Abstract
The dynamic shape of the endoplasmic reticulum (ER) is a reflection of its wide variety of critical cell biological functions. Consequently, perturbation of ER-shaping proteins can cause a range of human phenotypes. Here, we describe three affected children (from two consanguineous families) who carry homozygous loss-of-function mutations in LNPK (previously known as KIAA1715); this gene encodes lunapark, which is proposed to serve as a curvature-stabilizing protein within tubular three-way junctions of the ER. All individuals presented with severe psychomotor delay, intellectual disability, hypotonia, epilepsy, and corpus callosum hypoplasia, and two of three showed mild cerebellar hypoplasia and atrophy. Consistent with a proposed role in neurodevelopmental disease, LNPK was expressed during brain development in humans and mice and was present in neurite-like processes in differentiating human neural progenitor cells. Affected cells showed the absence of full-length lunapark, aberrant ER structures, and increased luminal mass density. Together, our results implicate the ER junction stabilizer lunapark in establishing the corpus callosum.
Keywords: lunapark, KIAA1715, endoplasmic reticulum, organelle morphology, epilepsy, corpus callosum hypoplasia, hypotonia, human genetics, recessive disease
Main Text
The endoplasmic reticulum (ER) has numerous cellular functions that require distinct properties and shapes.1, 2 Therefore, the dynamic transition between and maintenance of distinct states are a tightly regulated process that involves a number of effectors and regulators.3 Mutations in genes that encode these proteins have been implicated in a range of human disorders, including Alzheimer disease and hereditary spastic paraplegia (HSP), the latter of which has been studied extensively in connection with ER-shaping proteins.4, 5 For instance, heterozygous mutations in ATL1 (MIM: 606439), encoding Atlastin-1, cause autosomal-dominant spastic paraplegia 3A (SPG3A [MIM: 182600]) and are the second most common cause of HSP.4, 5, 6 SPG12 (MIM: 604805), SPG31 (MIM: 610250), and SPG72 (MIM: 515626) are likewise associated with mutations in REEP1 (MIM: 609139), RTN2 (MIM: 603183), and REEP2 (MIM: 609347), respectively.7, 8, 9 These and other mutations have provided a strong link between ER-shaping proteins and the health of neurons that have extended axonal projections, such as the corticospinal motor neurons that exhibit the hallmark axonopathy in HSP.5
The transmembrane protein lunapark (encoded by LNPK, also known as KIAA1715 [MIM: 610236]) is a more recent addition to the extended list of proteins that are involved in regulating ER shape.10 Lnpk, the mouse homolog of this gene, was originally described as part of the HoxD cluster that is critical for digit development and was named after the conserved L-N-P-A-R-K peptide motif found in its protein product.11, 12 The protein consists of an N-terminal domain important in N-myristoylation and ER targeting, two transmembrane domains, and a unique zinc-finger domain with a proposed role in oligomerization.13, 14 Current evidence and models suggest that lunapark mainly acts as a stabilizer of negative membrane curvature and thereby maintains three-way junctions of tubular ER through its localization to these structures.13, 15, 16 Although its cellular role has been described, its functional importance in mammals is currently unclear. The sole organismal phenotype to date was described in C. elegans and comprises locomotor defects and mislocalization of presynaptic proteins.17
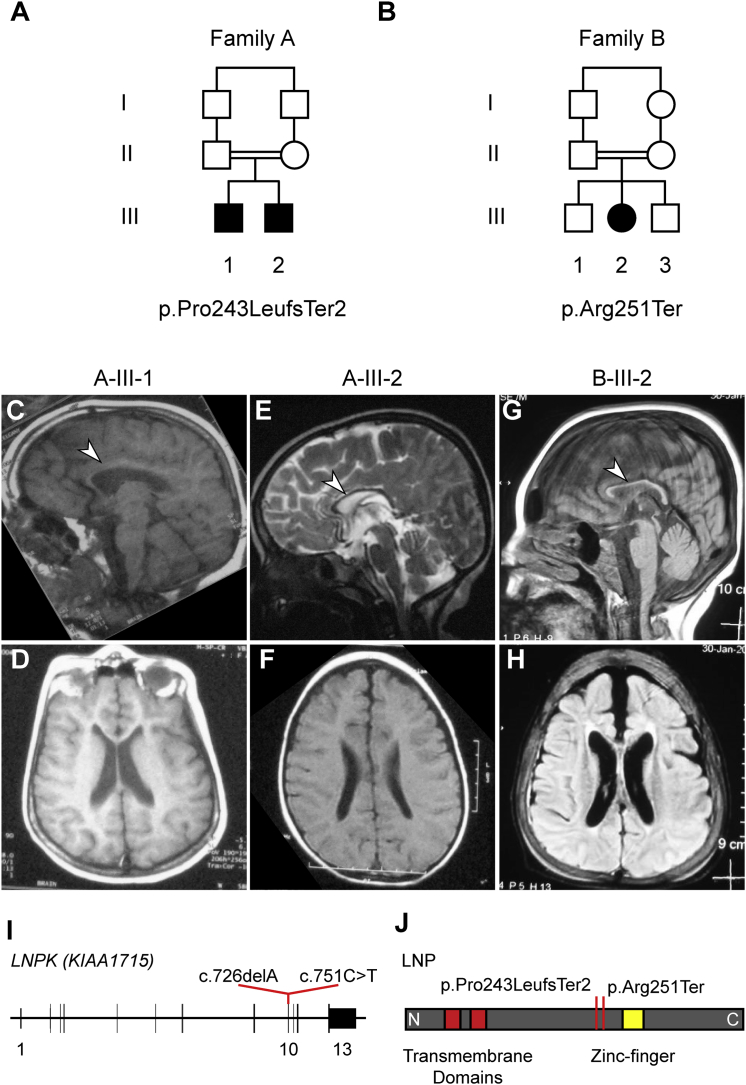
We identified two consanguineous families that carry loss-of-function (LoF) LNPK mutations that are associated with severe neurodevelopmental disease. Family A included two affected children (A-III-1 and A-III-2), both diagnosed with global developmental delay and seizures, from a first-cousin marriage (Figure 1A, Table 1, and Table S1). There were no reported prenatal or birth complications, and weight, length, and head circumference were unremarkable. At the last examination (15 and 7 years of age for A-III-1 and A-III-2, respectively), both individuals exhibited psychomotor developmental delay, intellectual disability, defects in social interactions, hyperactivity, and inattention. The older child was more severely affected and showed signs of regression and affected motor functions, including hypotonia, rigidity, and an inability to speak or walk, whereas the younger sibling suffered from only mild hypotonia and abnormal gait (Table 1 and Table S1). Both exhibited mainly myoclonic seizures as early as 2 years of age. Whereas the younger sibling’s epilepsy was controlled with anticonvulsants, the older sibling’s seizures were refractory (Table 1 and Table S1). Brain MRI of both children revealed no involvement of the cortical structures but did show corpus callosum hypoplasia in both and mild cerebellar vermis hypoplasia in the younger child (Figures 1C–1F).
