Abstract
Macrophages and inflammatory mediators have been implicated in ozone toxicity. In these studies, we used splenectomized (SPX) mice to assess the contribution of splenic monocytes to pulmonary inflammation and injury induced by ozone. Cells and tissue were collected 24–72 h after exposure of mice to air or ozone (0.8 ppm, 3 h). Following ozone exposure, increased numbers of pro-inflammatory CD11b + Ly6CHi and anti-inflammatory CD11b + Ly6CLo monocytes were observed in spleens of control (CTL) mice. CD11b + Ly6CHi and MMP-9+ pro-inflammatory macrophages were also observed in lungs of CTL mice after ozone, along with CD11b + Ly6CLo and mannose receptor (MR)+ anti-inflammatory macrophages. This was accompanied by increased lung expression of proteins involved in monocyte/macrophage trafficking including CCL3, CCL4, CCR1, and AT1R. Splenectomy resulted in decreases in pro-inflammatory macrophages in the lung and down regulation of CCR2, CCL2, and CCL4, but increases in CD11b + Ly6CLo anti-inflammatory macrophages. CD11b+Ly6G+Ly6C+ granulocytic (G)- and monocytic (M)-myeloid derived suppressor cells (MDSC)s were also detected in the lungs and spleens of CTL mice; these increased after ozone exposure. Splenectomy was associated with a decrease in G-MDSCs in the lung, with no effect on M-MDSCs. Changes in lung macrophage subpopulations and MDSCs in SPX mice were correlated with reduced ozone toxicity, as measured by decreases in bronchoalveolar lavage protein content and reduced 4-hydroxynonenal expression in the lung. These data suggest that the spleen is a source of pro-inflammatory/cytotoxic macrophages that contribute to ozone-induced lung injury, inflammation, and oxidative stress.
Keywords: ozone, macrophages, spleen, myeloid derived suppressor cells, inflammation.
Ozone is a ubiquitous urban air pollutant produced as a component of photochemical smog. It is a highly reactive molecule that induces cytotoxicity via its ability to generate free radicals and oxidize a variety of cellular components including proteins, lipids and DNA (Mudway and Kelly, 2000; Vallyathan and Shi, 1997). This leads to structural damage, predominately in alveolar epithelial regions of the lung, and to alterations in pulmonary mechanics (Alexeeff et al., 2007). Evidence suggests that macrophages accumulating in the lungs after ozone exposure contribute to both tissue injury and repair (Hollingsworth et al., 2007; Pendino et al., 1995; Sunil et al., 2015; Tighe et al., 2011). These activities are mediated by distinct macrophage subsets broadly classified as pro-inflammatory/cytotoxic M1 macrophages and anti-inflammatory/wound repair M2 macrophages (Byers and Holtzman, 2011; Laskin et al., 2011). The present studies were aimed at analyzing the origin of these cells, with a focus on the spleen as an extramedullary source.
The spleen is a lymphoid organ that functions to remove aging erythrocytes; it is also involved in immune homeostasis (Mebius and Kraal, 2005). The spleen has been shown to act as a reservoir of inflammatory monocytes that rapidly deploy to sites of injury (Kim et al., 2014; Swirski et al., 2009). Once localized at these sites, spleen monocytes differentiate into macrophages and become activated to release inflammatory mediators, which have been implicated in tissue injury (Swirski et al., 2009). Unlike bone marrow monocytes which depend on the chemokine receptor, CCR2, and its ligand, CCL2, to localize at inflammatory sites, splenic monocyte trafficking is mediated by angiotensin type I receptor (AT1R) and angiotensin II released from damaged tissues (Mellak et al., 2015). The present studies demonstrate that the spleen is a source of inflammatory lung macrophages responding to ozone-induced toxicity, and that in their absence, anti-inflammatory and wound repair macrophages predominate, resulting in attenuated tissue injury and oxidative stress. Elucidating the origin of cytotoxic/pro-inflammatory macrophages and mechanisms mediating their accumulation in the lung may lead to the development of novel approaches for reducing oxidant-induced lung injury.
MATERIALS AND METHODS
Animals and exposures
Female specific pathogen-free C57Bl6/J control (CTL) mice, which consisted of both sham-operated and wild-type mice, and splenectomized (SPX) mice (8–11 weeks; 17–22 g) were obtained from The Jackson Laboratories (Bar Harbor, Maine). Mice were utilized 3 weeks post-surgery. Animals were housed in filter-top microisolation cages and maintained on food and water ad libitum. Animals received humane care in compliance with the institution’s guidelines, as outlined in the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health. All animal protocols were approved by the Rutgers University Institutional Animal Care and Use Committee. Animals were exposed to air or ozone (0.8 ppm) for 3 h in a whole body Plexiglas chamber. Ozone was generated from oxygen gas via an ultraviolet light generator (Gilmont Instruments, Barrington, Illinois) and mixed with air. Concentrations inside the chamber were monitored using a photometric ozone analyzer (Teledyne Instruments, Thousand Oaks, California). Animals were euthanized 24–72 h after exposure by intraperitoneal injection of Nembutal (200 mg/kg).
Bronchoalveolar lavage collection and analysis
Bronchoalveolar lavage (BAL) was collected from all lobes of the lung by slowly instilling and withdrawing 1 ml of PBS into the lung 3 times through a cannula in the trachea. BAL fluid was centrifuged (300 × g, 8 min), supernatants collected, aliquoted, and stored at −80 °C until analysis. Cell pellets were resuspended in 1 ml PBS. The lung was then removed and 1 ml of PBS instilled and withdrawn 5 times while gently massaging the tissue. After centrifugation of the lavage fluid (400 × g, 6 min, 4 °C), the cell pellet was resuspended in 1 ml of PBS and combined with the first BAL cell suspension. Cells were washed twice with PBS, resuspended in 200 μl of buffer (PBS containing 2% FCS and 0.02% sodium azide), and viable cells enumerated using a hemocytometer following trypan blue staining. For differential analysis, cytospin preparations of BAL cells were stained with Geimsa. We found that BAL cells from both control and SPX mice consisted of >98% macrophages, and that this was unaltered by ozone exposure. In previous studies, we demonstrated that cells recovered using this procedure were phenotypically and functionally alveolar macrophages (Lavnikova et al., 1993; Prokhorova et al., 1994). Total protein was quantified in cell-free BAL fluid using a BCA Protein Assay kit (Pierce Biotechnologies Inc., Rockford, Illinois) with bovine serum albumin as the standard. Samples were analyzed in triplicate at 560 nm on a Vmax MAXline microplate reader (Molecular Devices, Sunnyvale, California).
Preparation of spleen cells and bone marrow cells
Spleens were removed and disaggregated in PBS using a Pasteur pipette. Bone marrow cells were harvested by flushing the femurs with 10–20 ml of ice cold PBS using a 20G needle. Cells were then repeatedly aspirated with a 10 ml pipet to disrupt clumps and centrifuged (400 × g, 6 min, 4 °C). Spleen cells and bone marrow cells were washed twice in PBS, resuspended in buffer, and viable cells enumerated.
Flow cytometric analysis
Cells were incubated for 10 min at 4 °C with anti-mouse CD16/32 (1:200, clone 93; Biolegend, San Diego, California) to block nonspecific binding and then with FITC-conjugated anti-mouse CD11b (1:200, clone M1/70; Biolegend), PE-conjugated anti-mouse Ly6C (1:200, clone HK1.4; Biolegend), PE/Cy7-conjugated anti-mouse F4/80 (1:200, clone BM8; Biolegend), AF 700-conjugated anti-mouse CD11c (1:200, clone N418; Biolegend) and AF 647-conjugated anti-mouse Ly6G (1:200, clone 1A8; Biolegend) antibodies or appropriate isotypic controls for 30 min at 4 °C. This was followed by incubation with eFluor 780-conjugated fixable viability dye for 30 min at 4 °C (1:1000, eBioscience, San Diego, California). Cells were analyzed on a Gallios flow cytometer (Beckman Coulter, Brea, California). Data were analyzed using Beckman Coulter Kaluza version 1.2 software. Viable cells were initially analyzed for expression of CD11b, followed sequentially by Ly6G, Ly6C, F4/80, and CD11c (Supplementary Data). Inflammatory cell subpopulations were identified as described previously (Sunil et al., 2015). The number of cells within each subpopulation was calculated from the percentage of positive cells relative to the total number of cells recovered.
