Abstract
Plants are associated with a complex microbiota that contributes to nutrient acquisition, plant growth, and plant defense. Nitrogen-fixing microbial associations are efficient and well characterized in legumes but are limited in cereals, including maize. We studied an indigenous landrace of maize grown in nitrogen-depleted soils in the Sierra Mixe region of Oaxaca, Mexico. This landrace is characterized by the extensive development of aerial roots that secrete a carbohydrate-rich mucilage. Analysis of the mucilage microbiota indicated that it was enriched in taxa for which many known species are diazotrophic, was enriched for homologs of genes encoding nitrogenase subunits, and harbored active nitrogenase activity as assessed by acetylene reduction and 15N2 incorporation assays. Field experiments in Sierra Mixe using 15N natural abundance or 15N-enrichment assessments over 5 years indicated that atmospheric nitrogen fixation contributed 29%–82% of the nitrogen nutrition of Sierra Mixe maize.
Author summary
Nitrogen is an essential nutrient for plants, and for many nonlegume crops, the requirement for nitrogen is primarily met by the use of inorganic fertilizers. These fertilizers are produced from fossil fuel by energy-intensive processes that are estimated to use 1% to 2% of the total global energy supply and produce an equivalent share of greenhouse gases. Because maize (Zea mays L.) is a significant recipient of nitrogen fertilization, a research goal for decades has been to identify or engineer mechanisms for biological fixation of atmospheric nitrogen in association with this crop. We hypothesized that isolated indigenous landraces of maize grown using traditional practices with little or no fertilizer might have evolved strategies to improve plant performance under low-nitrogen nutrient conditions. Here, we show that for one such maize landrace grown in nitrogen-depleted fields near Oaxaca, Mexico, 29%–82% of the plant nitrogen is derived from atmospheric nitrogen. High levels of nitrogen fixation are supported, at least in part, by the abundant production of a sugar-rich mucilage associated with aerial roots that provides a home to a complex nitrogen-fixing microbiome.
Introduction
Plants grow in close association with microbial communities that influence plant traits related to nutrient acquisition, plant development, plant defenses, and abiotic stress responses. The root-associated microbiota of plants has been characterized and shown to be much less complex than the microbiota of the surrounding soil, being enriched in Proteobacteria, Bacteroidetes, and Actinobacteria. These microbes are selected in part by plant cell wall features and metabolic cues from host cells [1,2]. Characterization of the rhizosphere microbiome associated with 27 modern maize (Z. mays) inbred lines also indicated substantial differences in relative abundance of microbial taxa between bulk soil and the rhizosphere, with the maize genotype contributing a small but significant influence on rhizosphere selectivity [3].
Nitrogen-fixing microbial associations with nonlegumes, especially cereals, have been a topic of intense interest for more than a century [4–7]. Nitrogen-fixing endophytes contribute to the nitrogen nutrition of sugarcane in some environments [8–10], but there is less evidence for the occurrence of efficient diazotrophic associations in other cereals. A 1-year study based on 15N dilution experiments in Miscanthus × giganteus suggested that this perennial bioenergy feedstock can acquire about 16% of its nitrogen from the air [11]. It has also been demonstrated that the model cereal, Setaria viridis, as well as Setaria italica (foxtail millet) can acquire a significant amount of fixed nitrogen from associations with Azospirillum brasilense[12,13]. Other examples of fixed atmospheric N2 being transferred to cereals include associations between Azoarcus sp. strain BH72 and Kallar grass [14], Herbaspirillum seropedicae and rice [15,16], and Klebsiella pneumoniae and wheat [17]. Because of its economic importance, the search for diazotrophic associations with maize (Z. mays) [18] has been a “holy grail” for decades, and several studies examined the contribution of nitrogen fixation by H. seropedicae and Azospirillum sp. to various maize accessions [19,20]. However, it is often difficult in these studies to distinguish the general plant growth–promoting benefits of these diazotrophic bacteria on yield from an actual transfer of fixed nitrogen to host plants. Five techniques are commonly used to evaluate nitrogen fixation: acetylene reduction assays (ARAs), 15N natural abundance, 15N enrichment, 15N2 gas enrichment, and nitrogen balance experiments [21,22]. All of these approaches have potential pitfalls, yet very few studies have compared different techniques or conducted assessments over multiple years to evaluate nitrogen fixation in nonlegumes.
Triplett suggested that it may be interesting to survey primitive maize landraces from the areas of maize origin to identify maize diazotrophic endophytes [5]. Estrada and colleagues [23] followed this suggestion and examined a landrace of maize in the Sierra Mixe region of Oaxaca, Mexico, and isolated a nitrogen-fixing endophyte from the resident maize landrace. The isolate was tentatively identified as a new species of Burkholderia, but the contribution of atmospheric dinitrogen (N2) to the nitrogen economy of the plant was not tested. This group also reported the isolation of a similar endophyte from field-grown teosinte plants and speculated that the Burkholderia strain might have formed a primitive symbiosis with teosinte that persisted during domestication of maize.
We also learned of isolated indigenous landraces of maize in the Sierra Mixe region of Oaxaca that were reportedly grown using traditional practices with little or no fertilizer and speculated that unique microbial community associations, not found in cultivated maize, might have evolved. This indigenous maize landrace is characterized by the extensive development of aerial roots that produce large amounts of mucilage. Mucilage associated with maize underground roots has been previously described [24,25], and it has been suggested that root exudates play a significant role in structuring rhizosphere microbial communities [26,27]. Indeed, it has been shown that pea root mucilage can serve as a sole carbon source for some rhizosphere bacteria, including Rhizobium sp., Burkholderia sp., and Pseudomonas sp. [28]. Aerial roots at the base of the maize shoot, also known as brace roots or nodal adventitious roots, can often reach the ground and are thought to provide anchorage to prevent lodging but may also contribute to nutrient and water uptake as well as gas exchange [29–31]. However, very little is known about the role of aerial roots that do not reach the ground and the mucilage that they produce. Here, we demonstrate that a Mexican maize landrace can acquire 29%–82% of its nitrogen from the air and that at least some of this N is fixed by diazotrophic bacteria present in the mucilage of aerial roots.
Results
Sierra Mixe maize morphology and mucilage
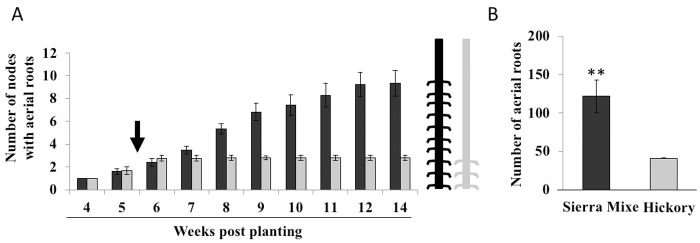
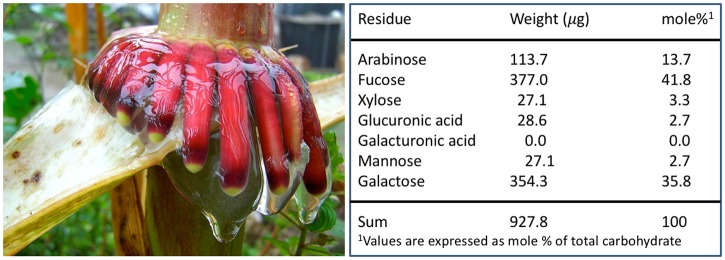
The Sierra Mixe maize varieties cultured locally—referred to as Rojo, Piedra Blanca, and Llano—share similar plant morphologies, growing to a height of over 5 meters and exhibiting extensive aerial root formation at each node. We compared the development of aerial roots in the Sierra Mixe maize with another tall maize variety, Hickory King (Fig 1A). Unlike most modern maize varieties in which aerial root formation ceases after the juvenile-to-adult transition (arrow, Fig 1A), aerial root formation in Sierra Mixe maize continued well after this transition, resulting in a 3- to 4-fold greater number of aerial roots (Fig 1B). Approximately midway through development (July to September), these maize aerial roots secrete significant amounts of mucilage that is rich in arabinose, fucose, and galactose when moisture is available (Fig 2). The sugars comprise a complex polysaccharide that presumably contributes to the viscosity of the mucilage and may be disassembled to provide monosaccharides to support microbial growth and metabolism. Mucilage produced by underground maize roots also contain high levels of fucose and arabinose [32], although at approximately one-half the concentration found in aerial root mucilage.
Fig 1. Physiological features of Sierra Mixe maize.
(A) The transition between juvenile and adult phases (black arrow) occurs 5 weeks after planting in Sierra Mixe maize (black bars) and in the tall maize heirloom Hickory King (gray bars). (B) Number of aerial roots observed on Sierra Mixe maize and Hickory King after 14 weeks of growth in the field in Madison, United States of America. Error bars represent standard errors; an asterisk indicates a significant difference between Sierra Mixe maize and Hickory King (Student t test, P < 0.01). (Data at DOI: 10.6084/m9.figshare.6534545).
Fig 2. Aerial root mucilage.
The aerial roots of Sierra Mixe maize (left) secrete large quantities of mucilage between 3 and 6 months after planting. The mucilage is carbohydrate rich, with the composition dominated by arabinose, fucose, and galactose (side panel).