Figure 1.
Clinical and Genetic Information for families A and B
(A and B) Family pedigrees for families A and B. Affected children in both families are from consanguineous parents (double line indicates first cousins).
(C–H) T1-weighted (D and F–H), T2-weighted (E), and FLAIR (C) MRI for individuals A-III-1 (C and D), A-III-2 (E and F), and B-III-2 (G and H). Shown are sagittal (C, E, and G) and axial (D, F, and H) images. Arrowheads indicate the corpus callosum hypoplasia common to all three affected children. Other brain regions appear largely unaffected with the exception of mild cerebellar affectation in A-III-2 and B-III-2.
(I) Schematic of LNPK depicts the genomic sequence spanning 13 exons. Red lines indicate the position of the two mutations in exon 10 and show their coordinates within the cDNA (GenBank: NM_030650.2).
(J) Schematic of the lunapark protein (LNP) depicts the location of the two transmembrane domains (red) and the zinc finger (yellow). N and C indicate the N and C termini of the protein, respectively. Red lines indicate the position of the two truncating mutations upstream of the zinc finger and the effect of the two mutations on the protein level (GenBank: NP_085153.1).
Table 1.
Mutations in LNPK Cause Various Phenotypes, Including Corpus Callosum Hypoplasia, Hypotonia, and Epilepsy
|
Individual |
|||
|---|---|---|---|
| A-III-1 | A-III-2 | B-III-2 | |
| Genomic mutation | chr2: 176804365GT>G (hg19a) | chr2: 176804365GT>G (hg19a) | chr2: 176804341G>A (hg19a) |
| cDNA mutation | c.726delA | c.726delA | c.751C>T |
| Protein variant | p.Pro243LeufsTer2 | p.Pro243LeufsTer2 | p.Arg251Ter |
| Gender | male | male | female |
| Origin | Egypt | Egypt | Pakistan |
| Consanguinity | first cousin | first cousin | first cousin |
| Age at diagnosis | 15 years | 7 years, 4 months | 14 months |
| Psychomotor Development | |||
| Gross motor skills | delayed | delayed | significantly delayed |
| Fine motor skills | delayed | delayed | significantly delayed |
| Language skills | absent | delayed | significantly delayed |
| Social skills | delayed | delayed | significantly delayed |
| Regression | progressive | stationary | progressive (bedridden) |
| Neurological Findings | |||
| Higher cognitive functions | severe intellectual disability, no speech, autistic features, very limited social interaction, hyperactivity, inattention, dementia | intellectual disability, a few unclear words, mild autistic features, hyperactive, inattention, minimal aggressiveness | vegetative state |
| Extrapyramidal symptoms | rigidity, drooling | no data | rigidity |
| Cerebellar deficits | no | no | bedridden |
| Motor deficits | hypertonia, only crawling | ambulatory | flaccid |
| Muscle tone | hypotonia with rigidity | mild hypotonia | flaccid |
| Reflexes | present | present | flaccid |
| Sensory | normal | normal | not possible |
| Gait | incapable | wide based | ataxia |
| Seizures | |||
| Age of onset | 2 years | 2 years | 1 year |
| Type(s) | myoclonic, tonic, and extension spasms | myoclonic | generalized tonic clonic |
| Most frequent type | myoclonic | myoclonic | generalized tonic clonic |
| Frequency | every several days | controlled | unknown |
| Treatment | valproate, levetiracetum, clonazepam | valproate, levetiracetum | valporate, carbamazepine |
| MRI Findings | |||
| Corpus callosum | hypoplasia | hypoplasia | hypoplasia |
| Cerebellum | normal | mild vermian hypoplasia | atrophy |
UCSC Genome Browser hg19.