Immunohistochemistry
A separate group of animals (n = 3 mice/treatment group) was used for this analysis. The lung was fixed in situ via the trachea with PBS containing 3% paraformaldehyde and 2% sucrose solution. After overnight at 4 °C, the tissue was washed 3 times in PBS/2% sucrose, transferred to 50% ethanol, and then paraffin embedded. Tissue sections (5 μm) were deparaffinized with xylene (4 min, × 2) followed by decreasing concentrations of ethanol (100–50%) and then water. After antigen retrieval using citrate buffer (10.2 mM sodium citrate, pH 6.0) and quenching of endogenous peroxidase with 3% H2O2 for 10 min, sections were incubated for 2 h at room temperature with 10–100% normal goat or rabbit serum to block nonspecific binding. This was followed by overnight incubation at 4 °C with rabbit antibody to mannose receptor-1 (MR-1, 1:1500; Abcam), AT1R (1:100; Abcam, Cambridge, MA), or matrix metalloproteinase-9 (MMP-9, 1:150; Santa Cruz Biotechnology, Dallas, Texas), or with goat anti-4-hydroxynonenal (4-HNE, 1:100; Abcam) antibody, or with the appropriate IgG controls (ProSci, Poway, California). Sections were then incubated with biotinylated secondary antibody (Vector Labs, Burlingame, California) for 30 min at room temperature. Binding was visualized using a Peroxidase Substrate Kit DAB (Vector Labs). Three random sections from each animal were analyzed.
Real-time PCR
Total RNA was isolated from the lung using an RNeasy kit (Qiagen, Valencia, California). RNA purity and concentration were measured using a NanoDrop spectrophotometer (ThermoFisher Scientific, Wilmington, DE). RNA was converted into cDNA using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, California). Standard curves were generated using serial dilutions from pooled randomly selected cDNA samples. Real-time PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems) on a ABI Prism 7300HT Sequence Detection System (Applied Biosystems). All PCR primer pairs were generated using Primer Express 2.0 (Applied Biosystems) and synthesized by Integrated DNA Technologies (Coralville, Iowa). Gene expression changes were normalized to GAPDH. Forward and reverse primer sequences used were: CCR2, TCCACGGCATACTATCAACATCTC and GGCC CC TTCATCAAGCTCTT; CCL2, TTGAATGTGAAGTTGACC CGTAA and GCTTGAGGTTGTGGAAAAG; CCL3, TCTTCTCAGCGCCATA TGGA and TCCGGCTGTAGGAGAAGCA; CCL4, TGCTCGTGGCTGC CTTCT and GAGGGTCAGAGCCCATTGG; CCR1, CTGAGGGCCCGA ACTGTTAC and GGCTAGGGCCCAGGTGAT; AT1R, CCATTGTCC ACCCGATGAA and TGACTTTGGCCACCAGCAT; CX3CR1, TCGGTCTCAGGCCTGGTACTACA and GGCTTCCGGCTGTTGGT; CCR5, TGA TAAGCTGCAAAAAGCTGAAGA and GTCAGAGATGGCCAGGTT GAG; GAPDH, TGAAGCAGGCATCTGAGGG and CGAAGGTGGAAG AGTGGGAG.
Western blotting
Bronchoalveolar lavage (BAL) proteins were separated using native and reducing gel electrophoresis as previously reported (Atochina-Vasserman, 2012; Guo et al., 2008). Briefly, denatured and native aliquots of BAL proteins were fractionated on denaturing 4–12% Novex Bis-Tris gels or 4–16% Bis-Tris NativePAGE gels (Invitrogen, San Diego, California), respectively, and then transferred to nitrocellulose membranes. Nonspecific binding was blocked by incubation of the blots with 10% nonfat milk for 1 h at room temperature. The blots were then incubated overnight at 4 °C with a mixture of rabbit polyclonal anti-SP-D antibody DV117 (a gift from Dr Amy Pastva, Duke University, North Carolina; 1:10 000) and rabbit polyclonal anti-surfactant protein (SP)-D antibody from Chemicon (Millipore, Billerica, Massachusetts; 1:4000), washed and then incubated for 1 h at room temperature with HRP-conjugated secondary antibody (1:5000), diluted in Tris-buffered saline/Tween-20. Immunoreactive bands were visualized using an ECL detection system (GE Healthcare Biosciences, Piscataway, New Jersey).
Statistical analysis
Spleen cell data were analyzed using one-way ANOVA; all other data were analyzed using 2-way ANOVA followed by Tukey’s post-hoc analysis. A P value of ≤.05 was considered statistically significant. Data from air-exposed animals were combined and analyzed together.
RESULTS
Effects of Ozone on Spleen Myeloid Cells
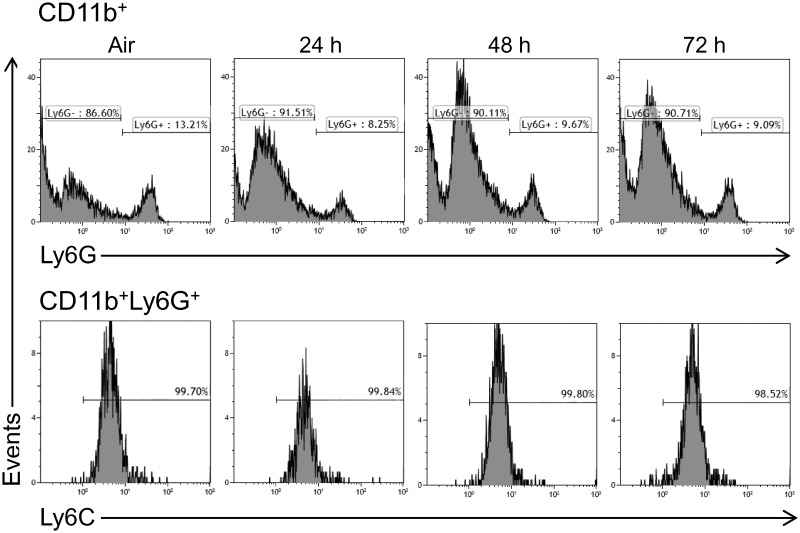
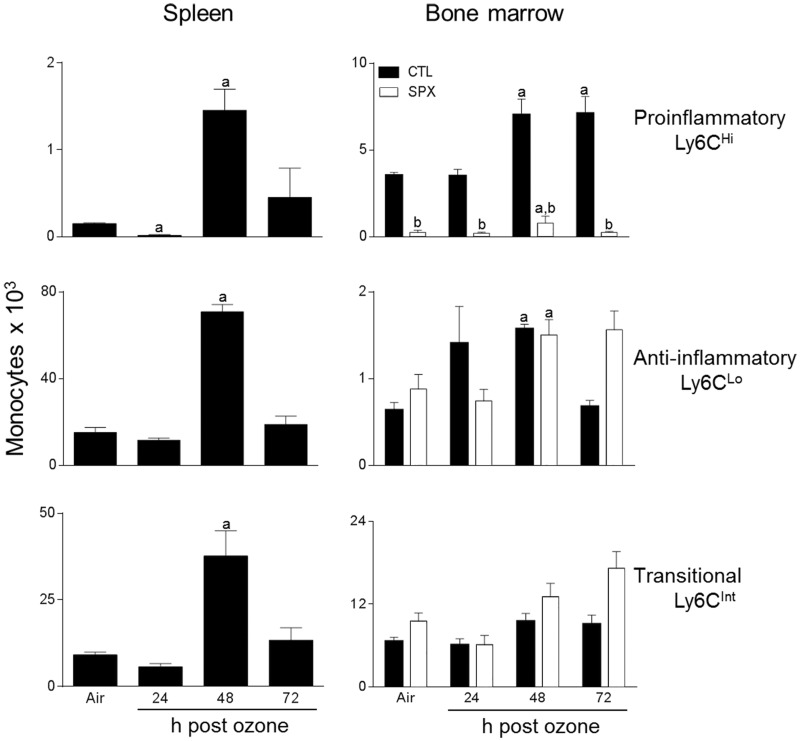
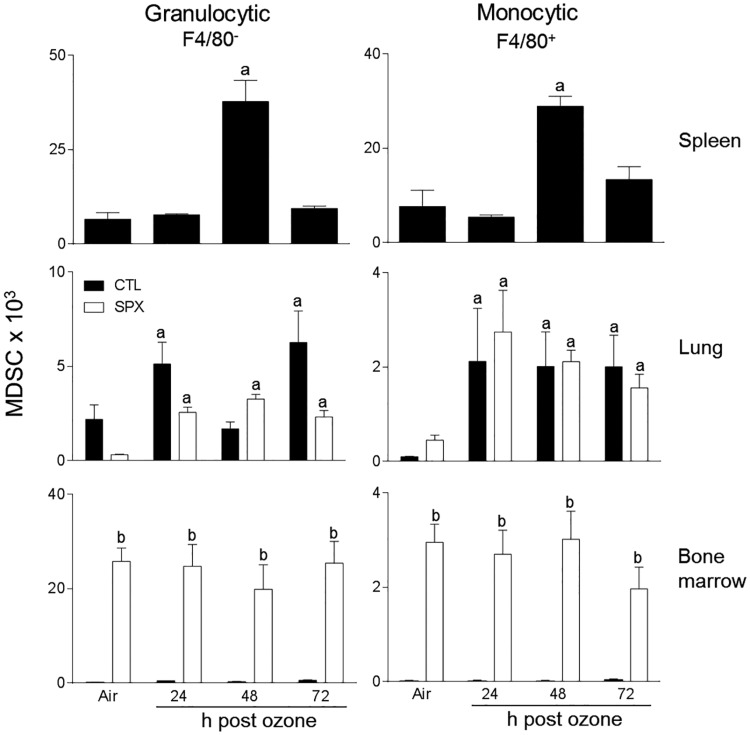
Treatment of control mice with ozone resulted in an increase in the total number of spleen cells at 48 h post-exposure, with no change in cell viability (Table 1 and not shown). To characterize these cells, we used techniques in flow cytometry, focusing on myeloid inflammatory cells, which were identified based on the forward and side scatter and expression of the β2 integrin, CD11b (Gordon and Taylor, 2005). CD11b+ myeloid cells were found to consist of Ly6G− (monocytic) and Ly6G+ (granulocytic) subpopulations (Figure 1). The majority of the cells (∼90%) were monocytic. We next analyzed their expression of Ly6C, a monocyte/macrophage activation marker, and F4/80, a marker of maturity (Mitchell et al., 2014). Figure 2 shows that Ly6G− F4/80+ spleen monocytes consisted of 3 subpopulations that expressed high (Ly6CHi), low (Ly6CLo), and intermediate (Ly6CInt) levels of Ly6C, which is characteristic of pro-inflammatory, anti-inflammatory, and transitional monocytes, respectively (Gordon and Taylor, 2005). Ozone exposure resulted in a significant increase in all 3 Ly6C positive monocyte subpopulations at 48 h (Figure 2, left panels). In contrast to Ly6G− spleen myeloid cells, the majority (>99%) of the Ly6G+ cells were highly positive for Ly6C, suggestive of a MDSC phenotype (Figure 1, lower panels) (Youn et al., 2008). F4/80− granulocytic (G-MDSC) and F4/80+ monocytic (M-MDSC) subpopulations were identified; both of these increased 48 h after ozone (Figure 3).