Sierra Mixe maize diazotrophic microbiota
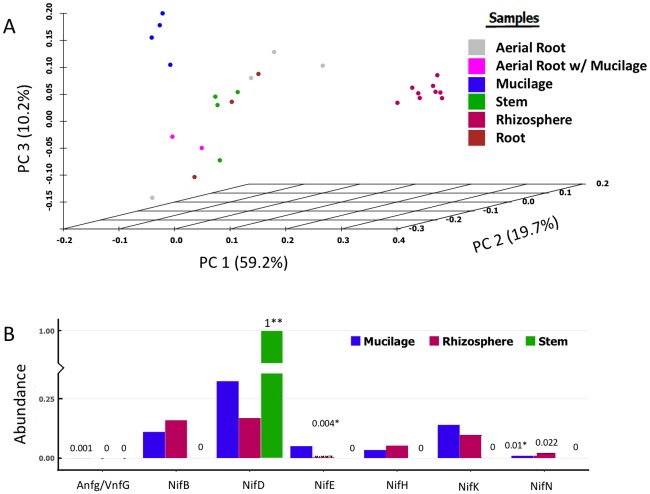
The microbiota associated with the underground and aerial roots, stems, and aerial root mucilage of Sierra Mixe maize grown in Sierra Mixe was investigated by amplifying and sequencing of 16S rRNA genes and by shotgun metagenome sequencing. The rhizosphere samples were the most diverse, and among plant samples, the aerial root mucilage had the highest diversity (S1 Fig) and a higher relative abundance of bacteroidetes and proteobacteria (beta and gamma) compared to other parts of the plants (S2 Fig). Many of the lineages that are overrepresented in mucilage include known plant-associated nitrogen fixers. A comparison of samples based on the total community composition showed a clustering of mucilage samples that were statistically distinct from the rest of the plant and rhizosphere samples (Fig 3A). Clustering of samples based upon the variance-stabilized abundance of sequence variants again indicates that while the rhizosphere samples were distinct and diverse, the mucilage samples were distant from the other plant tissues (S3 Fig). The complete list of all sequence variants identified in the Sierra Mixe maize and soil samples can be found at (DOI: 10.6084/m9.figshare.4789759). Additionally, the metagenomic data was searched for homologs of the 6 core nif genes as described by Dos Santos and colleagues [33]. This search revealed the presence of all 6 core nif genes in the metagenomes from mucilage and rhizosphere and only a subset of the 6 core nif genes from stem tissue (Fig 3B). A higher normalized abundance of most nif genes (except nifD) in mucilage than stem tissues suggests that the mucilage may be enriched in nitrogen-fixing microbes.
Fig 3. DNA sequencing–based characterization of the microbiome of Sierra Mixe maize.
(A) Samples clustered by PCoA on Bray-Curtis dissimilarity distance matrix. Bacterial communities were assessed using PCR amplification and sequencing of rRNA genes. Each point corresponds to an individual sample. Permanova tests run using adonis in vegan revealed mucilage samples were statistically distinct (e.g., mucilage versus rhizosphere P = 0.002, mucilage versus roots P = 0.03, mucilage versus aerial roots P = 0.03). (B) Metagenomic sequencing–based analysis of homologs of core nif genes (nifH, nifD, nifE, nifK, nifN, and nifB) and alternate nitrogenase (anfG/vnfG). Metagenomic samples were searched for homologs by mapping reads on reference nif trees. The number of hits was normalized to an estimate of the number of bacterial genes in the metagenomic sample (measured using the number of hits to the RecA hidden Markov model). *The lowest abundance of a core nif gene in mucilage and rhizosphere libraries. **Only core nif gene hit in stem library. anfG and vnfG alternate nitrogenase were observed only in mucilage library. PCoA, principal component analysis.
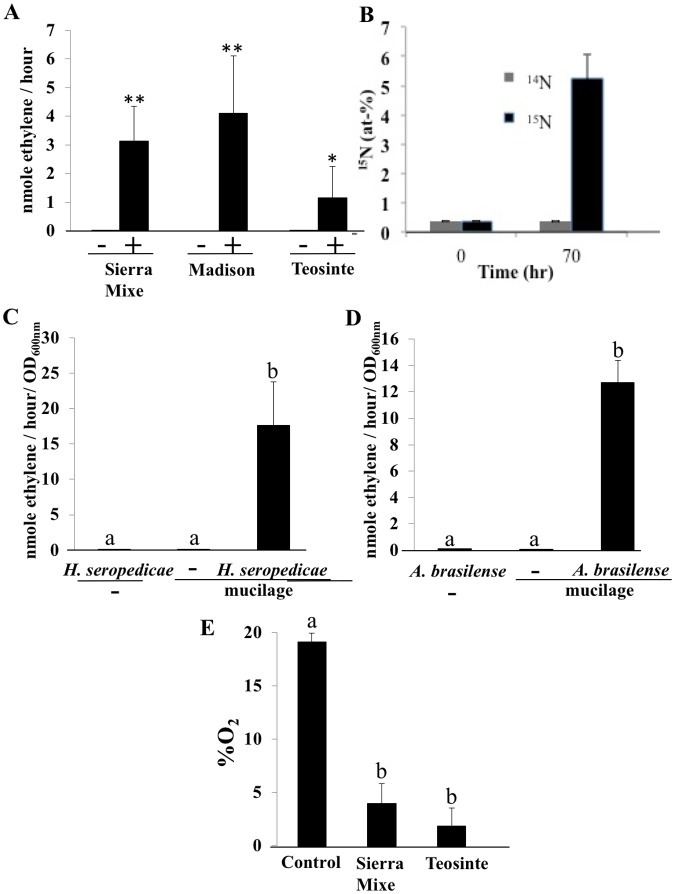
To assess the possibility that mucilage harbored a diazotrophic microbial community, the mucilage was tested for nitrogenase activity using ARAs [34] and by incorporation of 15N2 gas. ARA was used to assess leaves, stems, underground roots, aerial roots (with and without mucilage), and mucilage collected from Sierra Mixe maize plants grown either in Sierra Mixe, Mexico, or Madison, USA. No ARA activity was detected in underground roots, leaves, stems, or even aerial roots before mucilage production (S4 Fig). In contrast, significant ARA activity was detected in aerial roots harboring mucilage (S4 Fig) and in isolated mucilage from plants grown in either Sierra Mixe or Madison (Fig 4A). The ARA activity in mucilage isolated from Sierra Mixe maize that was grown in Madison suggests either that the Sierra Mixe maize seeds carry an endogenous inoculum of nitrogen-fixing bacteria or that Sierra Mixe maize can recruit adequate nitrogen-fixing bacteria from local environments. Nitrogen fixation by mucilage samples was also measured by direct incorporation of 15N2 in mucilage samples collected from Sierra Mixe maize (Fig 4B).
Fig 4. Nitrogenase and N2 fixation activity in mucilage produced by Sierra Mixe maize.
(A) Mucilage of various Sierra Mixe maize lines collected in Sierra Mixe or field-grown plants in Madison, USA, and of teosinte display strong acetylene reduction activity. (-, no acetylene; +, 10% acetylene). Asterisks indicate significant differences (*P < 0.05; **P < 0.01, Mann-Whitney test). (B) Nitrogen fixation in Sierra Mixe maize mucilage by 15N2 assimilation. Mucilage collected from Sierra Mixe maize grown in Sierra Mixe was incubated in gas-tight vials filled with 15N2 or 14N2 gas for 70 hours at 37 °C. 15N (atom % excess) was determined by IRMS. (C and D) H. seropedicae and A. brasilense display acetylene reduction activity when added to nonfixing mucilage, whereas the same mucilage supplemented with sterile medium (-) or the same bacteria without mucilage (-) do not. (E) Oxygen concentration at 8 mm inside of the mucilage. Means and standard errors are shown. Different letters indicate statistically supported groups (Kruskal-Wallis test). (Data at DOI: 10.6084/m9.figshare.6534545). IRMS, isotope-ratio mass spectrometry.
As with Sierra Mixe maize, a wild relative, Z. mays ssp. mexicana (teosinte), also produced extensive aerial roots (S5 Fig) but much smaller amounts of secreted mucilage. To test the ability of the teosinte mucilage to support nitrogen fixation, we collected mucilage from several plants of teosinte and measured endogenous nitrogenase activity using ARA. Acetylene reduction was readily observed in teosinte mucilage (Fig 4A), suggesting that production of mucilage that supports nitrogen fixation by an associated nitrogen-fixing microbiota may be an ancient trait of maize and potentially introgressed from Z. mays ssp. mexicana into the Sierra Mixe landrace postdomestication.
To assess the mucilage characteristics that support nitrogen fixation, we tested the ability of 2 phylogenetically distinct nitrogen-fixing bacteria to reduce acetylene when inoculated in the mucilage collected from aerial roots of Sierra Mixe maize. Before the experiment, the mucilage was frozen for 2 weeks at –80 °C and thawed, which abolished endogenous nitrogenase activity (Fig 4C). Two nitrogen-fixing bacteria, H. seropedicae, and A. brasilense, showed readily detectable ARA activity when added to the mucilage (Fig 4C and 4D). Bacterial nitrogenase is O2-sensitive and needs to be protected by a low-oxygen (<5%) environment or physiological protective mechanisms, as well as an abundant carbon source to derive energy for this process [35]. To determine if mucilage could fulfill these requirements, we measured the free-oxygen concentration in the mucilage of Sierra Mixe maize and teosinte at the depth of 8 mm and found it to be <5%, indicating that the mucilage can provide a microaerobic environment compatible with nitrogen fixation for these bacteria [36] (Fig 4D). Mucilage is also comprised of complex sugars that may be catabolized to provide free sugars—mainly arabinose, fucose, and galactose—capable of supporting bacterial growth and nitrogen fixation. To determine whether these properties of the mucilage are sufficient to support nitrogen fixation, we created an artificial medium mimicking these mucilage properties by using a low-N medium, solidified with 0.2% agar, that reduced oxygen concentration to levels almost as low as those found in the mucilage (S6A Fig) and supplemented with a mix of free sugars corresponding to the composition of the fully hydrolyzed mucilage carbohydrates. H. seropedicae, A. brasilense, and Burkholderia unamae (S6B, S6C and S6D Fig) showed significant ARA activity in this reconstructed mucilage, indicating that the low O2 and free sugars provided by the aerial root mucilage are sufficient to support nitrogen fixation by these diazotrophs.