Family B had one affected girl (B-III-2) and two healthy siblings from a first-cousin marriage (Figure 1B). There were no reported prenatal or birth complications, and weight, length, and head circumference were unremarkable. The girl’s last examination (at 13.5 years of age) revealed a significantly more severe clinical course than what was observed in family A: she was bedridden in a vegetative state and showed severe tremor and dysmetria with purposeful movement (Table 1 and Table S1). Her phenotype was slowly progressive, and she experienced frequent infections. She had generalized tonic-clonic epileptic seizures starting at 1 year of age, and her brain MRI showed corpus callosum hypoplasia and mild cerebellar atrophy; no abnormalities were seen in other brain areas (Figures 1G and 1H).
After informed consent was acquired from all participating individuals in accordance with the ethical standards of the responsible committee on human experimentation at the University of California, San Diego, whole-exome sequencing (WES) was performed on blood-derived DNA from both affected children from family A and the mother, father, and affected girl from family B. Blood-derived DNA from both parents in family A and both unaffected siblings in family B was used for segregation testing. For variant identification and prioritization, we employed our internal pipeline, which implemented minor allele frequency (public and internal), as well as PolyPhen-2 prediction and GERP scores as previously described (see also Supplemental Material and Methods).18, 19 Only one remaining candidate in family A segregated with the phenotype: a frameshift-causing deletion in exon 10 of LNPK (c.726delA [p.Pro243LeufsTer2] [GenBank: NM_030650.2]) (Figures 1I and 1J). Likewise, in family B, we identified only one high-impact variant that was located in LNPK (c.751C>T [GenBank: NM_030650.2]) and resulted in a LoF allele (p.Arg251Ter) (Figures 1I and 1J). Thus, the similar location of the LoF variants, as well as the matching phenotypes, rendered the LNPK mutation a likely disease-causing candidate.
Neither of the LNPK variants was reported in our unrelated cohort of over 5,000 individuals (the GME Variome) or in the Exome Aggregation Consortium (ExAC) Browser or gnomAD.19, 20 Consistent with the fact that this gene is sensitive to LoF mutations, no other homozygous LoF mutations have been reported in the ExAC Browser; likewise, its pNull score, a metric that reflects the likelihood that a given gene is neutral to both heterozygous and homozygous loss, is 0.00.20 Both variants are adjacent to or within a stretch of extended homozygosity (HomozygosityMapper; Figure S1) and are also predicted to cause LoF by MutationTaster.21, 22 The mutations in families A and B fall within the same exon and introduce premature stop codons that should both result in truncations of the protein product before the two zinc fingers (Figures 1I–1J and Figure S2).
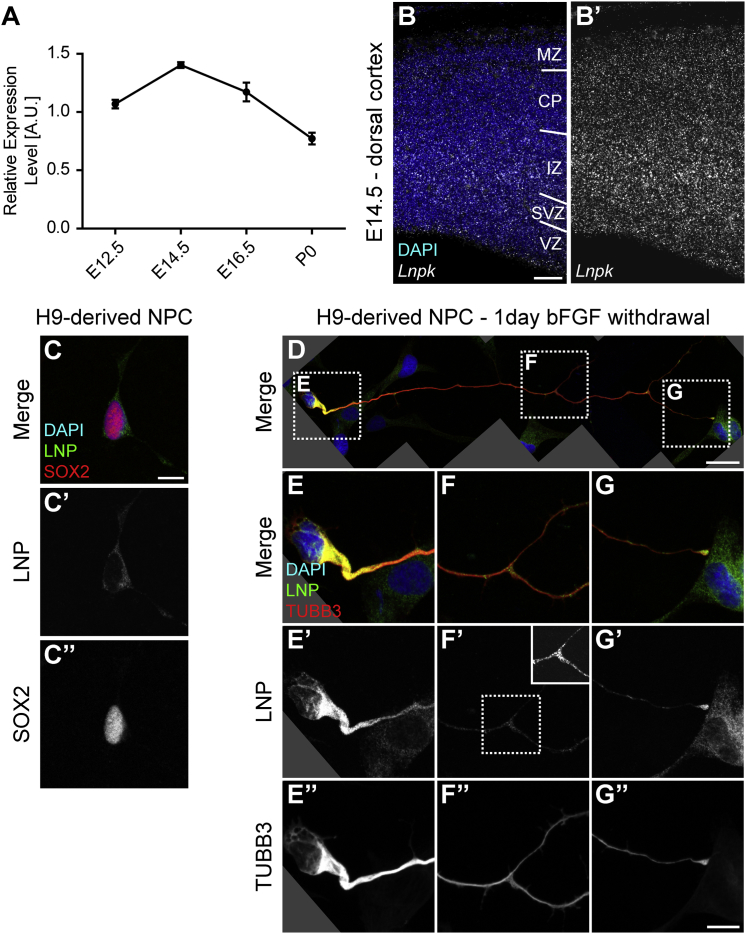
LNPK was found to be ubiquitously expressed in adult human tissues (Genotype-Tissue Expression [GTEx] data) and in human (BrainSpan data) and murine brain development at all assessed stages (Figure 2A and Figures S3 and S4). Spatially, at embryonic day 14.5 (E14.5), its expression was detected across all areas of the developing murine brain by in situ hybridization (Figure 2B and Figure S5). Both dorsal and ventral cortices showed similar localization of the transcript across the entire area, suggesting that Lnpk is expressed in both progenitor cells and postmitotic neurons in mice.
Figure 2.
Localization of Lunapark in Mice and Humans
(A) Graph showing the expression of mouse Lnpk in relation to the geometric mean of three housekeeping genes via quantitative RT-PCR. Time points range from embryonic day 12.5 (E12.5) to postnatal day 0 (P0). Graph shows mean ± SEM for each time point. n = 3 animals.