TABLE 1.
Effects of Ozone on Total Number of Spleen Cells
| Time | Spleen Cells × 106 |
|---|---|
| Air | 17.6 ± 1.7 |
| 24 h | 21.8 ± 1.2 |
| 48 h | 57.8 ± 6.3a |
| 72 h | 19.4 ± 0.4 |
Spleen cells were collected 24–72 h after exposure of mice to air or ozone and viable cells enumerated using a hemocytometer with trypan blue dye exclusion. Data are the mean ± SE (n = 3–4 mice/treatment group).
Significantly different (P < .05) from air-exposed animals.
FIG. 1.
Effects of ozone on spleen granulocytes and MDSCs. Spleen cells, collected 24–72 h after exposure of CTL mice to air or ozone, were immunostained with antibodies to CD11b, Ly6G, and Ly6C and then analyzed by flow cytometry. One representative histogram from 3 to 4 mice/treatment group is shown. Upper panel: CD11b+ spleen cells expressing Ly6G. Lower panel: CD11b + Ly6G+ spleen cells expressing Ly6C.
FIG. 2.
Effects of ozone on spleen and bone marrow monocytes. Cells, collected 24–72 h after exposure of CTL and SPX mice to air or ozone, were stained with antibodies to CD11b, Ly6G, Ly6C, and F4/80 or isotypic controls, and analyzed by flow cytometry. Monocytes were defined as CD11b + Ly6G−F4/80− and Ly6CHi (pro-inflammatory), Ly6CLo (anti-inflammatory), or Ly6CInt (transitional). Bars, mean ± SE (n = 3–4 mice/treatment group). aSignificantly different (P < .05) from air-exposed animals.
FIG. 3.
Effects of ozone on spleen, lung, and bone marrow MDSCs. Cells, collected 24–72 h after exposure of CTL and SPX mice to air or ozone, were stained with antibodies to CD11b, Ly6G, Ly6C, and F4/80 or isotypic controls, and analyzed by flow cytometry. MDSCs were defined as CD11b + Ly6G+ and F4/80− (granulocytic) or F4/80+ (monocytic). Bars, mean ± SE (n = 3–4 mice/treatment group for spleen cells and bone marrow cells; n = 10 mice/treatment group for lung cells). aSignificantly different (P < .05) from air-exposed animals; bSignificantly different (P < .05) from CTL.
Effects of Splenectomy on Lung Macrophage Subpopulations
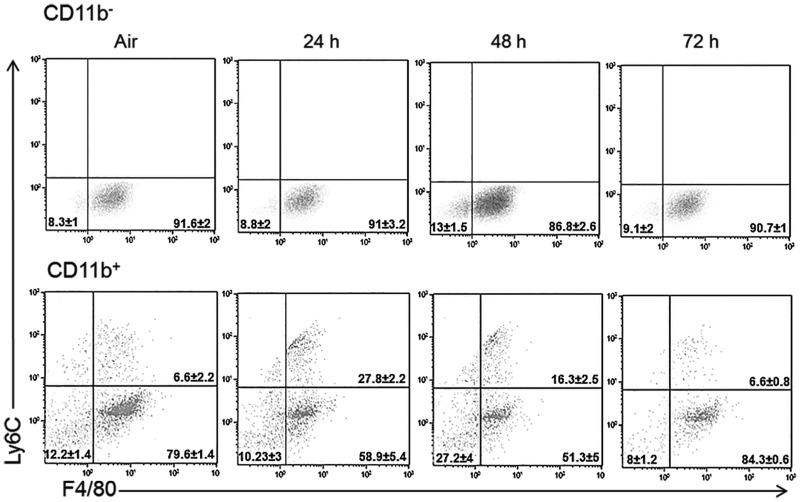
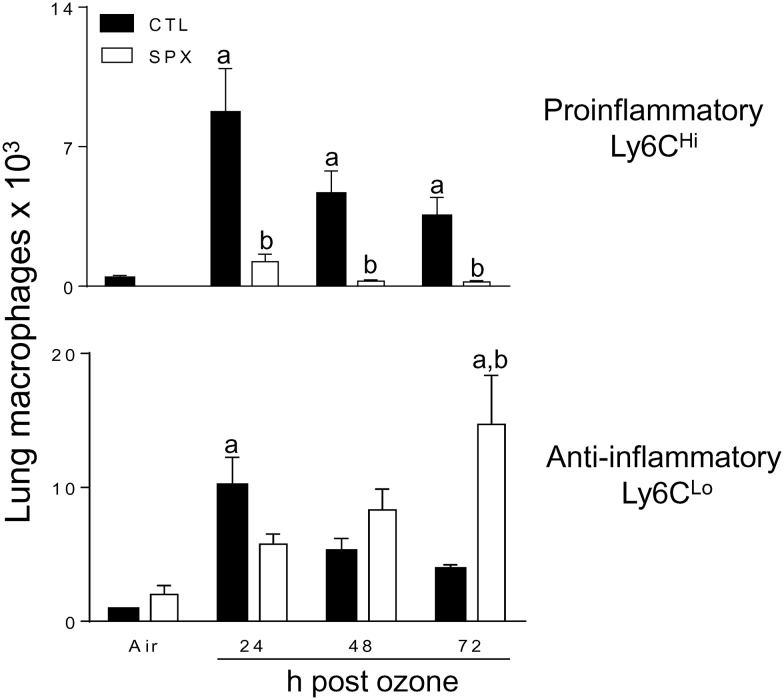
Earlier studies showed that both pro- and anti-inflammatory macrophages accumulate in the lungs after ozone exposure (Fakhrzadeh et al., 2002; Pendino et al.,1995; Sunil et al., 2012, 2015; Tighe et al., 2011). In further studies, we used SPX mice to determine if the spleen contributes to these inflammatory cell populations. In both air and ozone-exposed mice, differential staining of BAL cells revealed that >98% were macrophages. In air-exposed mice, one population was identified; these were characterized as CD11b−Ly6CLoF4/80 + CD11c+, which is consistent with a resident alveolar macrophage phenotype (Guth et al., 2009; Misharin et al., 2013; Zaynagetdinov et al., 2013). Ozone inhalation had no effect on resident alveolar macrophages in either CTL or SPX mice (Figure 4, upper panels). In contrast, a significant increase in the total number of CD11b+ cells was observed in the lungs after ozone (Figure 4, lower panels) (Sunil et al., 2015). As observed in the spleen, monocytic (Ly6G−) and granulocytic (Ly6G+) subpopulations of CD11b+ cells were identified in the lung (supplemental data). However, in the lung, only Ly6CHi pro-inflammatory and Ly6CLo anti-inflammatory macrophages were detected within the Ly6G− population (Sunil et al., 2015). The majority (>70%) of these macrophage subpopulations were F4/80 + CD11c+, indicative of a mature phenotype (Guth et al., 2009; Misharin et al., 2013; Zaynagetdinov et al., 2013). Increases in both pro-inflammatory and anti-inflammatory macrophages were observed in the lungs of CTL mice following ozone exposure; this was most prominent 24 h post-exposure (Figure 5). Splenectomy resulted in a significant decrease in Ly6CHi pro-inflammatory macrophages accumulating in the lung in response to ozone. Conversely, an increase in Ly6CLo anti-inflammatory macrophages was observed in lungs of SPX mice relative to CTL mice at 72 h post-ozone (Figure 5). Ly6G + Ly6C+ MDSCs were also identified in the lung, which consisted of G-MDSC and M-MDSC subpopulations (Figure 3). In CTL, as well as SPX mice, ozone caused a significant increase in both MDSC subpopulations. Whereas reduced numbers of G-MDSCs were observed in lungs of SPX mice 24 and 72 h after ozone relative to CTL mice, M-MDSCs were unchanged.