Based on ARA, we can conclude that mucilage from Sierra Mixe maize harbors native diazotrophs and can also support the N2-fixing activity of the exogenously inoculated diazotrophs, H. seropedicae, A. brasilense, and B. unamae. However, these data did not demonstrate that the aerial roots had the capacity to take up and assimilate the fixed N. To test whether atmospheric N2 that was fixed by mucilage-associated diazotrophs could be transferred to and utilized by the Sierra Mixe maize, a more direct 15N2 gas–enrichment experiment was used. Aerial roots, along with their generated mucilage, inoculated with A. brasilense Sp7 exhibited significant 15N2 gas incorporation in comparison to the 14N2 gas-treated roots (Fig 5), and isotope-ratio mass spectrometry (IRMS) analysis confirmed significant enrichment of 15N in chlorophyll (converted to pheophytin for analysis) of these roots compared to the negative controls (Fig 5).
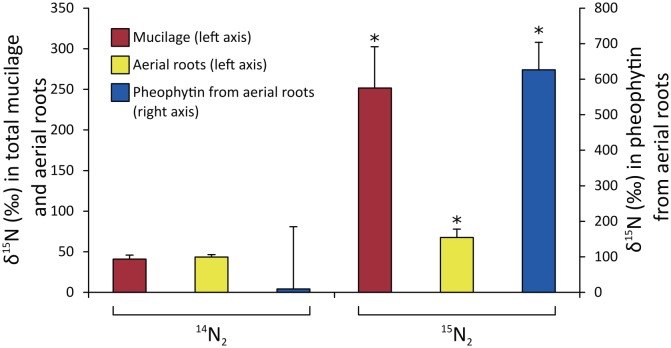
Fig 5. Analysis of Sierra Mixe maize samples for 15N2 enrichment in mucilage, aerial roots, and pheophytin from aerial roots.
Mucilage was generated from aerial roots and inoculated with A. brasilense Sp7. 15N2 gas was pumped in and, after incubation mucilage, was separated from the aerial roots. Mucilage alone and aerial roots alone were subjected to analysis by IRMS. Results revealed a significant enrichment of 15N2 in mucilage alone and aerial roots alone (left y-axis). Since the inoculated aerial roots may contain A. brasilense Sp7 attached to the surface, we extracted pheophytin from these aerial roots to test 15N2 incorporation in pheophytin. Results revealed a significant enrichment of 15N2 in these aerial roots, indicating that aerial roots are indeed the sites for transfer of fixed nitrogen to the plants (right y-axis). 14N2 samples were used as negative controls. n = 4 (aerial roots and mucilage), n = 3 (pheophytin). The asterisk (*) indicates a statistically significant difference (p < 0.05). (Data at DOI: 10.6084/m9.figshare.6534545) IRMS, isotope-ratio mass spectrometry.
Nitrogen fixation contributes to maize N nutrition
The transfer of 15N2 from mucilage to the aerial root tissue and chlorophyll demonstrated the potential of this diazotrophic community to contribute to the nitrogen nutrition of the plant, but a major question of this study is whether the mucilage-associated diazotrophic microbiota served to transfer fixed nitrogen to fulfill, at least in part, the reduced nitrogen requirements of Sierra Mixe maize under field conditions. The contribution of atmospheric nitrogen fixation to Sierra Mixe maize was first estimated in the field using natural abundance 15N measurements [37,38]. This method relies on the relative abundance of the stable isotope 15N in the atmosphere and soil, with 15N abundance being more abundant in the soil than in the air. As a consequence, plants that derive N from the atmosphere will exhibit reduced δ15N levels when compared to reference nonfixing plants. In 2006, samples of Sierra Mixe maize and reference plants from the Asteraceae and Ranunculaceae (families with no known nitrogen-fixing members) growing near each other were collected from each of 2 fields. In this preliminary experiment, Sierra Mixe maize δ15N was significantly lower than the reference plants, indicating the assimilation of atmospheric nitrogen (S1 Table). In 2010, 2011, and 2012, the methods from [38,39] were used in experiments in Sierra Mixe by sampling reference species of non-nitrogen-fixing plants growing near the Sierra Mixe maize plants and a conventional maize variety, Maiz Blanco Conasupo. In addition to determining δ15N from each maize and reference plant sample, leaf samples of each reference plant were used for 18S rRNA sequence analysis to identify the reference species (Table 1A). The δ15N values of Sierra Mixe maize grown in the field in Sierra Mixe were determined at a single developmental time point in 2010 and at 5 developmental time points in 2011 and 2012. In 2010, the δ15N values for the Sierra Mixe maize were significantly lower than those of the reference plant species and of the conventional maize variety, Maiz Blanco Conasupo (Table 1A), indicating that the Sierra Mixe maize was able to derive a significant part of its tissue nitrogen from atmospheric dinitrogen. Similar δ15N values from root and leaf samples also showed that the leaf samples analyzed were representative of the whole plant (S2 Table). In 2011 and 2012, the δ15N values of Sierra Mixe maize were significantly lower than the reference plants at 4 of the 5 developmental time points, suggesting that Sierra Mixe maize derived a portion of its tissue nitrogen from atmospheric nitrogen (Table 1B). The calculated percent of nitrogen derived from the atmosphere (%Ndfa) from the δ15N values in Table 1 ranged between 30% and 80% (S7 Fig).
Table 1. Natural abundance 15N determinations.
(A) δ15N values from Sierra Mixe maize, a conventional maize variety (Maiz Blanco Conasupo), and reference plants grown in Sierra Mixe, 3 months after planting. Values are given as mean and standard deviation. Statistical comparisons were made between the means of the reference plants, Maiz Blanco Conasupo, and Z. mays S. Mixe, using Student t tests (p < 0.05). Different letters indicate statistically supported groups. (B) δ15N values from Sierra Mixe maize plants grown in Fields 1 and 2 in Sierra Mixe during 2011 and 2012 at 2, 3, 4, 5, and 6 months after planting. Values are given as mean and standard deviation. Statistical comparisons were made between the reference plants mean and Z. mays S. Mixe means at each time point using Student t tests (p < 0.05). Different letters indicate statistically supported groups. Reference plants are listed in S3 Table. (Data at DOI: 10.6084/m9.figshare.6534545).
| A | B | |||
|---|---|---|---|---|
| Sample | Sample | 2011 δ N15 (‰) | 2012 δ N15 (‰) | |
| Rumex obtusifolius | 5.88 ± 0.44 | Field 1 | ||
| Ipomoea purpurea | 4.55 ± 029 | Reference plants mean | 5.86 ± 0.61 a | 4.14 ± 0.35 a |
| Bougainvillea sp. | 6.31 ± 0.49 | Z. mays S. Mixe 2nd Month | 4.57 ± 1.39 ab | 6.38 ± 1.09 a |
| Sambucus canadensis | 6.76 ± 0.09 | Z. mays S. Mixe 3rd Month | 2.36 ± 0.67 b | 0.78 ± 1.26 c |
| Hymenocallis chiapasiana | 4.08 ± 0.16 | Z. mays S. Mixe 4th Month | 3.92 ± 0.30 b | 3.27 ± 1.98 b |
| Physalis philadelphica | 6.12 ± 0.18 | Z. mays S. Mixe 5th Month | 4.04 ± 0.36 b | 3.77 ± 0.33 b |
| I. purpurea | 5.53 ± 0.20 | Z. mays S. Mixe 6th Month | 3.39 ± 0.20 b | 2.66 ± 0.22 b |
| Melampodiu perfoliatum | 4.29 ± 0.09 | |||
| Reference plants mean | 5.42 ± 0.21 a | Field 2 | ||
| Maiz Blanco Conasupo mean | 4.87 ± 0.57 a | Reference plants mean | 5.19 ± 0.64 a | 2.72 ± 0.26 a |
| Z. mays S. Mixe Field 1 | 3.05 ± 0.82 | Z. mays S. Mixe 2nd Month | 4.86 ± 1.39 a | −1.02 ± 0.90 bc |
| Z. mays S. Mixe Field 2 | 2.36 ± 0.94 | Z. mays S. Mixe 3rd Month | 2.10 ± 0.74 bc | −2.73 ± 1.07 c |
| Z. mays S. Mixe mean | 2.71 ± 0.60 b | Zea mays S. Mixe 4th Month | 2.35 ± 0.52 b | −0.61 ± 0.23 b |
| Zea mays S. Mixe 5th Month | 1.20 ± 0.22 c | 0.37 ± 0.31 b | ||
| Zea mays S. Mixe 6th Month | 2.74 ± 0.41 b | −0.41 ± 0.20 b |
The method of using natural abundance 15N and other species as reference plants to calculate %Ndfa is potentially limiting because of differences in root and shoot growth and phenology of reference and test plants and the limited range of δ15N found in soils. An alternative method, 15N enrichment, is similar to the natural abundance methods but enriches soil 15N by the addition of 15N fertilizer, thereby increasing the difference between soil and atmospheric δ15N and assay sensitivity. Several direct comparisons have indicated that both methods can give comparable but slightly different results [38,40].
Another method of estimating N2 fixation is the “N Difference” method, which determines the difference between the total N content of an N2-fixing plant and the total N content of a reference nonfixing plant. Total N is calculated by multiplying total N content (%) in a specific plant sample and the total biomass (kg/ha or kg) produced by the plant. The %Ndiff is calculated as described in Materials and methods.