(B) Image of in situ hybridization using RNAscope against the Lnpk mRNA transcript. Shown are DAPI and the in situ signal. B′ shows the Lnpk signal alone for better visualization. Abbreviations are as follows: VZ, ventricular zone; SVZ, subventricular zone; IZ, intermediate zone; CP, cortical plate; and MZ, marginal zone.
(C) Image shows an H9-derived NPC stained with DAPI and antibodies against LNP and SOX2. C′ and C″ show gray-scale images of the indicated channels.
(D–G) Images show an H9-derived NPC, driven toward differentiation by 1 day of bFGF withdrawal, stained with DAPI and antibodies against LNP and TUBB3 (Tuj). Shown are an overview (D), magnification of the soma (E), magnification of a process branch point (F), and magnification of the tip of the extending process (G). In addition to the merged image (E–G), gray-scale images of the LNP and TUBB3 channels are shown (E′–G′ and E″–G″, respectively). LNP appeared to be upregulated in differentiating NPCs and also accumulated in branch points and the tip of the extending neurite that was reminiscent of an axonal growth cone. The inlay in (F′) shows the boxed area with higher contrast for better visualization of the increased signal of LNP at the branching point. Scale bars represent 50 μm (B), 10 μm (C and G″), and 25 μm (D).
The human lunapark protein (LNP) was likewise present in human neural precursor cells (NPCs) derived from embryonic stem cells (Figure 2C). In a differentiated state, driven by the withdrawal of FGF for 1 day, NPCs exhibited an overall increase in LNP intensity (Figure 2D). In addition, neurite-like processes extended by the differentiating cells accumulated LNP at branching sites and at the growth-cone-like structure at the tip (Figures 2D–2G). This is consistent with the occurrence of tubular ER at the equivalent neuronal structures.23 Together, the spatiotemporal expression patterns of human LNPK and its mouse ortholog are consistent with a role in neurodevelopment and support the pathogenicity of LoF mutations in this gene.
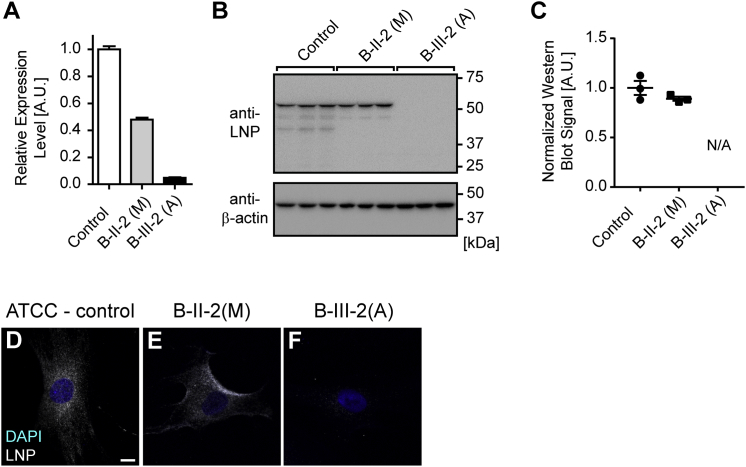
We obtained fibroblasts after acquiring informed consent from the affected girl and the mother in family B to test the impact of the premature stop variant (p.Arg251Ter). mRNA transcript levels, as assessed by quantitative RT-PCR, showed a notable reduction of the cells derived from the affected girl (B-III-2-(A)) (Figure 3A). Moreover, relative to the control fibroblasts, the maternal cells (B-II-2(M)) likewise exhibited reduced transcript levels of around 50%. Consequently, there was also no evidence of the full-length protein in whole-cell lysates from cells derived from the affected girl (Figures 3B and 3C). The protein also could not be detected by immunocytochemistry (Figures 3D–3F).
Figure 3.
The Premature-Stop-Codon Mutation in LNPK Results in Loss of Transcript and Full-Length Protein
(A) Graph showing the expression of human LNPK in relation to the geometric mean of three housekeeping genes via quantitative RT-PCR. Compared are control fibroblasts (control) and fibroblasts derived from the unaffected mother (B-II-2(M)) and the affected girl (B-III-2(A)) from family B. Consistent with NMD due to a premature stop codon, LNPK levels are reduced by approximately 50% in B-II-2(M) and almost completely lost in B-III-2(A). n = 3 independent experiments.
(B) Western blot images probing for full-length LNP and β-actin in parallel blots with the same input. Shown are triplicates for the three conditions described before. Neither full-length nor truncated LNP signal could be detected for B-III-2(A).
(C) Quantification of blots shown in (B). LNP was normalized to β-actin. For B-III-2(A), no peaks could be identified for quantification. Note that the severe transcript reduction is not reflected at the protein level for B-II-2(M). Shown are individual data points, as well as the mean ± SEM.
(D–F) Images show representative examples of fibroblasts stained with DAPI and the antibody detecting LNP for all three conditions. Results confirm the absence of full-length protein in cells derived from the affected girl. The scale bar in (D) represents 10 μm.