FIG. 4.
Effects of ozone on lung macrophage subpopulations. BAL cells, collected 24–72 h after exposure of CTL mice to air or ozone were stained with antibodies to CD11b, Ly6G, Ly6C, F4/80, and CD11c or isotypic controls, and analyzed by flow cytometry. Upper Panel: CD11b−Ly6G− cells expressing Ly6C, F4/80, and CD11c. Lower Panel: CD11b + Ly6G− cells expressing Ly6C, F4/80, and CD11c. One representative dot plot from 10 mice/treatment group is shown. The percentages represent the mean ± SE (n = 10 mice/treatment group).
FIG. 5.
Effects of splenectomy on lung macrophages responding to ozone. BAL cells, collected 24–72 h after exposure of CTL and SPX mice to air or ozone, were stained with antibodies to CD11b, Ly6G, Ly6C, F4/80, and CD11c or isotypic controls and analyzed by flow cytometry. Macrophages (MP) were defined as CD11b + Ly6G− F4/80 + CD11c+ and Ly6CHi (pro-inflammatory) or Ly6CLo (anti-inflammatory). Bars, mean ± SE (n = 10 mice/treatment group). aSignificantly different (P < .05) from air-exposed animals; bSignificantly different (P < .05) from CTL.
In further studies, we analyzed the effects of splenectomy on pro-inflammatory MMP-9+ and anti-inflammatory MR-1+ macrophages in histologic sections of the lung. In CTL mice, ozone inhalation resulted in increased numbers of MMP-9+ macrophages in the lung at 24 and 48 h post-exposure (Figure 6). These cells were reduced in SPX mice. Ozone also caused an increase in MR-1+ macrophages in lungs of CTL mice. Although numbers of MR-1+ macrophages were constitutively greater in SPX mice than in CTL mice, they were largely unaffected by ozone (Figure 7).
FIG. 6.
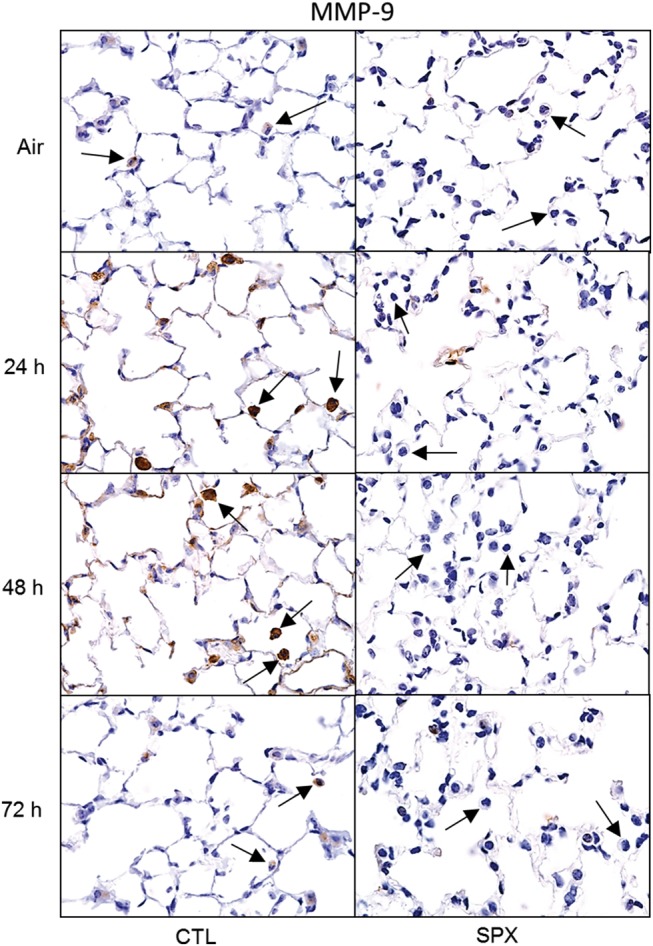
Effects of splenectomy on ozone-induced MMP-9 expression. Lung sections, prepared 24–72 h after exposure of CTL and SPX mice to air or ozone, were stained with antibody to MMP-9. Binding was visualized using a peroxidase DAB substrate kit. Arrows indicate alveolar macrophages. One representative lung section from 3 mice/treatment group is shown. Original magnification, 600×.
FIG. 7.
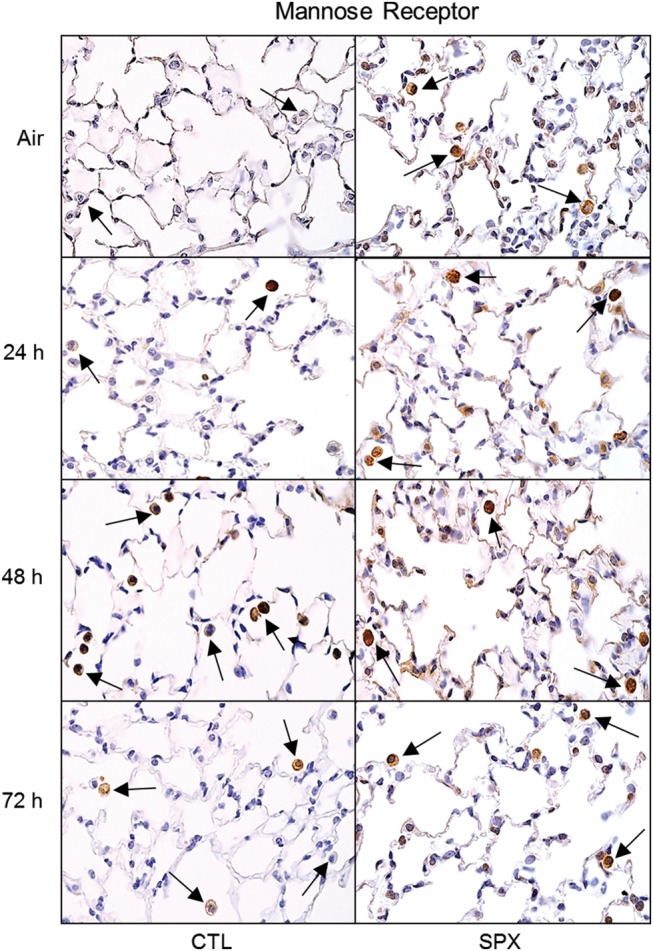
Effects of splenectomy on ozone-induced expression of mannose receptor-1. Lung sections, prepared 24–72 h after exposure of CTL and SPX mice to air or ozone, were stained with antibody to mannose receptor. Binding was visualized using a peroxidase DAB substrate kit. Arrows indicate alveolar macrophages. One representative lung section from 3 mice/treatment group is shown. Original magnification, 600×.