In 2016 and 2017, we assessed atmospheric nitrogen fixation using the 15N-isotope-enrichment method (1%–10% enrichment) at 3 vegetative growth stages—V9, V12, and Tassel—[41] in a random complete block design (5 replicates) in 3 low-N Sierra Mixe fields and at Tassel stage in 2017 in the same fields using the same design (Table 2). Field 3 (with a history of 0–1 year of maize production) yielded significant differences in Atom% 15N only for SM2 at the Tassel stage in 2016, but shoot N was significantly different in both 2016 and 2017. In 2016, Sierra Mixe maize landrace varieties exhibited significantly lower Atom%15N levels than the reference plants in Field 4, (with a history of 1–2 years of maize production) at Tassel and at V9 in 2016, and at Tassel in both years for shoot N. In both 2016 and 2017, Sierra Mixe maize landraces in Field 5 (over 3 years of continuous maize production) exhibited significantly lower Atom%15N and shoot N at all stages sampled, indicating a significant level of atmospheric nitrogen fixation in those experiments. The calculated %Ndfa ranged between 31% and 55%, and Ndiff ranged from 29%–82%. The correlation between Ndfa and Ndiff was 0.55 and 0.44 (P < 0.01) across locations in 2016 and 2017, respectively. Significant measures of N2 fixation were detected in 4 of 6 experiments by Atom% δ15N N determinations (Ndfa) and in 6 of 6 experiments by Ndiff determinations. Root and shoots exhibited significant differences in biomass, height, and stem diameter between control hybrids and local landraces (S4 Table). Soil analyses (S5 Table) showed that all sites were depleted in soil nitrogen yet produced a crop greater than 2,000 kg/ha. It is possible that differences between microbiota associated with the 3 fields in different stages of crop rotation (0 to >4 years of continuous maize production) account for differences observed among fields and between years, but further research is needed to answer this question.
Table 2. 15N-isotope-enrichment determinations in field trials.
Percent Ndfa and Ndiff were calculated for Sierra Mixe varieties when Atom% 15N excess or Shoot N for the whole plant were significantly different from reference varieties as assessed by ANOVA and single-degree-of-freedom contrasts (p = 0.05), respectively. Values followed by asterisks are significantly different from the reference varieties based on single-degree-of-freedom contrasts (p < 0.05). Percent Ndfa and Ndiff were not calculated for reference (dashes). (Data at DOI: 10.6084/m9.figshare.6534545).
| 2016 9-leaf | 2016 12-leaf | 2016 Tassel | 2017 Tassel | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atom 15N (%) | Ndfa (%) | Shoot N (kg/ha) | Ndiff (%) | Atom 15N (%) | Ndfa (%) | Shoot N (kg/ha) | Ndiff (%) | Atom 15N (%) | Ndfa (%) | Shoot N (kg/ha) | Ndiff (%) | Atom 15N (%) | Ndfa (%) | Shoot N (kg/ha) | Ndiff (%) | |
| Field 3 | ||||||||||||||||
| Reference | 0.60 | - | 5.1 | - | 0.28 | - | 21.3 | - | 0.21 | - | 12.0 | - | 0.24 | - | 5.9 | - |
| SM1 | 0.35 | 43.5 | 11.1 * | 46.4 | 0.19 | 30.2 | 31.7 | 29.1 | 0.18 | 21.5 | 27.3 | 46.5 | ||||
| SM2 | 0.57 | 10.3 | 6.6 | 22.0 | 0.24 | 14.9 | 27.5 | 23.3 | 0.15* | 29.0 | 34.2 * | 60.6 | 0.23 | 5.0 | 24.2 * | 75.0 |
| Field 4 | ||||||||||||||||
| Reference | 0.16 | - | 21.8 | - | 0.12 | - | 58.5 | - | 0.09 | - | 58.2 | - | 0.21 | - | 28.5 | - |
| SM1 | 0.17 | 5.6 | 31.5* | 28.5 | 0.07 | 32.9 | 84.5 | 29.2 | 0.05 * | 48.1 | 122.3 * | 48.7 | 0.17 | 15.0 | 30.2 | 13.0 |
| SM2 | 0.10 | 33.4 | 19.9 | 1.9 | 0.08 | 30.4 | 94.6 | 30.1 | 0.05 * | 43.2 | 101.8 | 37.6 | 0.19 | 6.0 | 51.3 * | 46.0 |
| Field 5 | ||||||||||||||||
| Reference | 0.45 | - | 6.2 | - | 0.23 | - | 16.4 | - | 0.18 | - | 9.8 | - | 0.41 | - | 8.4 | - |
| SM1 | 0.23 * | 48.5 | 14.9 * | 56.8 | 0.16 | 33.3 | 46.2 * | 61.1 | 0.08 * | 55.3 | 61.4 * | 82.2 | 0.27 * | 32.0 | 30.5 * | 70.0 |
| SM2 | 0.24 * | 46.4 | 10.5 | 37.9 | 0.12 * | 48.0 | 37.2 * | 52.7 | 0.08 * | 55.1 | 43.1 * | 76.6 | 0.27 * | 33.0 | 27.0 * | 71.0 |
Abbreviation: Ndfa, nitrogen derived from the atmosphere; Ndiff, total nitrogen difference.
Discussion
We have demonstrated that the mucilage associated with the aerial roots of Sierra Mixe maize can support a complex diazotrophic microbiota enriched for homologs of genes encoding nitrogenase subunits that harbor active nitrogenase activity, and that nitrogen is transferred efficiently from the nitrogen-fixing bacteria to the host plant tissues. Collectively, over several years and locations, the 15N natural abundance and 15N enrichment results of 2 selections of a Sierra Mixe indigenous maize landrace suggest that its nitrogen nutrition when grown in its native environment is partially fulfilled by fixation of atmospheric nitrogen. Nitrogen fixation is a particularly difficult phenotype to evaluate, as all the techniques available are prone to artifacts and can give different estimates for nitrogen fixation [42,43]. In this study, we used several classical techniques with Sierra Mixe maize and have shown over multiple locations and years that Sierra Mixe maize can fix nitrogen at rates (29%–82%) not previously reported, to our knowledge, in maize.
This study also revealed a new and important function for aerial roots and the mucilage they produce besides preventing lodging or water uptake [30]. This role in nitrogen fixation is probably the most important one for aerial roots that do not reach the ground. It will be interesting to explore if aerial roots produced by other cereals such as sorghum can perform a similar function [44]. We cannot rule out that diazotrophic activity in other parts of Sierra Mixe maize may also contribute to the acquisition of reduced nitrogen from the atmosphere. The developmental timing of the appearance of fixed atmospheric N2 in Sierra Mixe maize plants (Tables 1 and 2) before the extensive development of aerial roots suggests that there may, indeed, be additional sites of nitrogen fixation. However, we have not detected any significant nitrogenase activity outside of the aerial root mucilage.
The genetic basis of the trait or the source of the microbial inoculum, which may be either environmental or seed-borne, are unresolved. The observation that a teosinte species (Z. mays ssp. mexicana) also exhibits a similar diazotrophic activity in aerial root–associated mucilage suggests that this is an ancient trait that may have been introgressed and amplified in the Sierra Mixe landrace. It will be important, in the future, to determine the genetic basis of the trait, the identity of associated microbial diazotrophs, and the mechanisms of microbial recruitment. This research, together with other published research [5,18,23], suggests new avenues for research into potentially novel mechanisms of biological N2 fixation in maize. This could have a significant impact on maize crop productivity and nitrogen use efficiency, particularly in regions of the world where agriculture is characterized by poor soil nutrition.
Materials and methods
Plant material
Sierra Mixe maize seeds were obtained in Sierra Mixe region of Oaxaca, Mexico, from an open pollinated population. Z. mays ssp. mexicana (teosinte), LH123HT, Mo17, PHG39, LH82, PH207, and B73 seeds were obtained from USDA National Plant Germplasm System (NPGS) (accessions Ames 8083, PI601079, PI558532, PI600981, PI601170, PI601005, and PI550473, respectively). Maize line Hickory King was obtained from Victory Seeds (accession 3140041). Tornado-F21 and H-377 were obtained from Semillas Ceres in Oaxaca. SM1 and SM2 are different selections from the Sierra Mixe landrace. SM1 is a uniform population based on kernel size, shape, and color, and SM2 is a heterogeneous population representing the landrace.
Biological materials were accessed and utilized under an Access and Benefit Sharing Agreement between the Sierra Mixe community and BioN2, Inc., and with permission from the Mexican government. An internationally recognized certificate of compliance under the Nagoya Protocol (ABSCH-IRCC-MX-207343-3) has been issued for such activities.
Bacterial strains and media
A. brasilense Sp7 and B. unamae MTI-641 were kindly provided by Dr. G. Alexandre (University of Tennessee, Knoxville, USA) and Dr. A. Hirsch (University of California, Los Angeles, USA), respectively. H. seropedicae Z152 (ATCC 35894) was provided by the ATCC (http://www.atcc.org/). Bacteria were grown in liquid BSE medium.
Sample collection
The rhizosphere and plant tissues that include stem, leaf, aerial roots, underground roots, and mucilage of Sierra Mixe maize were sampled during seasons 2010, 2011, and 2012 from Fields 1 and 2 in Sierra Mixe. For plant endophyte analysis, tissues were surface sterilized by rinsing with mqH2O, shaken gently in 70% ethanol for 5 minutes, placed into 1% hypochlorite bleach, gently stirred for 10 minutes, rinsed 3 times in mqH20, and dried in a laminar flow cabinet. Roots and stems were also dissected to remove epidermal tissues before extraction. For seed endophyte analysis, embryo and endosperm of Sierra Mixe, Hickory King, and B73 were withdrawn from the seeds by hand using a razor blade in a laminar flow cabinet and were collected in 1.5 ml sterile microcentrifuge tubes. For this study, the rhizosphere was defined as a layer of soil covering the outer surface of the root system that could be washed from roots in a buffer/detergent solution. Roots were first separated and shaken to remove loosely adhering soil. All soils and plant material samples were used immediately for DNA extraction. Soil fertility analysis, which included physical parameters, soil reaction, and salinity, was performed in AgroLab (Pachuma, Mexico).
