochem Biophys Res Commun 2007;356:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitha-Rowe I, Petty WJ, Kitareewan S, Dmitrovsky E. Retinoid target genes in acute promyelocytic leukemia. Leukemia 2003;17:1723–1730. [DOI] [PubMed] [Google Scholar]
- 21.Li C, Wang J, Zhang H, Zhu M, Chen F, Hu Y, et al. Interferon-stimulated gene 15 (ISG15) is a trigger for tumorigenesis and metastasis of hepatocellular carcinoma. Oncotarget 2014;5:8429–8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiappinelli KB, Strissel PL, Desrichard A, Li H, Henke C, Akman B, et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell 2015;162:974–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ketscher L, Hannß R, Morales DJ, Basters A, Guerra S, Goldmann T, et al. Selective inactivation of USP18 isopeptidase activity in vivo enhances ISG15 conjugation and viral resistance. Proc Natl Acad Sci USA 2015; 112:1577–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malakhova OA, Kim KI, Luo JK, Zou W, Kumar KG, Fuchs SY, et al. UBP43 is a novel regulator of interferon signaling independent of its ISG15 isopeptidase activity. EMBO J 2006;25:2358–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.François-Newton V, Magno de Freitas Almeida G, Payelle-Brogard B, Monneron D, Pichard-Garcia L, Pierhler J, et al. USP18-based negative feedback control is induced by type I and type III interferons and specifically inactivates interferon α response. PLoS One 2011. ;6:e22200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honke N, Shaabani N, Zhang DE, Hardt C, Lang KS. Multiple functions of USP18. Cell Death Dis 2016;7:e2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cong XL, Lo MC, Reuter BA, Yan M, Fan JB, Zhang DE. Usp18 promotes conventional CD11b+ dendritic cell development. J Immunol 2012; 188:4776–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheon HJ, Borden EC, Stark GR. Interferons and their stimulated genes in the tumor microenvironment. Semin Oncol 2014;41:156–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YH, Kim WT, Jeong P, Ha YS, Kang HW, Yun SJ, et al. Novel combination markers for predicting survival in patients with muscle invasive bladder cancer: USP18 and DGCR2. J Korean Med Sci 2014;29:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fraile JM, Quesada V, Rodríguez D, Freije JMP, López-Otín C. Deubiquitinases in cancer: new functions and therapeutic options. Oncogene 2012;31:2373–2388. [DOI] [PubMed] [Google Scholar]
- 31.Sacco JJ, Coulson JM, Clague MJ, Urbé S. Emerging roles of deubiquitinases in cancer-associated pathways. IUBMB Life 2010;62:140–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah SJ, Blumen S, Pitha-Rowe I, Kitareewan S, Freemantle SJ, Feng Q, et al. UBE1L represses PML/RAR-alpha by targeting the PML domain for ISG15ylation. Mol Cancer Ther 2008;7:905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fenq Q, Sekula D, Guo Y, Liu X, Black CC, Galimberti F, et al. UBE1L causes lung cancer growth suppression by targeting cyclin D1. Mol Cancer Ther 2008;7:3780–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burks J, Reed RE, Desai SD. ISGylation governs the oncogenic function of ki-ras in breast cancer. Oncogene 2014;33:794–803. [DOI] [PubMed] [Google Scholar]
- 35.Shahidul Makki M, Cristy Ruteshouser E, Huff V. Ubiquitin specific protease 18 (Usp18) is a WT1 transcriptional target. Exp Cell Res 2013;319:612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burkart C, Arimoto K, Tang T, Cong X, Xiao N, Liu YC, et al. Usp18 deficient mammary epithelial cells create an antitumor environment driven by hypersensitivity to IFN-λ and elevated secretion of Cxcl10. EMBO Mol Med 2013;5:967–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan M, Luo JK, Ritchie KJ, Sakai I, Takeuchi K, Ren R, et al. Ubp43 regulates BCR-ABL leukemogenesis via the type 1 interferon receptor signaling. Blood 2007;110:305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santin I, Moore F, Grieco FA, Marchetti P, Brancolini C, Eizirik DL. USP18 is a key regulator of the interferon-driven gene network modulating pancreatic beta cell inflammation and apoptosis. Cell Death Dis 2012;3:e419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manini I, Sgorbissa A, Potu H, Tomasella A, Brancolini C. The deISGylase USP18 limits TRAIL-induced apoptosis through the regulation of TRAIL levels. Cancer Biol Ther 2013; 14:1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mao H, Wang M, Cao B, Zhou H, Zhang Z, Mao X. Interferon-stimulated gene 15 induces cancer cell death by suppressing the NF-kB signaling pathway. Oncotarget 2016;7:70143–70151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong B, Li H, Lu Y, Zhang M, Zheng Y, Qian J, et al. USP18 is crucial for IFN-γ-mediated inhibition of B16 melanoma tumorigenesis and antitumor immunity. Mol Cancer 2014; 13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chinyengetere F, Sekula DJ, Lu Y, Giustini AJ, Sanglikar A, Kawakami M, Ma T, et al. Mice null for the deubiquitinase USP18 spontaneously develop leiomyosarcomas. BMC Cancer 2015; 15:886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mustachio LM, Kawakami M, Lu Y, Rodriguez-Canales J, Mino B, Behrens C, et al. The ISG15-specific protease USP18 regulates stability of PTEN. Oncotarget 2017;8:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Potu H, Sgorbissa A, Brancolini C. Identification of USP18 as an important regulator of the susceptibility to IFN-alpha and drug-induced apoptosis. Cancer Res 2010;70:655–665. [DOI] [PubMed] [Google Scholar]
- 45.Pfoh R, Lacdao IK, Saridakis V. Deubiquitinases and the new therapeutic opportunities offered to cancer. Endocr Relat Cancer 2015;22:T35–T54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Arcy P, Wang X, Linder S. Deubiquitinase inhibition as a cancer therapy. Pharmacol Ther 2015;147:32–54. [DOI] [PubMed] [Google Scholar]
- 47.Basters A, Geurink PP, Röcker A, Witting KF, Tadayon R, Hess S, et al. Structural basis of the specificity of USP18 toward ISG15. Nat Struct Mol Biol 2017;24:270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Mazurkiewicz M, Hillert EK, Olofsson MH, Pierrou S, Hillertz P, et al. The proteasome deubiquitinase inhibitor VLX1570 shows selectively for ubiquitin-specific protease-14 and induces apoptosis of multiple myeloma cells. Sci Rep 2016;6:26979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farshi P, Deshmukh RR, Nwankwo JO, Arkwright RT, Cvek B, Liu J, et al. Deubiquitinases (DUBs) and DUB inhibitors: a patent review. Expert Opin Ther Pat 2015;25:1191–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.