Effects of Splenectomy on Ozone-Induced Expression Chemokines and Chemokine Receptors Involved in Monocyte/Macrophage Trafficking
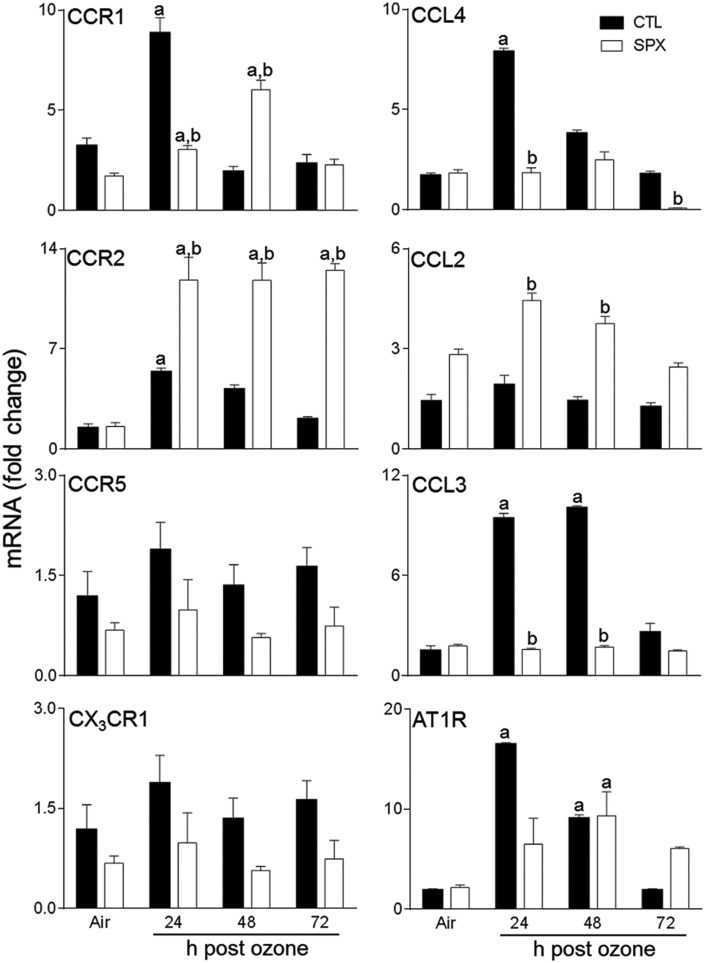
In CTL mice, chemokines CCL3 and CCL4 and chemokine receptors, CCR1 and CCR2 were upregulated in the lung 24 h post-ozone (Figure 8). In contrast, ozone had no effect on expression of CCL2, CCR5, or CX3CR1. Splenectomy resulted in increased expression of CCR2 and CCL2, when compared with CTL mice, at all post-ozone exposure times. Conversely, ozone-induced upregulation of CCR1, CCL3, and CCL4 was blunted in SPX mice. Splenectomy had no significant effect on CCR5 or CX3CR1 gene expression, or on their response to ozone (Figure 8). We also analyzed expression of AT1R, which is important in monocyte trafficking from the spleen (Mellak et al., 2015; Swirski et al., 2009). In CTL mice, increased numbers of AT1R+ cells were observed in the lung at 24 and 48 h after ozone (Figure 9); this was correlated with increased AT1R mRNA expression (Figure 8). Ozone-induced increases in AT1R+ macrophages were delayed in SPX mice relative to CTL mice. Whereas increases in AT1R mRNA expression were also delayed in SPX mice treated with ozone, levels remained upregulated for 72 h.
FIG. 8.
Effects of splenectomy on ozone-induced expression of inflammatory genes. mRNA, prepared from lungs 24–72 h after exposure of CTL and SPX mice to air or ozone, was analyzed by real-time PCR. Data are presented as fold change relative to GAPDH. Bars, mean ± SE (n = 3 mice/treatment group). aSignificantly different (P < .05) from air-exposed animals. bSignificantly different (P < .05) from CTL mice.
FIG. 9.
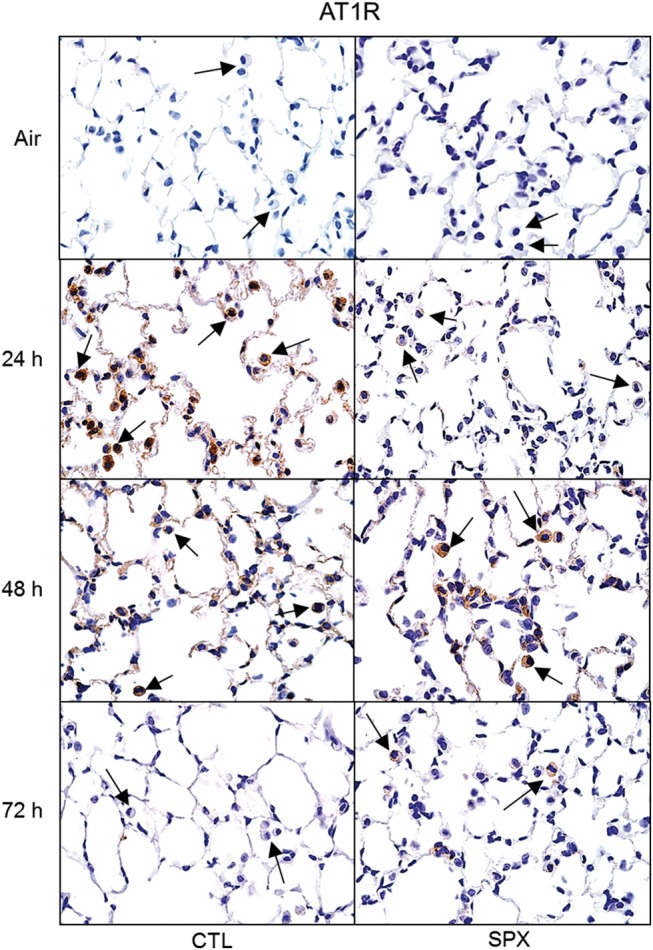
Effects of splenectomy on ozone-induced expression of AT1R. Lung sections, prepared 24–72 h after exposure of CTL and SPX mice to air or ozone, were stained with antibody to AT1R. Binding was visualized using a peroxidase DAB substrate kit. Arrows indicate alveolar macrophages. One representative lung section from 3 mice/treatment group is shown. Original magnification, 600×.
Effects of Splenectomy on Bone Marrow Monocytes and MDSCs
We next analyzed the effects of ozone on bone marrow monocytes in CTL and SPX mice. Three subpopulations of CD11b+ monocytes expressing high, low, and intermediate levels of Ly6C were identified in the bone marrow (Figure 2, right panels). The majority of these cells were immature F4/80− (data not shown). In CTL mice, ozone inhalation resulted in an increase in Ly6CHi monocytes at 48 and 72 h post-exposure (Figure 3). Conversely, Ly6CLo monocytes increased in the bone marrow after ozone exposure, whereas Ly6CInt transitional monocytes were unchanged. Whereas splenectomy resulted in a decrease in Ly6CHi monocytes, increases in Ly6CLo and Ly6CInt monocytes were noted at 72 h post-ozone. Monocytic (F4/80+) and granulocytic (F4/80−) Ly6G + Ly6C+ MDSCs were also identified in the bone marrow (Figure 3). Significantly greater numbers of these cells were observed in SPX mice; however, they were not altered by ozone exposure.