Plant phenotyping
The number of nodes with aerial roots was monitored weekly (greenhouse) or after 14 weeks (field). The total number of aerial roots was quantified after 14 weeks. The disappearance of leaf wax and appearance of trichomes were monitored weekly to determine the transition between juvenile stage and adult stage in Sierra Mixe and Hickory King maize.
Greenhouse and field experiments, Madison, USA
For experiments in the greenhouse, seeds of Sierra Mixe of Fields 1 and 2 and Hickory King were surface sterilized and germinated as described previously. After 1 week, the seedlings are transplanted in 40-liter pots filled with a mix of sand and perlite (v:v) and grown in a high-ceiling greenhouse at the Biotron facility (University of Wisconsin, Madison, USA). Plants were watered twice a day for 2 minutes with half-strength of Hoagland solution. For experiments in the field, 3 independent plots of 20 plants per genotype were planted, with 3 border rows (B73) between each genotype. Sierra Mixe and Teosinte plants that were grown in Madison for the ARA were planted in the same field at the same time. This experiment was replicated in 3 different field plots.
Mucilage glycosyl composition
Glycosyl composition analysis was performed by combined gas chromatography/mass spectrometry (GC/MS) of the per-O-trimethylsilyl (TMS) derivatives of the monosaccharide methyl glycosides produced from the sample by acidic methanolysis. Methyl glycosides were first prepared from dry mucilage samples by methanolysis in 1 M HCl in methanol at 80 °C (18–22 hours), followed by re-N-acetylation with pyridine and acetic anhydride in methanol (for detection of amino sugars). The samples were then per-O-trimethylsilylated by treatment with Tri-Sil (Pierce) at 80 °C (0.5 hours). GC/MS analysis of the TMS methyl glycosides was performed on an HP 6890 GC interfaced to a 5975b MSD, using an All Tech EC-1 fused silica capillary column (30 m × 0.25 mm ID). The analysis was performed at the Complex Carbohydrate Center Research Center (CCRC) of University of Georgia, Athens, USA.
DNA extraction
DNA (80–150 ng μl−1) was extracted from 100 mg of the rhizosphere plant tissues using a DNA isolation kit (Mo Bio Laboratories, Carlsbad, USA). The PCR control for microbial DNA isolation was performed on 16S rRNA genes. PCR was performed using eubacterial primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′), and the product was approximately a 1,450 bp fragment. Amplification was carried out with 1 μM of each primer in 3 mM MgCl2, 20 μM of each dNTP, 1.25 units of Taq polymerase (Promega) in a total volume of 20 μl of 1X reaction buffer (Promega, Madison, USA). PCR conditions included an initial denaturation at 95 °C for 3 minutes followed by 35 cycles of denaturation at 94 °C for 1 minute, annealing at 56 °C for 1 minute, and elongation at 72 °C for 1.5 minutes, with a final elongation at 72 °C for 7 minutes. DNA was resolved using an agarose gel run at 100 V for 30 minutes for analysis of total DNA and amplification of PCR products, respectively. Gels were visualized by ethidium bromide staining under UV light in a gel documentation system. The products obtained were purified with a NucleoSpin Gel extraction kit (Clontech, Palo Alto, USA).
Illumina-based 16S rRNA gene sequencing
16S rRNA gene PCR and sequencing of rhizosphere and plant tissues were carried out using the Caporaso protocol [45]. We extended the Caporaso approach [46] to a dual barcode scheme for each sample and replaced the Golay barcodes with a different set of Illumina-compatible barcodes that were designed to balance base composition and tolerate up to 4 sequencing errors in barcode sequences. The forward primer used was AATGATACGGCGACCACCGAGATCTACAC[Barcode]TATGGTAATTGTGTGCCAGCMGCCGCGGTAA, and the reverse primer was CAAGCAGAAGACGGCATACGAGAT[Barcode]AGTCAGTCAGCCGGACTACHVGGGTWTCTAAT. The barcodes used were designed to allow pooling of multiple samples within a single MiSeq run. Ten cycles of PCR with barcoded primers were performed at low annealing temperature (55 °C); samples were then pooled and cleaned using a Qiagen column to remove the unincorporated primers. At this stage, an additional 10 or 20 cycles of PCR were performed on the pool using the Illumina paired-end flowcell primers with a higher annealing temperature (65 °C). The resulting PCR product was subjected to QC with an Agilent Bioanalyzer and estimated concentration using KAPA Biosystems qPCR kit. The samples were diluted to the appropriate loading concentration for a MiSeq run, spiked with 25% phiX control library, and sequenced using an Illumina MiSeq instrument with the manufacturer’s standard 150 nucleotides paired-end dual-index sequencing protocol and the custom sequencing primers.
The Illumina sequences were obtained from 2 MiSeq runs (2X150 bp paired-end) and were demultiplexed using a custom script (https://figshare.com/s/04997ae7f7d18b53174a). The 822,804 reads thus obtained were trimmed to a Phred-equivalent of 20 and filtered for adaptor contamination using BBDuk (of the BBTool packages https://sourceforge.net/projects/bbmap/). Because of the low quality of the reverse mate pair, reads of the forward mate were used in the analysis. The reads were then preprocessed and analyzed using DADA2 [47]. Prescribed standard filtering parameters were used, such as PhiX contamination check and removal of reads with more than 2 errors or ambiguous bases or with an expected error greater than 2. Chimeras were identified and removed using the removeBimeraDenovo function of DADA2. The clean reads were then collapsed into sequence variants and classified using RDP training set (version 14). The sequence variants that were classified as chloroplast or mitochondria were removed from further analyses. From samples with library size ranging from 84 to 20,597 reads (mean of 4,703), 995 unique sequence variants were identified.
Alpha and Beta diversity metrics were generated using the Phyloseq 3.4.2 R packages [48]. Alpha diversity was calculated using Shannon and Simpson indices. Additionally, a PCoA plot based upon Bray-Curtis dissimilarity matrix was used to visualize the differences in samples. The sequence variant table was used to generate a heat map following the variance-stabilizing transformation in DESeq2 [49]. Permanova tests were run using the adonis function from the vegan 3.4.3.R package (https://CRAN.R-project.org/package=vegan) performed on the NMDS ordination.
Metagenomic sequencing
Illumina sequencing libraries from the same DNA extractions as above were made using an adaptation of the Nextera transposase-based library construction method with multiplex barcoding. Samples were then sequenced on the MiSeq and HiSeq instruments. Illumina sequences thus obtained were demultiplexed and trimmed using Trimmomatic (ver 0.33) [50] with the following parameter: Illuminaclip 2:30:10, Headcrop:15, Leading:20, Trailing:20, Sliding window:4:20, and Minlen:100. The reads were then screened for PhiX and maize sequences (genomic, chloroplast, and mitochondrial) using Bowtie2 aligner [51] against the PhiX genome (Genbank acc# NC_001422.1) and Z. mays cultivar B73 draft genome (RefSeq assembly acc# GCF_000005005.2). The clean reads were assigned taxonomy using Kaiju [52] with the nr database. To calculate the beta diversity of the samples, we used Phylosift (ver 1.0.1) [53], which identifies and places reads matching 37 conserved phylogenetic marker genes on a reference tree. From these placements, an Edge-PCA analysis [54] was carried using Guppy [54].
Nif gene search
Peptide sequences of the 6 core nif genes (nifH, nifD, nifE, nifK, nifN, nifB) and alternate nitrogenase (anfG, vnfG) from known diazotrophs as previously published [33] were retrieved from GenPept as a reference. A multiple-sequence alignment of these sequences was generated as a reference alignment using ClustalW2 [55]. A blast search (E-value < 0.001) of 6 frame-translated metagenomic reads was conducted against these reference sequences. The hits were then aligned against the multiple sequences alignment of reference using clustal-Omega [56] followed by generation of phylogenetic trees for every individual nif gene, using Fasttree2.1 [57] with a WAG model of amino acid evolution and gamma20 likelihood. The reads were assigned as belonging to the nif genes if they were inside the clade of the reference sequences. Each read that had significant similarity to one of the core nif genes was further analyzed by phylogenetic analysis to confirm its assignment as one of the 6 core nif genes. The counts of nif genes thus obtained were normalized by recA counts (determined using recA TIGRFAM HMM [58] with HMMER3 and an E-value cutoff of e−10).
Acetylene Reduction Assay ARA
For ARA with the mucilage, 2 ml of freshly collected mucilage from 1 or 2 aerial roots (Sierra Mixe) or several plants (teosinte) grown in the field were introduced in 14.5 ml vials (Wheaton, Millville, USA) that were tightly closed. For ARA with added bacteria, A. brasilense, H. seropedicae, and B. unamae were grown in BSE medium for 48 hours. Then, bacteria were collected by centrifugation (5 minutes, 4,000 × g) and suspended in the Fahraeus medium. The 14.5 ml vials, each containing 5 ml of mucilage previously stored for several months at –20 °C to reduce endogenous nitrogen-fixing bacteria, were inoculated with the bacterial suspension at a final OD600nm = 0.01. Control tubes were prepared either without bacteria or with 5 ml of Fahraeus medium instead of mucilage. Then, 850 μl of acetylene (Airgas) was injected into each vial. OD600nm was measured for each tube after 72 hours. For both conditions, controls without acetylene were performed in parallel. For ARA with aerial roots, 1 aerial root without mucilage was introduced in each 14.5 ml vial (10 replicates). One ml of acetylene (Airgas) was injected into each vial. For ARA with seedlings, Sierra Mixe seedlings were inoculated with A. brasilense and grown for 3 weeks. Plants were then transferred to 500 ml jars, and 50 ml of acetylene was injected in each jar. For ARA with underground roots, pieces of roots (about 10 cm long) were collected from plants grown in pots and introduced into 14.5 ml vials (3 replicates). Ethylene quantification was made by injecting 1 ml of the air phase, sampled after 72 hours, on a gas chromatography (GC-2010 Shimadzu) equipped with a Rt-Alumina BOND/KCL column (Restek).