Although the depletion of mRNA transcript, presumably through nonsense-mediated decay (NMD), suggested a loss of protein product, we could not completely exclude the presence of mutant protein. Although the currently employed antibody demonstrated the loss of full-length protein, it was unable to detect the truncated protein products (data not shown). Thus, we wanted to assess whether the mutant proteins were stable if ectopically expressed. After transfection of expression vectors for wild-type LNP and both truncations, the two mutants showed a punctate pattern reminiscent of wild-type LNP (Figure S6). This was consistent with the retained integrity of the transmembrane domains and with previous work that demonstrated that localization to the ER depends on the N terminus of LNP.14 The expressed constructs were more stable than the wild-type (Figures S6A and S6B), suggesting that any residual mRNA could be transcribed into a functional protein with decreased cellular turnover. Despite these findings, the lack of mRNA transcripts suggests that the main pathogenic mechanism of these truncation mutations is a loss of protein; yet, we cannot exclude a contribution of the truncated protein to the phenotype in any way given that both mutants appear to be stable if expressed.
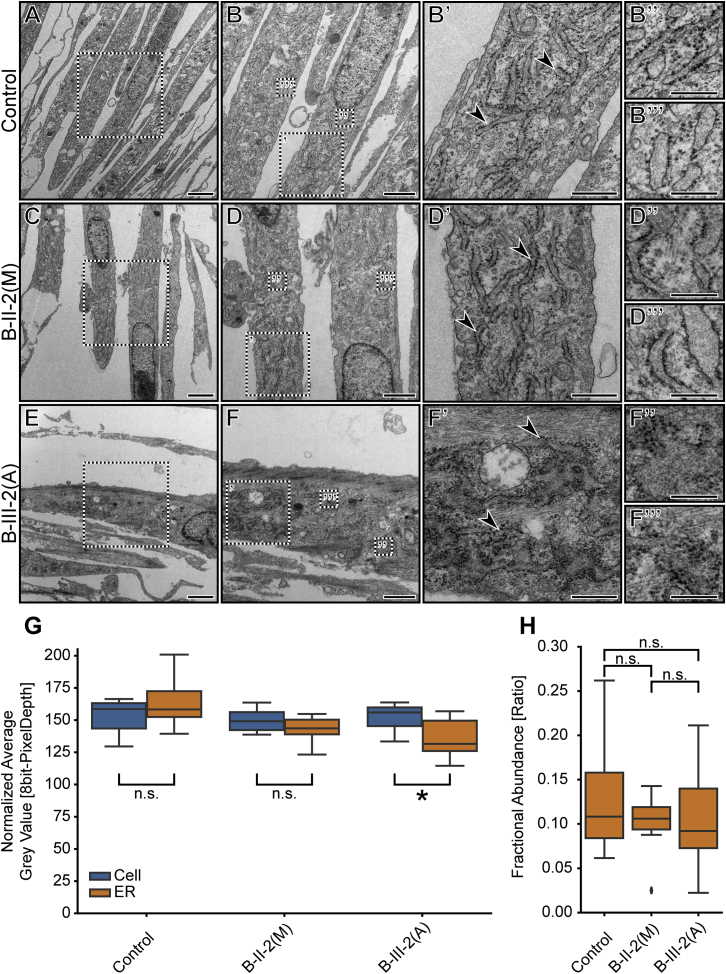
The effects of lunapark perturbation have been previously demonstrated by RNAi knockdown and genetic knockout in cell lines.13, 15 Its loss resulted in a reduction of tubular ER structures and an increased sheet-like appearance at the cellular periphery. This phenotype was observed in various cell lines in which each cell has a large surface area, ideal for imaging ER defects. Fibroblasts are less suited for these analyses because they typically have a spindle-like shape and less peripheral, tubular ER than the cell lines typically employed for these analyses. Thus, standard light microscopy would not provide sufficient resolution for assessing ER phenotypes in these cells. Therefore, we used electron microscopic assessment of the ER of pelleted cells to detect any potential phenotypes caused by loss of LNPK (Figure 4). All three fibroblast lines—control, B-II-2(M), and B-III-(A)—showed some variability with regard to ER structure as a result of both cellular variability and the angle of sectioning for individual cells. Nevertheless, aberrant ER structures were much more frequently present in the affected child’s cells (9/15) than in the maternal (3/14) or control (1/17) cells (Figures 4A–4F and Figure S7). The observed phenotype mainly consisted of a more sheet-like, less compact appearance, a reduced contrast of the membranous structure surrounding the ER, and decreased brightness of the lumen, indicative of higher mass density within the ER (Figure 4F).24 This is similar to what has been described in a mouse model with increased ER stress.25
Figure 4.
Fibroblasts Harboring a Homozygous, Truncating Mutation in LNPK Exhibit Aberrant ER Shape and Increased Luminal Mass Density
(A–F) Electron microscopy images of control fibroblasts (control) and fibroblasts derived from the unaffected mother (B-II-2(M)) and the affected girl (B-III-2(A)) from family B. Higher-magnification images (B, D, and F) also contain boxed regions that are shown on the right with the corresponding ′, ″, and ″′ labels. Arrowheads in (B′), (D′), and (F′) indicate rough ER that was used for the subsequent analysis and point out the disturbed ER morphology in (F′). Scale bars represent 2 μm (A, C, and E), 1 μm (B, D, and F), 500 nm (B′, D′, and F′), and 250 nm (B″, B″′, D″, D″′, F″, and F″′).