Effects of Splenectomy on Ozone-Induced Lung Injury and Oxidative Stress
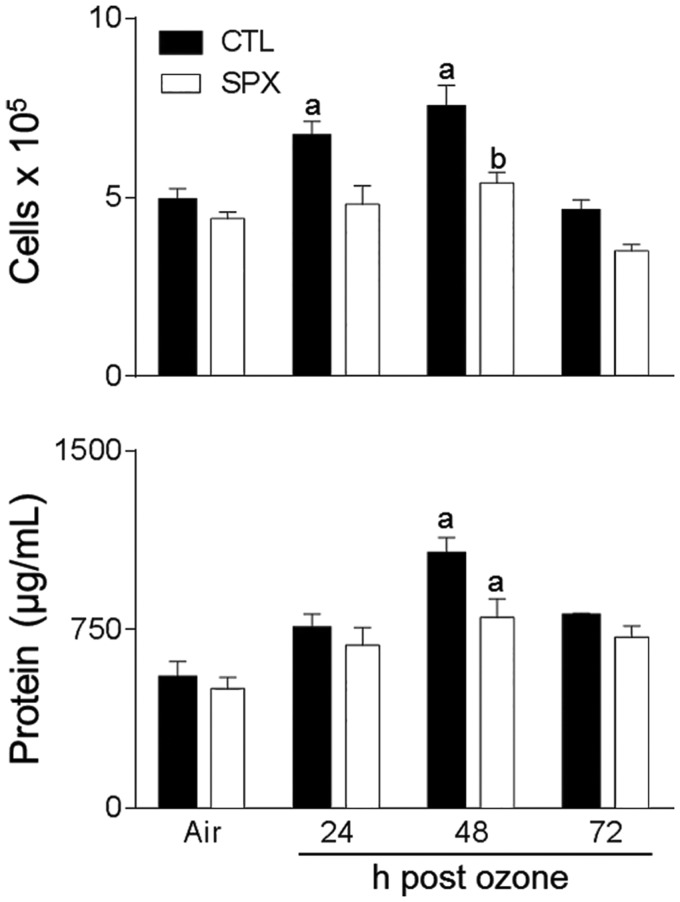
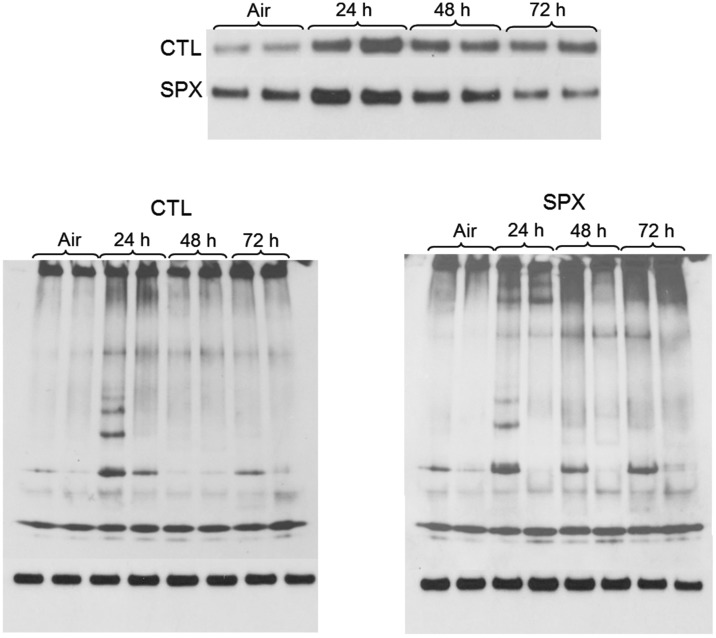
In additional studies, we determined if ozone-induced changes in inflammatory cells in the lungs of SPX mice were correlated with alterations in toxicity. Treatment of CTL mice with ozone resulted in significant increases in BAL cell and protein content (Figure 10). This was reduced in SPX mice. Ozone-induced oxidative stress, as measured by the lipid peroxidation end product 4-HNE, was also reduced in SPX mice relative to CTL mice (Figure 11). In contrast, splenectomy had no effect on ozone-induced increases in total SP-D levels in BAL or on structural alterations in SP-D, as reflected by the presence of multiple lower molecular weight SP-D bands in reducing gels (Figure 12).
FIG. 10.
Effects of splenectomy on ozone-induced alterations in BAL cell number and protein. BAL was collected 24–72 h after exposure of CTL and SPX mice to air or ozone. Upper panel: Viable cells were enumerated using trypan blue dye exclusion. Lower panel: Cell-free supernatants were analyzed in triplicate for protein using a BCA protein assay kit. Bars, mean ± SE (n = 8 mice/treatment group). aSignificantly different (P < .05) from air-exposed animals; bSignificantly different (P < .05) from CTL.
FIG. 11.
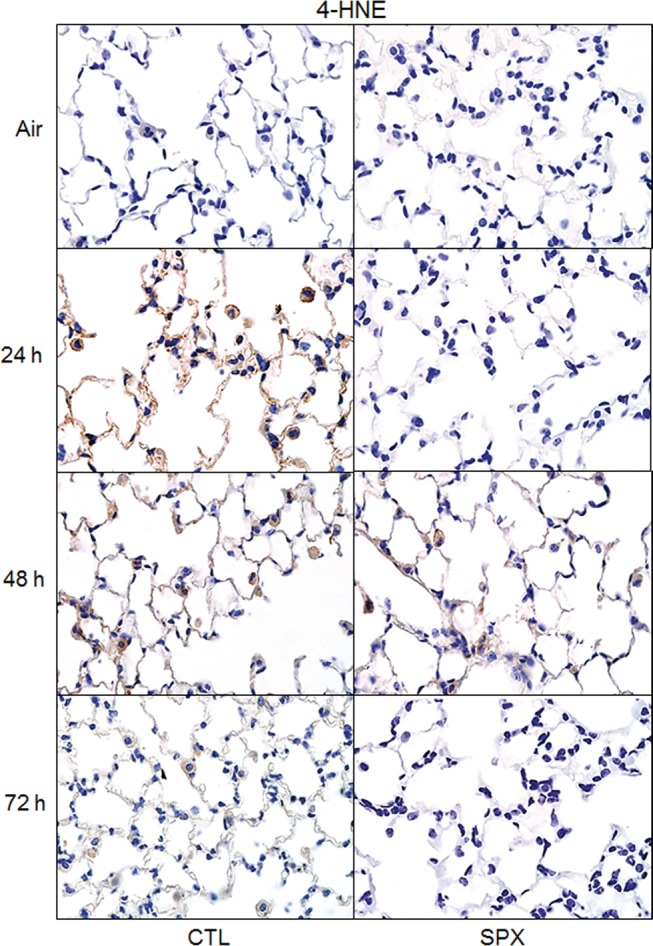
Effects of splenectomy on ozone-induced 4-HNE. Lung sections, prepared 24–72 h after exposure of CTL and SPX mice to air or ozone, were stained with antibody to 4-HNE. Binding was visualized using a peroxidase DAB substrate kit. One representative section from 3 mice/treatment group is shown. Original magnification, 600×.
FIG 12.
Effects of splenectomy on ozone-induced alterations in BAL SP-D structure. BAL was collected 24–72 h after exposure of CTL and SPX mice to air or ozone. BAL SP-D protein was analyzed by western blotting. Upper panel: SP-D protein analyzed in denaturing gels. Lower panels: SP-D protein analyzed in native gels. Each lane represents BAL analysis of one animal.
DISCUSSION
Earlier studies have shown that the spleen is a source of inflammatory monocytes that rapidly accumulate at sites of tissue injury (Bao et al., 2010; Kim et al., 2014; Swirski et al., 2009). Once localized at these sites, they develop into macrophages with cytotoxic and pro-inflammatory activities, contributing to the pathogenesis of acute ischemic brain injury and myocardial infarction (Bao et al., 2010; Swirski et al., 2009). Similarly, the present studies demonstrate that the spleen functions as a reservoir of pro-inflammatory monocytes that contribute to ozone toxicity in the lung. This is based on our findings that ozone-induced lung injury and oxidative stress are reduced in SPX mice, a response associated with decreased numbers of pro-inflammatory macrophages in the lung. Conversely, increased numbers of a subset of anti-inflammatory macrophages are observed in lungs of SPX mice after ozone exposure. These data suggest that reduced ozone toxicity in SPX mice is a result of a shift in the balance from pro-inflammatory/cytotoxic macrophages to anti-inflammatory/wound repair macrophages.
Splenic Ly6CHi monocytes have been reported to be pro-inflammatory/cytotoxic, whereas Ly6CLo monocytes function to suppress inflammation and promote wound healing (Swirski et al., 2009). Following ozone exposure, we found that both Ly6CHi and Ly6CLo monocyte subpopulations increased in the spleen. However, this response was transient, occurring at 48 h, a time consistent with an increase in the total number of spleen cells. It should be noted, however, that spleen monocytes responding to ozone represent only about 20% of the total spleen cell population. Similar transient increases in monocytes in the spleen have been described in mice after thermal injury to the skin, and after Plasmodium chabaudi exposure, suggesting that this may be a general response to tissue injury or infection (Helmby et al., 2000; Noel et al., 2007). Conversely, a reduction in splenic Ly6CHi pro-inflammatory monocytes has been reported after post-ischemic brain injury in mice (Bao et al., 2010; Kim et al., 2014). This may be due to egress of these cells from the spleen after injury. Immature Ly6CInt monocytes were also identified in the spleen. These cells are thought to be in transition from a Ly6CHi to a Ly6CLo phenotype (Lin et al., 2009). The fact that this monocyte subpopulation increased in the spleen 48 h after ozone exposure suggests that these cells may be important in replenishing pools of anti-inflammatory monocytes deployed from the spleen after lung injury. This is supported by findings that the spleen rapidly replenishes its monocytic reservoir after injury (Swirski et al., 2009).