Mucilage 15N2 assimilation
The enrichment of mucilage in 15N atom was achieved by removing 4 ml of headspace gas and replacing it with 4 ml of either 15N2 (Sigma-Aldrich) or 14N2 nitrogen gas directly into a vial containing 1.0 mL of mucilage. Mucilage was collected from Sierra Mixe maize plants grown in Sierra Mixe and stored at 4 °C for up to 2 weeks between sampling and the determination of 15N2 assimilation. The mucilage samples were incubated at 37 °C for 0 and 70 hours in the presence of 15N2. 15N2 assimilation was stopped by freezing the mucilage samples at −20 °C. The samples were then freeze-dried and weighed. The 15N2 analysis in the mucilage samples was performed at the UC Davis Stable Isotope Facility (Davis, USA) and the UW-Madison Soil Science Facility (Madison, USA). Statistical analysis was performed using SYSTAT version 10 (Chicago, USA).
Measurement of free-oxygen concentration
For measurement in collected mucilage, 2 ml of mucilage was introduced in a 15 ml tube. The probe (robust oxygen mini probe, Pyroscience) was introduced 8 mm deep in the mucilage and oxygen measurements performed until stabilization of the signal was observed. Control corresponds to free-oxygen concentration in the liquid Fahraeus medium. One-point calibration was made in aerated water, as advised by the manufacturer.
15N2 gas–enrichment experiments
Aerial roots were collected from Sierra Mixe maize grown at the Biotron greenhouse facility (University of Wisconsin, Madison, USA). Mucilage was generated from each of these aerial roots by incubating them in 5 ml of water at room temperature for 48 hours. Mucilage, along with the aerial roots, was inoculated with A. brasilense Sp7. Then, 10%–15% (v/v) of 15N2 gas was pumped into the vials, and the samples were incubated at 30 °C for 48 hours. After incubation of mucilage alone, or aerial roots alone, pheophytin extracted from these aerial roots was subjected to IRMS analysis. To obtain pheophytin, chlorophyll was extracted from aerial roots and converted to pheophytin by acid treatment, following as described [59]. 14N2-treated mucilage and aerial roots were used as negative controls.
15N natural abundance
The proportion (%) of nitrogen derived from biological nitrogen fixation (%Ndfa) was estimated from the 15N natural abundance (expressed in delta units, ‰) of the Sierra Mixe maize (δ15Nfixing plant) and that of the reference plant species (δ15Nref). In each of the 2011 and 2012 field seasons in Sierra Mixe, 90–114 individual maize samples (depending on the year) and 270 reference plant samples, representing 8–10 species (depending on the year) of non-nitrogen-fixing plants, were analyzed. For the single time point in 2010, 12 individual maize samples and 33 reference plant samples, representing 8 species of non-nitrogen-fixing plants, were analyzed. The reference plant species in the field were identified using universal 18S PCR analysis from DNA sampled using FTA Plant Saver card (GE Life Sciences, Pittsburg, USA) simultaneously with tissue samples collected for 15N analysis. PCRs were performed using Sigma’s Extract-N-Amp Plant PCR Kit according to the manufacturer for sequencing and BLASTN comparison. For 15N natural abundance, the third-youngest leaf of Sierra Mixe maize or reference plants was collected from Field 3 and 4 from the second to the sixth month postplanting and analyzed for N-isotope composition. Total organic nitrogen was determined by Kjeldahl digestion followed by steam distillation. Analysis for natural 15N abundance was carried out as described by Bremer and van Kessel [38]. The 15N analysis was performed at UCD Stable Isotope Facility (http://stableisotopefacility.ucdavis.edu/13cand15n.html). The percentage of nitrogen derived from nitrogen fixation (%Ndfa) was calculated as follows:
where “δ15N” is stable nitrogen isotopes, “ref” is the value from non-N-fixing reference plants, “fixing plant” is Sierra Mixe maize, and “B” is the 15N abundance in the air, assumed to be 0.0‰.
15N-enrichment field experiments
In 2016, 3 locations were chosen: Field 3, land that had not been planted to crops for over 10 years; Field 4, land that had maize for 1 year; and Field 5, land with continuous maize. A randomized complete block design trial was established at each site, with 5 replicates with 4 varieties. Each plot consisted of 6 matas surrounded by a common border of SM2 on all sides and a double border on outside rows. A mata is the traditional planting design in the Sierra Mixe region, similar to a hill plot in which multiple plants are seeded together. Each mata was planted with 5 seeds and thinned to 3 seeds for 18 plants per plot. Matas were planted in a grid 80 × 100 cm from each other. 15N was applied at a dose of 0.36 grams per plot in a liquid solution, reaching the desired enrichment of 1% for all 3 fields, with 50 ml added per mata.
In 2017, the same 3 locations were planted with same design and entries, except Field 3 consisted only of H377 and SM2 entries. In all 3 fields, 15N was applied at a dose of 0.95 grams per plot, reaching the desired enrichment of greater than 1%. A solution was spread evenly over each plot using a garden watering can, such that the whole experimental area received an equal amount of enriched 15N. Plants were covered with plastic bags at the time of application (V5) to ensure that 15N was not directly applied to the leaves and that the 15N was uniformly available to all plants.
Soil samples were taken from a 0–60 cm depth in each plot, blended, and sent for analysis at UC Davis Soil lab. Means were calculated across locations. In 2016, at V9 and V12, 1 mata (3 plants) was sampled; and at Tassel, 4 matas (12 plants) were sampled. In 2017, a single sampling of 6 matas (18 plants) was sampled at Tassel. For each sampling, plants were dug out to include all roots. Because of the high rainfall (2,100 mm concentrated in the growing season from June to October), roots were shallow for both reference and test varieties. Each plant was photographed, and data were recorded for the number of plants, plant height, the total fresh weight of shoots, and roots and stem diameter. Whole plants were chopped, ground, subsampled, and dried in an oven to record total dry weight for shoots and roots. Well-blended subsamples were taken and shipped to Davis to measure Total N and 15N. Total nitrogen and Atom% 15N were determined for each plot at each time point for the shoot. Total organic nitrogen was determined by Kjeldahl digestion followed by steam distillation. The 15N analysis was performed at UCD Stable Isotope Facility (http://stableisotopefacility.ucdavis.edu/13cand15n.html).
%Ndfa was calculated using the 15N-enrichment method [40] as
Atom% excess is calculated with sample value obtained from the UC Davis Stable Isotope Facility– 0.37 (n in air).
Nitrogen difference (%NDiff) method was calculated as
The average of Tornado F21 and H377 was used as the reference to calculate %Ndfa and %Ndiff. For Ndiff calculations, the area harvested was adjusted based on matas harvested at each time point.
Field data analyses
Data were analyzed using the R lme4 package. Data were checked for outliers and subjected to ANOVA and mean separation using Least Significant Difference (p = 0.05) for each location. LSDs were calculated only when ANOVA F-tests were significant at p = 0.05. For %Ndfa and %Ndiff, single-degree-of-freedom contrasts were calculated to compare test varieties to the mean of the reference varieties (P > 0.05). Pearson correlation coefficients were calculated between %Ndfa and %Ndiff.
Supporting information
(EPS)
(EPS)
The heat map depicts the abundances of (A) 1,000 most abundant SVs and (B) 20 most abundant SVs in the dataset that were transformed using variance-stabilizing transformation in DESeq2. The libraries are Mucilage (Blue; OLMC00, OLMD00, OLMV00, OLMX00), Aerial Root (Gray; OLAR00, OLAR02, OLAR04, OLAR05), Aerial Root with Mucilage (Pink; OLAR01, OLAR03), Stem (Green; OLST00, OLST01, OLST02, OLST03), Underground Root (Brown; OLUR01, OLUR02, OLUR03), and Rhizosphere (Magenta; OXRZ11, OXRZ12, OXRZ13, OXRZ21, OXRZ22, OXRZ23, OXRZ32, OXRZ31, OXRZ33).
(EPS)
Significant nitrogenase activity was only found on aerial roots with mucilage.
(EPS)
(A) Number of nodes with aerial roots and (B) number of aerial roots observed on teosinte, Sierra Mixe maize, and Hickory King after 14 weeks. Bar = standard error of the mean. Different letters indicate statistically supported groups according to the Kruskal-Wallis test.
(EPS)
(A) Oxygen measured at 3 depths in Fahraeus medium with (black bars) or without (gray bars) 0.2% agar. (B) Effect of the different sugars present in the mucilage on the ability of H. seropedicae, (C) A. brasilense, and (D) B. unamae to reduce acetylene.
(EPS)
Plants grown in Sierra Mixe during 2010 (light gray bars), 2011 (dark grey bars), and 2012 (black bars) were evaluated for %Ndfa; values were calculated using δ15N values in Table 1. Bar = standard error of the mean. %Ndfa, percent of nitrogen derived from the atmosphere.
(EPS)
From each location, 6 leaf samples were randomly sampled from Sierra Mixe maize plants and 6 leaf samples from each of 2 reference plants. The third emergent leaf of each maize plant was sampled. Reference plants were selected from the most abundant weed species within each sample location, and from a plant family (Asteraceae and Ranunculaceae) that is neither actinorhizal nor leguminous nor has members known to associate with diazotrophic bacteria. δ15N was determined for each plant sampled, and %Ndfa was calculated for Sierra Mixe maize according to the equation 2 in [19]. Values are given as mean and s.e. Different letters indicate statistically supported groups (one-way ANOVA, P < 0.05). %Ndfa, percent of nitrogen derived from the atmosphere.
