Introduction
Testicular tumors are relatively uncommon and accounts for only 1–2% of all tumors in men. Spermatocytic seminoma (SS), a special form of seminoma, is a rare entity and constitutes only 2% of all seminomas.1 Less than 400 cases of SS have been reported till date and most of them are elderly with mean age of presentation is 54 years.2 We hereby report a case of SS, incidentally detected in a young individual, who presented with a large unilateral primary vaginal hydrocele.
Case report
A 30 years old healthy male patient presented with painless, progressive left hemi-scrotal swelling of six months duration. It was associated with dull ache on prolonged standing and exertion but there was no association with fever, dysuria or trauma to the testis. General and systemic examinations were unremarkable. Scrotal examination revealed 14 cm × 8 cm × 6 cm fluctuant, non-tender, negatively transilluminant left hemiscrotal swelling (Fig. 1). Testis and epididymis were not palpable due to large hydrocele and examination of contralateral inguino-scrotal region was normal.
Fig. 1.
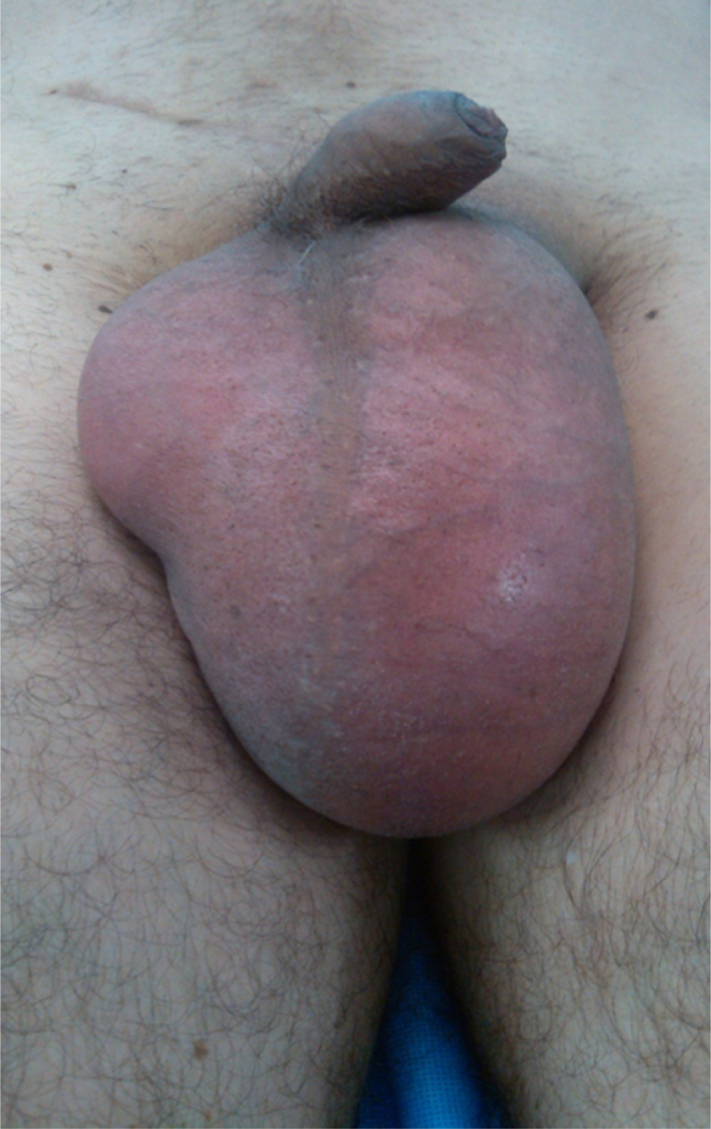
14 cm × 8 cm × 6 cm fluctuant, non-tender, trans-illuminant negative left hemiscrotal swelling.
Ultrasound scrotum revealed enlarged left testis with hypervascular, multiple, hypoechoic nodular lesions varying in size from 2.5 to 3 cm and tense ipsilateral hydrocoele. Tunica albuginea, epididymis and cord were sonologically normal.
Patient was further evaluated with tumor markers (β-HCG, LDH and AFP), Chest radiograph and abdominal ultrasound which were found to be normal. After clinical, radiological and laboratory assessment testicular malignancy with secondary hydrocoele was suspected and planned for orchidectomy for tissue diagnosis.