(G) Graph depicting the normalized average gray value of cellular positions that were classified as either cellular (excluding ER; cell) or rough ER (ER) for the three conditions introduced above. Points were selected by unbiased stereology (see Material and Methods). The average gray value was used as a proxy for luminal mass density. Statistical analysis for simple effects within rows (two-way ANOVA and Sidak’s multiple-comparison test) showed a significant difference between the ER gray values of the control and both B-II-2(M) (p = 0.0018) and B-III-2(A) (p < 0.0001) but not between the two lines from family B (p = 0.5662) or between any cellular gray values (p > 0.95 for all). Statistical analysis for effects within a column (two-way ANOVA and Sidak’s multiple-comparison test) showed a significant difference between the cellular and ER gray values for B-III-2(A) (∗p = 0.0106) but not for either the control (n.s., p = 0.1930) or B-II-2(M) (n.s., p = 0.4789). n ≥ 10 EM images.
(H) Graph depicting the fractional abundance of ER positions in relation to the sum of all positions with a cell. Points were selected by unbiased stereology (see Material and Methods) and the same as used for (G). The fractional abundance was used as a proxy for volume (size) ER changes relative to the cell. No significant differences (n.s.) could be observed between any of the conditions (one-way ANOVA and Tukey’s multiple-comparison test).
We confirmed the increased luminal mass density by blinded, unbiased stereology, where we assessed the average gray values at points that were classified as either ER or part of the remaining cellular area (Figure 4G).26 When we compared the overall cellular brightness (non-ER) with the specific ER signal within each category, only the affected fibroblasts were significantly darker in the ER lumen (Figure 4G). Employing the same, unbiased stereology dataset, we found no detectable difference between the fractional abundance of ER and all assessed points within the cellular area (Figure 4H). Together, these data suggest that defects in ER shape and luminal composition in fibroblasts, but not defects in the overall ER size relative to the cellular size, are due to loss of LNPK (according to the fractional abundance measurement).
Here, we have described two unrelated, consanguineous families with three affected individuals who exhibited a similar phenotypic spectrum comprising hypotonia, movement disorders, epilepsy, and corpus callosum hypoplasia. In both families, the phenotype segregates with a LoF mutation in LNPK, encoding the protein lunapark.
The cellular role of lunapark was unknown until a recent study demonstrated its role in ER structure.10 Before this, its function was explored only once, in the context of C. elegans, where its localization was dependent upon a kinesin microtubule motor, and its deletion resulted in motor defects and mislocalization of presynaptic proteins.17 Although the authors were not able to link these phenotypes to a molecular function, the additional insights provided more recently permit connections from the phenotypes in C. elegans to the protein’s localization to tubular ER junctions and its function in regulating ER shape.13, 15, 16 Animals with the lnp1 deletion exhibited subtle motor defects, reminiscent of the movement phenotypes in the three affected individuals.17 Furthermore, a presynaptic vesicle defect, as described by the authors, might contribute to the observed epilepsy phenotype.
How does LoF of lunapark result in human phenotypes? Axonal ER fulfills many roles in developing and mature axons and requires extensive, active shaping and maintenance.23 This is exemplified by the aforementioned defects in ER-shaping molecules that were implicated in spastic paraplegia almost two decades ago.5, 6 This finding suggested that neurons with extended axons are uniquely susceptible to perturbations of this organelle.5 In addition to the maintenance of extended processes, the same regulation of the tubular structure of ER also appears to be critical for their formation: Zhu et al. demonstrated that the SPG3A protein Atlastin-1 was not only localized to active growth cones but also critical for axon extension.27 Lunapark has been described by several lines of evidence to stabilize nascent three-way junctions of tubular ER.13, 15, 16 Its localization to extending processes, as demonstrated in differentiating NPCs in this manuscript, is consistent with current knowledge of the localization of tubular ER structure in growing axons.23
More recent work has also suggested the involvement of lunapark in the formation of stacked membrane discs and the integrity of nuclear pore complexes.28, 29 It is unclear to what extent the multiple functions assigned to lunapark could contribute to the range of phenotypes found in the three affected children. Yet, given the similarities to disorders associated with other genes that encode ER-shaping proteins, we propose that this cellular function is responsible for the observed phenotypes. Although there are differences in clinical presentation, the phenotypes of the three affected children resembled those found for dominant-negative mutations in ATL1 in SPG3A.30 Although the hallmark spasticity is largely absent, limb weakness, delayed motor development, and variable impairment of mobility are shared. Structurally, Orlacchio et al. have described a case where a heterozygous ATL1 variant resulted in a thin corpus callosum.31 Moreover, epilepsy and intellectual disability have both been described in other forms of hereditary spastic paraplegia.5
Given the strong connection between ER-shaping molecules and human disease, it would be interesting to screen for hitherto unknown genes that are involved in this pathway and test their relevance in human disease. We and others have performed a similar approach for cilia genes to yield new insights into biology and disease.32, 33
The function of lunapark at the organismal level has been poorly understood, and most functional insights derive from in vitro analysis of cell lines and unicellular yeast.10, 13, 14, 15 The identification of homozygous LoF mutations in individuals with corpus callosum hypoplasia suggests that its function, similar to that of Atlastin-1, is important for neurite extension, maintenance, or both. In future studies, it will be interesting to see what exact role lunapark has during the dynamic extension of growing axons and how this differs from or confirms the insights obtained from cell lines.
Declaration of Interests
The authors declare no competing interests.