Consistent with previous findings, ozone inhalation resulted in increased numbers of CD11b+ infiltrating myeloid cells in the lung (Sunil et al., 2015; Tighe et al., 2011). The majority of the cells were Ly6G−, indicating a monocytic phenotype; this is in accord with our findings that >98% of BAL cells are macrophages. In CTL mice, maximal accumulation of Ly6CHi pro-inflammatory and Ly6CLo anti-inflammatory macrophages in the lung was observed 24 and 48 h post ozone exposure. The observation that this occurs earlier than peak accumulation of monocytic precursors in the spleen indicates that transient increases in the spleen may mainly function to replace depleted reservoirs. This is supported by our findings that only about 20% of the spleen monocytes migrate to the lung after ozone exposure. Splenectomy resulted in reduced numbers of Ly6CHi, as well as MMP-9+ pro-inflammatory macrophages in the lung. These findings are consistent with previous reports demonstrating that the spleen is a source of pro-inflammatory monocytic precursors that respond to tissue injury (McKim et al., 2016; Kim et al., 2014). In contrast, a reduction in Ly6CHi macrophages has been described in cardiac tissue after splenectomy in a model of myocardial infarction (Swirski et al., 2009). These data suggest that the contribution of splenic monocytes to injury and infection likely depends on the disease model and the nature of the pathology. Following ozone exposure, Ly6CLo anti-inflammatory macrophages increased in the lungs of SPX mice relative to CTL mice, indicating that the origin of these cells is distinct from pro-inflammatory macrophages. Interestingly, MR-1+ anti-inflammatory macrophages were constitutively greater in SPX mice relative to CTL mice, however, these cells were unaffected by ozone exposure. These findings provide support for the notion that there are multiple subpopulations of anti-inflammatory macrophages and that their origins are distinct (Gordon and Taylor, 2005; Wynn et al., 2013). Decreases in pro-inflammatory lung macrophages responding to ozone in SPX mice resulted in a predominance of anti-inflammatory macrophages in the lung. A similar shift in the phenotype of macrophages responding to ozone and reduced toxicity has been described in mice lacking galectin-3 (Sunil et al., 2015). Conversely, in the absence of CX3CR1+ M2 macrophages, mice have been reported to be hypersensitive to ozone, a response correlated with increased numbers of pro-inflammatory M1 macrophages in the lung (Tighe et al., 2011). Taken together, these data support the idea that ozone toxicity is due, in part, to an imbalance between pro-inflammatory and anti-inflammatory macrophages in the lung (Laskin et al., 2011).
Following ozone exposure, increases in immature pro-inflammatory Ly6CHi monocytes were also observed in the bone marrow, consistent with enhanced myelopoiesis in response to tissue injury (Courties et al., 2015). In SPX mice, pro-inflammatory Ly6CHi monocytes decreased in the bone marrow, whereas anti-inflammatory Ly6CLo and transitional Ly6CInt monocytes increased following ozone exposure. Increased numbers of anti-inflammatory macrophages in the lungs of SPX mice may be a consequence of increased release of these cells from the bone marrow. This may be a compensatory response to the loss of the splenic myeloid reservoir (Tripp et al., 1997). A similar decrease in bone marrow monocytes has been reported in patients who have undergone splenectomy (Franco et al., 1996).
Myeloid derived suppressor cells (MDSCs) are a heterogeneous population of leukocytes known to negatively regulate inflammatory responses to injury and infection (Nagaraj and Gabrilovich, 2010). Previous studies have shown that MDSCs increase in the lungs after Mycobacterium tuberculosis, Pseudomonas aeruginosa, or Pneumocystis pneumonia infection (Obregon-Henao et al., 2013; Rieber et al., 2013; Zhang et al., 2012). Similarly, we observed increased numbers of G-MDSCs and M-MDSCs in the lung, as well as the spleen after ozone exposure. Whereas splenectomy had no effect on lung M-MDSCs, G-MDSCs were reduced, suggesting distinct origins of these MDSC subpopulations. Our findings are consistent with reports that the spleen is a source of G-MDSCs (Levy et al., 2015). Elevated numbers of G-MDSCs and M-MDSCs were also observed in bone marrow of SPX mice, when compared with CTL mice, which may be due to a compensatory increase in the generation of these cells in the absence of the spleen. The role of MDSCs in ozone-induced injury and inflammation is unknown. Lung MDSCs have been implicated in the resolution of inflammation via downregulation of toll-like receptor (TLR) 4 (Bunt et al., 2009). TLR4 has been reported to play a role in ozone-induced lung injury and inflammation (Connor et al., 2012; Hollingsworth et al., 2007). MDSCs may act to reduce TLR4 in the lungs after ozone, which contributes to down regulating inflammation and promoting wound repair; however, this remains to be investigated.
Macrophage trafficking to sites of injury is regulated by locally released chemokines and chemokine receptors present on responding cells. We found that ozone-induced upregulation of chemokines CCL4 and CCL3, and the chemokine receptor CCR1 was correlated with an accumulation of pro-inflammatory macrophages in the lung, suggesting potential signaling pathways mediating their trafficking. Splenectomy resulted in a blunted response of CCR1, CCL4, and CCL3 to ozone, but an increased response of CCR2 and CCL2. Evidence suggests that CCR2 and CCL2 are required for pro-inflammatory Ly6CHi monocyte egress from the bone marrow (Serbina and Pamer, 2006). Decreases in pro-inflammatory Ly6CHi monocytes in the bone marrow of SPX mice may be due to upregulation of CCR2 on these cells, which facilitates their release from the bone marrow. AT1R is involved in splenic monocyte migration to sites of injury (Mellak et al., 2015). Splenic AT1R+ monocytes have been reported to play a role in the pathogenesis of myocardial infarction; thus, reduced monocyte accumulation in the myocardium was observed in mice treated with angiotensin-converting enzyme inhibitor (Nahrendorf et al., 2010). Following ozone exposure, increases in AT1R+ macrophages were observed in the lungs in CTL mice. Findings that ozone-induced accumulation of these cells in the lungs and expression of AT1R mRNA were delayed in SPX mice indicate that the early responding AT1R+ macrophages originate in the spleen. The origin of the AT1R+ macrophages appearing in the lung at later times post ozone in SPX mice remains to be determined.
Decreases in pro-inflammatory macrophages in the lungs of ozone treated SPX mice were correlated with reduced oxidative stress, as measured by the appearance of 4-HNE, a lipid peroxidation end product generated by free radicals following ozone exposure (Grimsrud et al., 2008; Kirichenko et al., 1996). BAL protein content, a marker of alveolar epithelial barrier dysfunction, was also decreased in ozone-exposed SPX mice. These data support the idea that the spleen is a source of cytotoxic/pro-inflammatory precursors and/or mediators that contribute to ozone-induced lung injury and inflammation. Conversely, ozone-induced structural alterations in SP-D were unaffected by splenectomy. These results were surprising because previous studies showed that reactive nitrogen species (RNS) released by pro-inflammatory macrophages mediate post-translational modifications of SP-D (Atochina-Vasserman, 2012; Groves et al., 2012; Malaviya et al., 2015). These data suggest that there are alternative sources of RNS in the lungs of SPX mice that modify SP-D. In this regard, earlier studies have shown that Type II pneumocytes generate nitric oxide in response to inflammatory cytokines following ozone exposure (Punjabi et al., 1994). Moreover, RNS generated from lung epithelial cells can contribute to SP-D modifications (Bove and van der Vliet, 2006).
In conclusion, the present studies demonstrate that the spleen contributes a subpopulation of inflammatory macrophages to pools of lung leukocytes responding to ozone. Findings that splenectomy protected mice from ozone-induced injury and oxidative stress are consistent with the idea that the spleen is a source of pro-inflammatory/cytotoxic monocytes. This is supported by our observation that peak accumulation of pro-inflammatory/cytotoxic macrophages in the lung precedes ozone-induced injury and oxidative stress. Identification of the origin of inflammatory macrophages contributing to ozone toxicity may be important in the development of novel therapeutics aimed at selectively targeting these cells and reducing lung injury.
FUNDING
This work was supported by the National Institutes of Health [grant numbers ES004738, AR055073, ES007148, HL086621, and ES005022].