(DOCX)
Data are from a single sampling date (May 2012) in Sierra Mixe maize, with 30 replicates analyzed for each sample reported.
(DOCX)
(DOCX)
Numbers followed by different letters are significantly different based on Least Significant difference at p = 0.05.
(DOCX)
(A) Macroelements and soil characteristics. (B) Microelements for fields in 2017.
(DOCX)
Acknowledgments
This paper is dedicated to the life and memory of Cristobal Heitmann. Cristobal’s energy and enthusiasm was a major catalyst in completing this research. He died tragically while the manuscript was under review. We thank Vicente Vasquez and Maria del Refugio Vasquez for assistance in developing the program in Mexico; Carmen Ortega and Saulon Zamora for sample collection and field trial assistance; Shawn Kaeppler, Natalia de Leon, and Jillian Foerster for field assistance; Nguyet Dao for microbial 15N-fixation assays; Harry Read for processing 15N2-enrichment samples; John Zhang for assistance with library construction; and Armando Garcia-Llanos for assistance in sample processing. We thank the Comisiriado of the Sierra Mixe, Mexico, for their support and access to community genetic resources.
Abbreviations
- %Ndfa
percent of nitrogen derived from the atmosphere
- %Ndiff
percent total nitrogen difference
- ARA
acetylene reduction assay
- CCRC
Complex Carbohydrate Center Research Center
- GC/MS
gas chromatography/mass spectrometry
- IRMS
isotope-ratio mass spectrometry
- NPGS
National Plant Germplasm System
- PCoA
principal component analysis
- TMS
trimethylsilyl
Data Availability
All numerical data are now posted at DOI: 10.6084/m9.figshare.6534545 and all DNA/RNA sequence data at https://figshare.com/s/04997ae7f7d18b53174a.
Funding Statement
Mars, Incorporated http://www.mars.com/global. The research was funded by an unrestricted gift and a grant to ABB. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The research was funded by grants to ABB from BioN2, Incorporated. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Preliminary research was supported by a Dissertation Research Grant from UC Mexus to KS. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bulgarelli D, Rott M, Schlaeppi K, van Themaat EVL, Ahmadinejad N, Assenza F, et al. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature. 2012;488(7409):91–5. 10.1038/nature11336 [DOI] [PubMed] [Google Scholar]
- 2.Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, Malfatti S, et al. Defining the core Arabidopsis thaliana root microbiome. Nature. 2012;488(7409):86–+. 10.1038/nature11237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peiffer JA, Spor A, Koren O, Jin Z, Tringe SG, Dangl JL, et al. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(16):6548–53. 10.1073/pnas.1302837110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roesch LFW, Camargo FAO, Bento FM, Triplett EW. Biodiversity of diazotrophic bacteria within the soil, root and stem of field-grown maize. Plant and Soil. 2008;302(1–2):91–104. 10.1007/s11104-007-9458-3 [DOI] [Google Scholar]
- 5.Triplett EW. Diazotrophic endophytes: Progress and prospects for nitrogen fixation in monocots. Plant and Soil. 1996;186(1):29–38. 10.1007/bf00035052 [DOI] [Google Scholar]
- 6.Mus F, Crook M, Garcia K, Costas A, Geddes B, Kouri E, et al. Symbiotic Nitrogen Fixation and the Challenges to Its Extension to Nonlegumes. Applied and Environmental Microbiology. 2016;82(13):3698–710. 10.1128/AEM.01055-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beatty P, Good A. Future Prospects for Cereals That Fix Nitrogen. Science. 2011;333(6041):416–7. 10.1126/science.1209467 [DOI] [PubMed] [Google Scholar]
- 8.Urquiaga S, Xavier RP, de Morais RF, Batista RB, Schultz N, Leite JM, et al. Evidence from field nitrogen balance and N-15 natural abundance data for the contribution of biological N-2 fixation to Brazilian sugarcane varieties. Plant and Soil. 2012;356(1–2):5–21. 10.1007/s11104-011-1016-3 [DOI] [Google Scholar]
- 9.Luo T, Ou-Yang XQ, Yang LT, Li YR, Song XP, Zhang GM, et al. Raoultella sp strain L03 fixes N-2 in association with micropropagated sugarcane plants. Journal of Basic Microbiology. 2016;56(8):934–40. 10.1002/jobm.201500738 [DOI] [PubMed] [Google Scholar]
- 10.Sevilla M, Burris R, Gunapala N, Kennedy C. Comparison of benefit to sugarcane plant growth and N-15(2) incorporation following inoculation of sterile plants with Acetobacter diazotrophicus wild-type and Nif(-) mutant strains. Molecular Plant-Microbe Interactions. 2001;14(3):358–66. 10.1094/MPMI.2001.14.3.358 [DOI] [PubMed] [Google Scholar]
- 11.Keymer D, Kent A. Contribution of nitrogen fixation to first year Miscanthus x giganteus. Global Change Biology Bioenergy. 2014;6(5):577–86. 10.1111/gcbb.12095 [DOI] [Google Scholar]
- 12.Pankievicz V, do Amaral F, Santos K, Agtuca B, Xu Y, Schueller M, et al. Robust biological nitrogen fixation in a model grass-bacterial association. Plant Journal. 2015;81(6):907–19. 10.1111/tpj.12777 [DOI] [PubMed] [Google Scholar]
- 13.Okon Y, Heytler P, Hardy R. N2-fixation by Azospirillum-brasilense and its incorporation into host Setaria-italica. Applied and Environmental Microbiology. 1983;46(3):694–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hurek T, Handley L, Reinhold-Hurek B, Piche Y. Azoarcus grass endophytes contribute fixed nitrogen to the plant in an unculturable state. Molecular Plant-Microbe Interactions. 2002;15(3):233–42. 10.1094/MPMI.2002.15.3.233 [DOI] [PubMed] [Google Scholar]
- 15.Boddey R, DeOliviera O, Urquiaga S, Reis V, DeOliveres F, Baldini V, et al. Biological nitrogen-fixation associated with sugar-cane and rice—contributions and prospects for improvement. Plant and Soil. 1995;174(1–2):195–209. 10.1007/BF00032247 [DOI] [Google Scholar]
- 16.Gyaneshwar P, James E, Reddy P, Ladha J. Herbaspirillum colonization increases growth and nitrogen accumulation in aluminium-tolerant rice varieties. New Phytologist. 2002;154(1):131–45. 10.1046/j.1469-8137.2002.00371.x [DOI] [Google Scholar]
- 17.Iniguez A, Dong Y, Triplett E. Nitrogen fixation in wheat provided by Klebsiella pneumoniae 342. Molecular Plant-Microbe Interactions. 2004;17(10):1078–85. 10.1094/MPMI.2004.17.10.1078 [DOI] [PubMed] [Google Scholar]
- 18.Montanez A, Abreu C, Gill P, Hardarson G, Sicardi M. Biological nitrogen fixation in maize (Zea mays L.) by N-15 isotope-dilution and identification of associated culturable diazotrophs. Biology and Fertility of Soils. 2009;45(3):253–63. 10.1007/s00374-008-0322-2 [DOI] [Google Scholar]
- 19.Santos C, Vieira J, Sellstedt A, Normand P, Moradas-Ferreira P, Tavares F. Modulation of Frankia alni ACN14a oxidative stress response: activity, expression and phylogeny of catalases. Physiologia Plantarum. 2007;130(3):454–63. 10.1111/j.1399-3054.2007.00868.x [DOI] [Google Scholar]
- 20.deSalamone I, Dobereiner J, Urquiaga S, Boddey R. Biological nitrogen fixation in Azospirillum strain-maize genotype associations as evaluated by the N-15 isotope dilution technique. Biology and Fertility of Soils. 1996;23(3):249–56. 10.1007/s003740050168 [DOI] [Google Scholar]
- 21.Boddey R, Alves B, Urquiaga S, Malik K, Mirza M, Ladha J. Evaluation of biological nitrogen fixation associated with non-legumes. Nitrogen Fixation With Non-Legumes. 1998;79:287–305. [Google Scholar]
- 22.Hardarson G. Methods for enhancing symbiotic nitrogen-fixation. Plant and Soil. 1993;152(1):1–17. 10.1007/BF00016329 [DOI] [Google Scholar]
- 23.Estrada P, Mavingui P, Cournoyer B, Fontaine F, Balandreau J, Caballero-Mellado J. A N-2-fixing endophytic Burkholderia sp associated with maize plants cultivated in Mexico. Canadian Journal of Microbiology. 2002;48(4):285–94. 10.1139/w02-023 [DOI] [PubMed] [Google Scholar]
- 24.McCully ME, Boyer JS. The expansion of maize root-cap mucilage during hydration .3. Changes in water potential and water content. Physiologia Plantarum. 1997;99(1):169–77. 10.1111/j.1399-3054.1997.tb03445.x [DOI] [Google Scholar]
- 25.Sealey LJ, McCully ME, Canny MJ. The expansion of maize root-cap mucilage during hydration. 1. Kinetics. Physiologia Plantarum. 1995;93(1):38–46. 10.1034/j.1399-3054.1995.930107.x [DOI] [Google Scholar]
- 26.Delaux PM, Radhakrishnan GV, Jayaraman D, Cheem J, Malbreil M, Volkening JD, et al. Algal ancestor of land plants was preadapted for symbiosis. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(43):13390–5. 10.1073/pnas.1515426112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dennis PG, Miller AJ, Hirsch PR. Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? Fems Microbiology Ecology. 2010;72(3):313–27. 10.1111/j.1574-6941.2010.00860.x [DOI] [PubMed] [Google Scholar]
- 28.Knee EM, Gong FC, Gao MS, Teplitski M, Jones AR, Foxworthy A, et al. Root mucilage from pea and its utilization by rhizosphere bacteria as a sole carbon source. Molecular Plant-Microbe Interactions. 2001;14(6):775–84. 10.1094/MPMI.2001.14.6.775 [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Fu Y, Huang J, Wu C, Zheng C. Transcript profiling during the early development of the maize brace root via Solexa sequencing. Febs Journal. 2011;278(1):156–66. 10.1111/j.1742-4658.2010.07941.x [DOI] [PubMed] [Google Scholar]