Acknowledgments
We are indebted to the families for their participation in this study. This work was supported by NIH grants R01NS048453 and R01NS052455, the Simons Foundation Autism Research Initiative 275275, and the Howard Hughes Medical Institute (to J.G.G.). M.W.B. was supported by an EMBO Long-Term Fellowship (ALTF 174-2015), which is co-funded by the Marie Curie Actions of the European Commission (LTFCOFUND2013 and GA-2013-609409). The authors thank Susan Ferro-Novick and Peter Novick for reagents and their critical reading of the manuscript. We would also like to thank the following core services: the University of California, San Diego Neuroscience Core and Jennifer Santini for microscopy assistance (NIH grant NS047101), Dr. Marilyn Farquhar for the use of the electron microscopy facility, Ying Jones for electron microscopy sample preparation, and Timothy Meerloo for assistance with microscopy. The authors thank the Broad Institute (NIH grants U54HG003067 to E. Lander and UM1HG008900 to D. MacArthur), Yale University (NIH grant 5UM1HG006504-07), and Baylor College of Medicine (NIH grant 5UM1HG006542-07) for sequencing support and analysis. The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the NIH Office of the Director and by the National Cancer Institute, National Human Genome Research Institute, National Heart, Lung, and Blood Institute, National Institute on Drug Abuse, National Institute of Mental Health, and National Institute of Neurological Disorders and Stroke. The data used for the analyses described in this manuscript were obtained from the GTEx Portal on March 3, 2018.
Published: July 19, 2018
Footnotes
Supplemental Data include Supplemental Material and Methods, seven figures, and two tables and can be found with this article online at https://doi.org/10.1016/j.ajhg.2018.06.011.
Accession Numbers
The accession number for the data reported in this article is dbGaP: phs000288.v1.p1.
Web Resources
BrainSpan: Atlas of the Developing Human Brain, http://www.brainspan.org/
Exome Aggregation Consortium (ExAC) Browser, http://exac.broadinstitute.org/
Genome Aggregation Database (gnomAD), http://gnomad.broadinstitute.org/
GME Variome, http://igm.ucsd.edu/gme
GTEx Portal, https://www.gtexportal.org/home/
HomozygosityMapper, http://www.homozygositymapper.org/
OMIM, http://www.omim.org
MutationTaster, http://www.mutationtaster.org/
Python, https://www.python.org
SimulConsult measurement resources, http://www.simulconsult.com/resources/measurement.html
Supplemental Data
References
- 1.Schwarz D.S., Blower M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016;73:79–94. doi: 10.1007/s00018-015-2052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voeltz G.K., Rolls M.M., Rapoport T.A. Structural organization of the endoplasmic reticulum. EMBO Rep. 2002;3:944–950. doi: 10.1093/embo-reports/kvf202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H., Hu J. Shaping the endoplasmic reticulum into a social network. Trends Cell Biol. 2016;26:934–943. doi: 10.1016/j.tcb.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Westrate L.M., Lee J.E., Prinz W.A., Voeltz G.K. Form follows function: The importance of endoplasmic reticulum shape. Annu. Rev. Biochem. 2015;84:791–811. doi: 10.1146/annurev-biochem-072711-163501. [DOI] [PubMed] [Google Scholar]
- 5.Blackstone C. Hereditary spastic paraplegia. Handb. Clin. Neurol. 2018;148:633–652. doi: 10.1016/B978-0-444-64076-5.00041-7. [DOI] [PubMed] [Google Scholar]
- 6.Zhao X., Alvarado D., Rainier S., Lemons R., Hedera P., Weber C.H., Tukel T., Apak M., Heiman-Patterson T., Ming L. Mutations in a newly identified GTPase gene cause autosomal dominant hereditary spastic paraplegia. Nat. Genet. 2001;29:326–331. doi: 10.1038/ng758. [DOI] [PubMed] [Google Scholar]
- 7.Züchner S., Wang G., Tran-Viet K.N., Nance M.A., Gaskell P.C., Vance J.M., Ashley-Koch A.E., Pericak-Vance M.A. Mutations in the novel mitochondrial protein REEP1 cause hereditary spastic paraplegia type 31. Am. J. Hum. Genet. 2006;79:365–369. doi: 10.1086/505361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montenegro G., Rebelo A.P., Connell J., Allison R., Babalini C., D’Aloia M., Montieri P., Schüle R., Ishiura H., Price J. Mutations in the ER-shaping protein reticulon 2 cause the axon-degenerative disorder hereditary spastic paraplegia type 12. J. Clin. Invest. 2012;122:538–544. doi: 10.1172/JCI60560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esteves T., Durr A., Mundwiller E., Loureiro J.L., Boutry M., Gonzalez M.A., Gauthier J., El-Hachimi K.H., Depienne C., Muriel M.P. Loss of association of REEP2 with membranes leads to hereditary spastic paraplegia. Am. J. Hum. Genet. 2014;94:268–277. doi: 10.1016/j.ajhg.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S., Novick P., Ferro-Novick S. ER network formation requires a balance of the dynamin-like GTPase Sey1p and the Lunapark family member Lnp1p. Nat. Cell Biol. 2012;14:707–716. doi: 10.1038/ncb2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montavon T., Soshnikova N., Mascrez B., Joye E., Thevenet L., Splinter E., de Laat W., Spitz F., Duboule D. A regulatory archipelago controls Hox genes transcription in digits. Cell. 2011;147:1132–1145. doi: 10.1016/j.cell.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 12.Spitz F., Gonzalez F., Duboule D. A global control region defines a chromosomal regulatory landscape containing the HoxD cluster. Cell. 2003;113:405–417. doi: 10.1016/s0092-8674(03)00310-6. [DOI] [PubMed] [Google Scholar]