Radical orchidectomy was performed through left inguinal approach. Residual cut ends of cord and vessels were marked with titanium clips and deep ring was closed with non-absorbable suture. Delivery of the testis into the inguinal wound was difficult due to large fluid filled sac. Hence, fluid was aspirated using 20 Fg needle through inguinal incision, taking adequate precaution to prevent spillage of contents in and around the operative field. Sac was thick walled, therefore, excised along with testis and resected specimen was sent for HPE evaluation.
Gross examination of specimen revealed testis of size 7 cm × 5.5 cm × 3 cm and weight of 158 g (Fig. 2). On cut section multiple well circumscribed nodules, largest measuring 3 cm in diameter, were seen without any area of calcification, necrosis or hemorrhage. Compressed normal testis was seen at the lower end (Fig. 3).
Fig. 2.
Radical Orchidectomy specimen.
Fig. 3.
Cut section showing multiple nodular lesions, varying in size from 2.5 to 3 cm with compressed normal testis at lower pole.
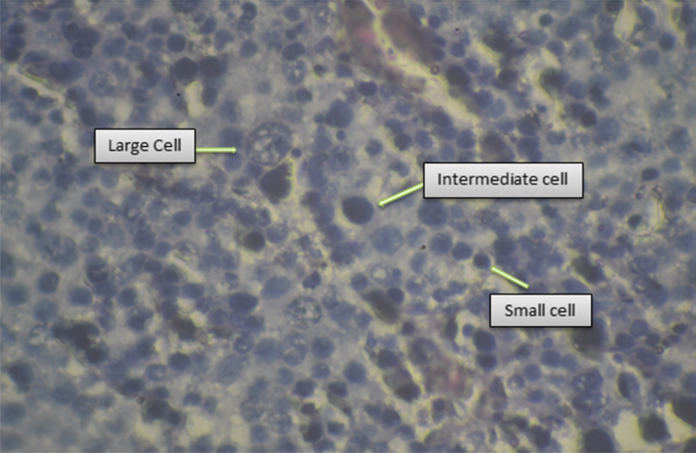
Microscopic examination of the tissue sections showed diffuse proliferation of polymorphous cells of three types: Large cells had uniform round nuclei with spireme chromatin, intermediate cells had perfectly round nuclei with evenly dispersed chromatin and eosinophilic cytoplasm and small cells were lymphocyte like with uniformly hyperchromatic nuclei and scant cytoplasm (Fig. 4). Mitotic figures were 1–2 per HPF. There was no fibrovascular stroma, lymphocytic infiltrates, granuloma/multinucleate giant cells, sarcomatous dedifferentiation or intratubular germ cell neoplasm observed on histology. Tunica albuginea and cut end of spermatic cord were free from tumor cells. Immunohistochemistry evaluation subsequently revealed CD 117 positivity and negative for LCA.
Fig. 4.
Spermatocytic seminoma showing admixture of large cells with round nuclei and spireme chromatin, medium sized cells with evenly distributed chromatin and small lymphocyte-like cells (H&E stain ×40).
After histopathological/IHC evaluation, a final diagnosis of SS (PT1N0M0S0, stage I) was made and patient is kept under regular follow up at Oncology centre. No recurrence or metastasis has been observed till date (nine months post surgery).
Discussion
SS was first described in 1946 by Masson (“le seminoma spermatocytaire”). It originates from premeiotic germ cells3, 4 and affects elderly male. It has never been found in pediatric age group and the youngest documented age of presentation is 19 years. Differentiation of SS from other histologically similar types of tumors like classical seminoma, embryonal cell carcinoma and lymphoma is important because as compared to other forms, SS has more indolent course, rarely metastasize and adequately treated with orchidectomy alone without any form of adjuvant therapy.5 Adjuvant treatment is only indicated in tumors with high mitotic index, sarcomatous changes or when associated with lymphoma. Only two cases of metastasis has been reported till date and both of them had tumors with sarcomatous changes.6
SS differs from classical seminoma being more common in elderly, occurs only in fully descended testis, has no ovarian counterpart7 and has never been found at extragonadal sites without involvement of testis. Serum markers are usually not elevated.8 Histological/IHC difference includes lack of fibrous stroma, lymphocytic and/or granulomatous stromal reaction, abundant glycogen, PLAP positivity and intratubular germ cell component. The cytoplasm is typically dense and amphophilic which is clear in classical seminoma.