- 30.Hoppe D, McCully M, Wenzel C. The nodal roots of Zea—Their development in relation to structural features of the stem. Canadian Journal of Botany-Revue Canadienne De Botanique. 1986;64(11):2524–37. 10.1139/b86-335 [DOI] [Google Scholar]
- 31.Hochholdinger F, Woll K, Sauer M, Dembinsky D. Genetic dissection of root formation in maize (Zea mays) reveals root-type specific developmental programmes. Annals of Botany. 2004;93(4):359–68. 10.1093/aob/mch056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacic A, Moody SF, Clarke AE. Structural-analysis of secreted root slime from maize (Zea-mays-L). Plant Physiology. 1986;80(3):771–7. 10.1104/pp.80.3.771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dos Santos PC, Fang Z, Mason SW, Setubal JC, Dixon R. Distribution of nitrogen fixation and nitrogenase-like sequences amongst microbial genomes. Bmc Genomics. 2012;13 10.1186/1471-2164-13-162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vessey JK. Measurement of nitrogenase activity in legume root-nodules—In defense of the acetylene-reduction assay. Plant and Soil. 1994;158(2):151–62. 10.1007/bf00009490 [DOI] [Google Scholar]
- 35.Hunt S, Layzell DB. Gas-exchange of legume nodules and the regulation of nitrogenase activity. Annual Review of Plant Physiology and Plant Molecular Biology. 1993;44:483–511. 10.1146/annurev.pp.44.060193.002411 [DOI] [Google Scholar]
- 36.Marchal K, Vanderleyden J. The "oxygen paradox" of dinitrogen-fixing bacteria. Biology and Fertility of Soils. 2000;30(5–6):363–73. 10.1007/s003740050017 [DOI] [Google Scholar]
- 37.Boddey RM, Polidoro JC, Resende AS, Alves BJR, Urquiaga S. Use of the N-15 natural abundance technique for the quantification of the contribution of N-2 fixation to sugar cane and other grasses. Australian Journal of Plant Physiology. 2001;28(9):889–95. 10.1071/pp01058 [DOI] [Google Scholar]
- 38.Bremer E, Vankessel C. Appraisal of the N-15 natural-abundance method for quantifying dinitrogen fixation. Soil Science Society of America Journal. 1990;54(2):404–11. 10.2136/sssaj1990.03615995005400020018x [DOI] [Google Scholar]
- 39.Teixeira FCP, Reinert F, Rumjanek NG, Boddey RM. Quantification of the contribution biological nitrogen fixation to Cratylia mollis using the N-15 natural abundance technique in the semi-arid Caatinga region of Brazil. Soil Biology & Biochemistry. 2006;38(7):1989–93. 10.1016/j.soilbio.2005.11.013 [DOI] [Google Scholar]
- 40.Doughton JA, Saffigna PG, Vallis I, Mayer RJ. Nitrogen-fixation in chickpea. 2. Comparison of N-15 enrichment and N-15 natural-abundance methods for estimating nitrogen-fixation. Australian Journal of Agricultural Research. 1995;46(1):225–36. 10.1071/ar9950225 [DOI] [Google Scholar]
- 41.S.W. R, J.J. H, G.O. B. How a corn plant develops. Iowa State Univ. Coop Ext. Serv. Spec. Rep. 48: Iowa State Univ., Ames.; 1986.
- 42.Wilson S, Bottjer D, Church M, Karl D. Comparative Assessment of Nitrogen Fixation Methodologies, Conducted in the Oligotrophic North Pacific Ocean. Applied and Environmental Microbiology. 2012;78(18):6516–23. 10.1128/AEM.01146-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hudd G, Lloydjones C, Hillcottingham D. Comparison of acetylene-reduction and N-15 techniques for the determination of nitrogen-fixation by field bean (Vicia-faba) nodules. Physiologia Plantarum. 1980;48(1):111–5. 10.1111/j.1399-3054.1980.tb03227.x [DOI] [Google Scholar]
- 44.Li R, Han Y, Lv P, Du R, Liu G. Molecular mapping of the brace root traits in sorghum (Sorghum bicolor L. Moench). Breeding Science. 2014;64(2):193–8. 10.1270/jsbbs.64.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caporaso J, Lauber C, Walters W, Berg-Lyons D, Lozupone C, Turnbaugh P, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4516–22. 10.1073/pnas.1000080107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caporaso J, Lauber C, Walters W, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. Isme Journal. 2012;6(8):1621–4. 10.1038/ismej.2012.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nature Methods. 2016;13(7):581–+. 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMurdie P, Holmes S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE. 2013;8(4). 10.1371/journal.pone.0061217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Love M, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014;15(12). 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bolger A, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Langmead B, Salzberg S. Fast gapped-read alignment with Bowtie 2. Nature Methods. 2012;9(4):357–U54. 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Menzel P, Ng K, Krogh A. Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nature Communications. 2016;7 10.1038/ncomms11257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Darling A, Jospin G, Lowe E, Matsen FI, Bik H, Eisen J. PhyloSift: phylogenetic analysis of genomes and metagenomes. Peerj. 2014;2 10.7717/peerj.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsen F, Evans S. Edge Principal Components and Squash Clustering: Using the Special Structure of Phylogenetic Placement Data for Sample Comparison. PLoS ONE. 2013;8(3). 10.1371/journal.pone.0056859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Larkin M, Blackshields G, Brown N, Chenna R, McGettigan P, McWilliam H, et al. Clustal W and clustal X version 2.0. Bioinformatics. 2007;23(21):2947–8. 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
- 56.Sievers F, Wilm A, Dineen D, Gibson T, Karplus K, Li W, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology. 2011;7 10.1038/msb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Price M, Dehal P, Arkin A. FastTree 2-Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE. 2010;5(3). 10.1371/journal.pone.0009490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haft D, Selengut J, White O. The TIGRFAMs database of protein families. Nucleic Acids Research. 2003;31(1):371–3. 10.1093/nar/gkg128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kahn ML, Parra-Colmenares A, Ford CL, Kaser F, McCaskill D, Ketchum RE. A mass spectrometry method for measuring N-15 incorporation into pheophytin. Analytical Biochemistry. 2002;307(2):219–25. 10.1016/s0003-2697(02)00046-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(EPS)
(EPS)
The heat map depicts the abundances of (A) 1,000 most abundant SVs and (B) 20 most abundant SVs in the dataset that were transformed using variance-stabilizing transformation in DESeq2. The libraries are Mucilage (Blue; OLMC00, OLMD00, OLMV00, OLMX00), Aerial Root (Gray; OLAR00, OLAR02, OLAR04, OLAR05), Aerial Root with Mucilage (Pink; OLAR01, OLAR03), Stem (Green; OLST00, OLST01, OLST02, OLST03), Underground Root (Brown; OLUR01, OLUR02, OLUR03), and Rhizosphere (Magenta; OXRZ11, OXRZ12, OXRZ13, OXRZ21, OXRZ22, OXRZ23, OXRZ32, OXRZ31, OXRZ33).
(EPS)
Significant nitrogenase activity was only found on aerial roots with mucilage.
(EPS)
(A) Number of nodes with aerial roots and (B) number of aerial roots observed on teosinte, Sierra Mixe maize, and Hickory King after 14 weeks. Bar = standard error of the mean. Different letters indicate statistically supported groups according to the Kruskal-Wallis test.
(EPS)
(A) Oxygen measured at 3 depths in Fahraeus medium with (black bars) or without (gray bars) 0.2% agar. (B) Effect of the different sugars present in the mucilage on the ability of H. seropedicae, (C) A. brasilense, and (D) B. unamae to reduce acetylene.
(EPS)
Plants grown in Sierra Mixe during 2010 (light gray bars), 2011 (dark grey bars), and 2012 (black bars) were evaluated for %Ndfa; values were calculated using δ15N values in Table 1. Bar = standard error of the mean. %Ndfa, percent of nitrogen derived from the atmosphere.
(EPS)
From each location, 6 leaf samples were randomly sampled from Sierra Mixe maize plants and 6 leaf samples from each of 2 reference plants. The third emergent leaf of each maize plant was sampled. Reference plants were selected from the most abundant weed species within each sample location, and from a plant family (Asteraceae and Ranunculaceae) that is neither actinorhizal nor leguminous nor has members known to associate with diazotrophic bacteria. δ15N was determined for each plant sampled, and %Ndfa was calculated for Sierra Mixe maize according to the equation 2 in [19]. Values are given as mean and s.e. Different letters indicate statistically supported groups (one-way ANOVA, P < 0.05). %Ndfa, percent of nitrogen derived from the atmosphere.
(DOCX)
Data are from a single sampling date (May 2012) in Sierra Mixe maize, with 30 replicates analyzed for each sample reported.
(DOCX)
(DOCX)
Numbers followed by different letters are significantly different based on Least Significant difference at p = 0.05.
(DOCX)
(A) Macroelements and soil characteristics. (B) Microelements for fields in 2017.
(DOCX)
Data Availability Statement
All numerical data are now posted at DOI: 10.6084/m9.figshare.6534545 and all DNA/RNA sequence data at https://figshare.com/s/04997ae7f7d18b53174a.