- 13.Wang S., Tukachinsky H., Romano F.B., Rapoport T.A. Cooperation of the ER-shaping proteins atlastin, lunapark, and reticulons to generate a tubular membrane network. eLife. 2016;5 doi: 10.7554/eLife.18605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moriya K., Nagatoshi K., Noriyasu Y., Okamura T., Takamitsu E., Suzuki T., Utsumi T. Protein N-myristoylation plays a critical role in the endoplasmic reticulum morphological change induced by overexpression of protein Lunapark, an integral membrane protein of the endoplasmic reticulum. PLoS ONE. 2013;8:e78235. doi: 10.1371/journal.pone.0078235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen S., Desai T., McNew J.A., Gerard P., Novick P.J., Ferro-Novick S. Lunapark stabilizes nascent three-way junctions in the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA. 2015;112:418–423. doi: 10.1073/pnas.1423026112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shemesh T., Klemm R.W., Romano F.B., Wang S., Vaughan J., Zhuang X., Tukachinsky H., Kozlov M.M., Rapoport T.A. A model for the generation and interconversion of ER morphologies. Proc. Natl. Acad. Sci. USA. 2014;111:E5243–E5251. doi: 10.1073/pnas.1419997111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghila L., Gomez M. The evolutionarily conserved gene LNP-1 is required for synaptic vesicle trafficking and synaptic transmission. Eur. J. Neurosci. 2008;27:621–630. doi: 10.1111/j.1460-9568.2008.06049.x. [DOI] [PubMed] [Google Scholar]
- 18.Dixon-Salazar T.J., Silhavy J.L., Udpa N., Schroth J., Bielas S., Schaffer A.E., Olvera J., Bafna V., Zaki M.S., Abdel-Salam G.H. Exome sequencing can improve diagnosis and alter patient management. Sci. Transl. Med. 2012;4:138ra78. doi: 10.1126/scitranslmed.3003544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott E.M., Halees A., Itan Y., Spencer E.G., He Y., Azab M.A., Gabriel S.B., Belkadi A., Boisson B., Abel L., Greater Middle East Variome Consortium Characterization of Greater Middle Eastern genetic variation for enhanced disease gene discovery. Nat. Genet. 2016;48:1071–1076. doi: 10.1038/ng.3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seelow D., Schuelke M. HomozygosityMapper2012--Bridging the gap between homozygosity mapping and deep sequencing. Nucleic Acids Res. 2012;40:W516–W520. doi: 10.1093/nar/gks487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarz J.M., Rödelsperger C., Schuelke M., Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods. 2010;7:575–576. doi: 10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- 23.Luarte A., Cornejo V.H., Bertin F., Gallardo J., Couve A. The axonal endoplasmic reticulum: One organelle-many functions in development, maintenance, and plasticity. Dev. Neurobiol. 2018;78:181–208. doi: 10.1002/dneu.22560. [DOI] [PubMed] [Google Scholar]
- 24.Bozzola J.J. Wiley Online Library; 1992. Electron microscopy. [Google Scholar]
- 25.Harding H.P., Zeng H., Zhang Y., Jungries R., Chung P., Plesken H., Sabatini D.D., Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in Perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 26.Howard V., Reed M. Garland Science; 2004. Unbiased stereology: Three-dimensional measurement in microscopy. [Google Scholar]
- 27.Zhu P.P., Soderblom C., Tao-Cheng J.H., Stadler J., Blackstone C. SPG3A protein atlastin-1 is enriched in growth cones and promotes axon elongation during neuronal development. Hum. Mol. Genet. 2006;15:1343–1353. doi: 10.1093/hmg/ddl054. [DOI] [PubMed] [Google Scholar]
- 28.Casey A.K., Chen S., Novick P., Ferro-Novick S., Wente S.R. Nuclear pore complex integrity requires Lnp1, a regulator of cortical endoplasmic reticulum. Mol. Biol. Cell. 2015;26:2833–2844. doi: 10.1091/mbc.E15-01-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S., Powers R.E., Gold V.A., Rapoport T.A. The ER morphology-regulating lunapark protein induces the formation of stacked bilayer discs. Life Sci. Alliance. 2018;1 doi: 10.26508/lsa.201700014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dürr A., Camuzat A., Colin E., Tallaksen C., Hannequin D., Coutinho P., Fontaine B., Rossi A., Gil R., Rousselle C. Atlastin1 mutations are frequent in young-onset autosomal dominant spastic paraplegia. Arch. Neurol. 2004;61:1867–1872. doi: 10.1001/archneur.61.12.1867. [DOI] [PubMed] [Google Scholar]
- 31.Orlacchio A., Montieri P., Babalini C., Gaudiello F., Bernardi G., Kawarai T. Late-onset hereditary spastic paraplegia with thin corpus callosum caused by a new SPG3A mutation. J. Neurol. 2011;258:1361–1363. doi: 10.1007/s00415-011-5934-z. [DOI] [PubMed] [Google Scholar]
- 32.Wheway G., Schmidts M., Mans D.A., Szymanska K., Nguyen T.T., Racher H., Phelps I.G., Toedt G., Kennedy J., Wunderlich K.A., UK10K Consortium. University of Washington Center for Mendelian Genomics An siRNA-based functional genomics screen for the identification of regulators of ciliogenesis and ciliopathy genes. Nat. Cell Biol. 2015;17:1074–1087. doi: 10.1038/ncb3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roosing S., Hofree M., Kim S., Scott E., Copeland B., Romani M., Silhavy J.L., Rosti R.O., Schroth J., Mazza T. Functional genome-wide siRNA screen identifies KIAA0586 as mutated in Joubert syndrome. eLife. 2015;4:e06602. doi: 10.7554/eLife.06602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.