Lymphoma of the testis also develops in older population but bilateral involvement is commoner (40%) than SS (<5%). Histologically, Lymphoma has a predominant interstitial growth pattern with a relatively monotonous cell population that lacks the lacy distribution of chromatin and may be confused with monomorphic type of SS, which has a sheet like arrangement of large tumor cells with round nuclei, prominent nucleoli, and frequent mitotic figures and they are negative for leukocyte common antigen (LCA).9
Embryonal carcinoma occurs in young adult (around 31 years) and typically present with painful palpable testicular mass. Up to two fifth of patients already have metastasis at diagnosis. It has solid, glandular, tubular and papillary cell patterns and lacks three cell types as seen in SS. Cell borders are also ill defined and foci of coagulative necrosis with much more crowded nuclei are seen. Mitotic rate is comparatively high and IHC shows positivity for CD30, PLAP and Cytokeratin.
In the reported case, primary hydrocele and testicular tumor were present as two separate entities. Patient presented with large hydrocele with clinically impalpable testis, which was transilluminant negative due to thick sac. Suspicious nodular lesions of the testis were detected sonologically, which turned out to be SS on HPE/IHC. Tumor was confined to the testis, albuginea was intact and metastatic work up was negative. Radical orchidectomy which was contemplated for histological diagnosis turned out to be therapeutic too.
Conclusion
SS is a rare testicular tumor and differs from classical seminoma, Lymphoma and embryonal cell carcinoma in cells of origin, demographic profile, mode of spread and treatment modalities. It has a benign clinical course and occurrence in young individual is extremely rare. Radical orchidectemy is mandatory for diagnosis and adjuvant treatment is required only in selective cases. Periodical clinical review, ultrasound scrotum, CT abdomen, chest radiograph and serum markers (β-HCG, LDH and AFP) are required to detect recurrence and metastasis. Prognosis is good for stage I disease and 10 year survival is more than 95%.
Conflicts of interest
The authors have none to declare.
References
- 1.Carrière P., Baade P., Fritschi L. Population based incidence and age distribution of spermatocytic seminoma. J Urol. 2007;178(July (1)):125–128. doi: 10.1016/j.juro.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Rajpert-De Meyts E., Jacobsen G.K., Bartkova J. The immunohistochemical expression pattern of Chk2, p53, p19INK4d, MAGE-A4 and other selected antigens provides new evidence for the premeiotic origin of spermatocytic seminoma. Histopathology. 2003;42(3):217–226. doi: 10.1046/j.1365-2559.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 3.Waheeb R., Hofmann M.C. Human spermatogonial stem cells: a possible origin for spermatocytic seminoma. Int J Androl. 2011;34(August (4 Pt 2)):e296–e305. doi: 10.1111/j.1365-2605.2011.01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reuter V.E. Origins and molecular biology of testicular germ cell tumors. Mod Pathol. 2005;18(February (suppl 2)):S51–S60. doi: 10.1038/modpathol.3800309. [DOI] [PubMed] [Google Scholar]
- 5.Talerman A. Spermatocytic seminoma: clinicopathological study of 22 cases. Cancer. 1980;45(8):2169–2176. doi: 10.1002/1097-0142(19800415)45:8<2169::aid-cncr2820450827>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 6.Mostofi F.K. Tumor markers and pathology of testicular tumors. Prog Clin Biol Res. 1984;153:69–87. [PubMed] [Google Scholar]
- 7.Xu N., Li F., Tian R. A rare case of bilateral sequential spermatocytic seminoma. World J Surg Oncol. 2013;11(1):175. doi: 10.1186/1477-7819-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung P.W., Bayley A.J., Sweet J. Spermatocytic seminoma: a review. Eur Urol. 2004;April doi: 10.1016/j.eururo.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Cummings O.W., Ulbright T.M., Eble J.N., Roth L.M. Spermatocytic seminoma: an immunohistochemical study. Hum Pathol. 1994;25(1):54–59. doi: 10.1016/0046-8177(94)90171-6. [DOI] [PubMed] [Google Scholar]