Supplementary Material
REFERENCES
- Alexeeff S. E., Litonjua A. A., Suh H., Sparrow D., Vokonas P. S., Schwartz J. (2007). Ozone exposure and lung function: effect modified by obesity and airways hyperresponsiveness in the VA normative aging study. Chest 132, 1890–1897. [DOI] [PubMed] [Google Scholar]
- Atochina-Vasserman E. N. (2012). S-nitrosylation of surfactant protein D as a modulator of pulmonary inflammation. Biochim. Biophys. Acta. 1820, 763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y., Kim E., Bhosle S., Mehta H., Cho S. (2010). A role for spleen monocytes in post-ischemic brain inflammation and injury. J Neuroinflammation 7, 92.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove P. F., van der Vliet A. (2006). Nitric oxide and reactive nitrogen species in airway epithelial signaling and inflammation. Free Radic. Biol. Med. 41, 515–527. [DOI] [PubMed] [Google Scholar]
- Bunt S. K., Clements V. K., Hanson E. M., Sinha P., Ostrand-Rosenberg S. (2009). Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J. Leukoc. Biol. 85, 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers D. E., Holtzman M. J. (2011). Alternatively activated macrophages and airway disease. Chest 140, 768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor A. J., Laskin J. D., Laskin D. L. (2012). Ozone-induced lung injury and sterile inflammation. Role of toll-like receptor 4. Exp. Mol. Pathol. 92, 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courties G., Herisson F., Sager H. B., Heidt T., Ye Y., Wei Y., Sun Y., Severe N., Dutta P., Scharff J., et al. (2015). Ischemic stroke activates hematopoietic bone marrow stem cells. Circ. Res. 116, 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhrzadeh L., Laskin J. D., Laskin D. L. (2002). Deficiency in inducible nitric oxide synthase protects mice from ozone-induced lung inflammation and tissue injury. Am. J. Respir. Cell Mol. Biol. 26, 413–419. [DOI] [PubMed] [Google Scholar]
- Franco V., Florena A. M., Campesi G. (1996). Intrasinusoidal bone marrow infiltration: A possible hallmark of splenic lymphoma. Histopathology 29, 571–575. [DOI] [PubMed] [Google Scholar]
- Gordon S., Taylor P. R. (2005). Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964. [DOI] [PubMed] [Google Scholar]
- Grimsrud P. A., Xie H., Griffin T. J., Bernlohr D. A. (2008). Oxidative stress and covalent modification of protein with bioactive aldehydes. J. Biol. Chem. 283, 21837–22841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves A. M., Gow A. J., Massa C. B., Laskin J. D., Laskin D. L. (2012). Prolonged injury and altered lung function after ozone inhalation in mice with chronic lung inflammation. Am. J. Respir. Cell Mol. Biol. 47, 776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C. J., Atochina-Vasserman E. N., Abramova E., Foley J. P., Zaman A., Crouch E., Beers M. F., Savani R. C., Gow A. J. (2008). S-nitrosylation of surfactant protein-D controls inflammatory function. PLoS Biol. 6,e266.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth A. M., Janssen W. J., Bosio C. M., Crouch E. C., Henson P. M., Dow S. W. (2009). Lung environment determines unique phenotype of alveolar macrophages. Am. J. Physiol. Lung Cell Mol. Physiol. 296, L936–L946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmby H., Jonsson G., Troye-Blomberg M. (2000). Cellular changes and apoptosis in the spleens and peripheral blood of mice infected with blood-stage Plasmodium chabaudi chabaudi AS. Infect. Immun. 68, 1485–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth J. W., Maruoka S., Li Z., Potts E. N., Brass D. M., Garantziotis S., Fong A., Foster W. M., Schwartz D. A. (2007). Ambient ozone primes pulmonary innate immunity in mice. J. Immunol. 179, 4367–4375. [DOI] [PubMed] [Google Scholar]
- Kim E., Yang J., Beltran C. D., Cho S. (2014). Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J. Cereb. Blood Flow Metab. 34, 1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichenko A., Li L., Morandi M. T., Holian A. (1996). 4-hydroxy-2-nonenal-protein adducts and apoptosis in murine lung cells after acute ozone exposure. Toxicol. Appl. Pharmacol. 141, 4116–4124. [DOI] [PubMed] [Google Scholar]
- Laskin D. L., Sunil V. R., Gardner C. R., Laskin J. D. (2011). Macrophages and tissue injury: Agents of defense or destruction? Annu. Rev. Pharmacol. Toxicol. 51, 267–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavnikova N., Prokhorova S., Helyar L., Laskin D. L. (1993). Isolation and partial characterization of subpopulations of alveolar macrophages, granulocytes, and highly enriched interstitial macrophages from rat lung. Am. J. Respir. Cell Mol. Biol. 8, 384–392. [DOI] [PubMed] [Google Scholar]
- Levy L., Mishalian I., Bayuch R., Zolotarov L., Michaeli J., Fridlender Z. G. (2015). Splenectomy inhibits non-small cell lung cancer growth by modulating anti-tumor adaptive and innate immune response. Oncoimmunology 4, e998469.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. L., Castano A. P., Nowlin B. T., Lupher M. L., Jr, Duffield J. S. (2009). Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J. Immunol. 183, 6733–6743. [DOI] [PubMed] [Google Scholar]
- Malaviya R., Gow A. J., Francis M., Abramova E. V., Laskin J. D., Laskin D. L. (2015). Radiation-induced lung injury and inflammation in mice: role of inducible nitric oxide synthase and surfactant protein D. Toxicol. Sci. 144, 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim D. B., Patterson J. M., Wohleb E. S., Jarrett B. L., Reader B. F., Godbout J. P., Sheridan J. F. (2016). Sympathetic release of splenic monocytes promotes recurring anxiety following repeated social defeat. Biol. Psychiatry 79, 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebius R. E., Kraal G. (2005). Structure and function of the spleen. Nat. Rev. Immunol. 5, 606–616. [DOI] [PubMed] [Google Scholar]
- Mellak S., Ait-Oufella H., Esposito B., Loyer X., Poirier M., Tedder T. F., Tedgui A., Mallat Z., Potteaux S. (2015). Angiotensin II mobilizes spleen monocytes to promote the development of abdominal aortic aneurysm in Apoe-/- mice. Arterioscler. Thromb. Vasc. Biol. 35, 378–388. [DOI] [PubMed] [Google Scholar]
- Misharin A. V., Morales-Nebreda L., Mutlu G. M., Budinger G. R., Perlman H. (2013). Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am. J. Respir. Cell Mol. Biol. 49, 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A. J., Roediger B., Weninger W. (2014). Monocyte homeostasis and the plasticity of inflammatory monocytes. Cell Immunol. 29, 22–31. [DOI] [PubMed] [Google Scholar]
- Mudway I. S., Kelly F. J. (2000). Ozone and the lung: a sensitive issue. Mol. Aspects Med. 21, 1–48. [DOI] [PubMed] [Google Scholar]
- Nagaraj S., Gabrilovich D. I. (2010). Myeloid-derived suppressor cells in human cancer. Cancer J. 16, 348–353. [DOI] [PubMed] [Google Scholar]
- Nahrendorf M., Pittet M. J., Swirski F. K. (2010). Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 121, 2437–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel J. G., Osterburg A., Wang Q., Guo X., Byrum D., Schwemberger S., Goetzman H., Caldwell C. C., Ogle C. K. (2007). Thermal injury elevates the inflammatory monocyte subpopulation in multiple compartments. Shock 28, 684–693. [DOI] [PubMed] [Google Scholar]
- Obregon-Henao A., Henao-Tamayo M., Orme I. M., Ordway D. J. (2013). Gr1intCD11b+ myeloid-derived suppressor cells in Mycobacterium tuberculosis infection. PLoS One 8, e80669.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendino K. J., Meidhof T. M., Heck D. E., Laskin J. D., Laskin D. L. (1995). Inhibition of macrophages with gadolinium chloride abrogates ozone-induced pulmonary injury and inflammatory mediator production. Am. J. Respir. Cell Mol. Biol. 13, 125–132. [DOI] [PubMed] [Google Scholar]
- Prokhorova S., Lavnikova N., Laskin D. L. (1994). Functional characterization of interstitial macrophages and subpopulations of alveolar macrophages from rat lung. J. Leukoc. Biol. 55, 141–146. [DOI] [PubMed] [Google Scholar]
- Punjabi C. J., Laskin J. D., Pendino K. J., Goller N. L., Durham S. K., Laskin D. L. (1994). Production of nitric oxide by rat type II pneumocytes: increased expression of inducible nitric oxide synthase following inhalation of a pulmonary irritant. Am. J. Respir. Cell Mol. Biol. 11, 165–172. [DOI] [PubMed] [Google Scholar]
- Rieber N., Brand A., Hector A., Graepler-Mainka U., Ost M., Schafer I., Wecker I., Neri D., Wirth A., Mays L., et al. (2013). Flagellin induces myeloid-derived suppressor cells: implications for Pseudomonas aeruginosa infection in cystic fibrosis lung disease. J. Immunol. 190, 1276–1284. [DOI] [PubMed] [Google Scholar]
- Serbina N. V., Pamer E. G. (2006). Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7, 311–317. [DOI] [PubMed] [Google Scholar]
- Sunil V. R., Francis M., Vayas K. N., Cervelli J. A., Choi H., Laskin J. D., Laskin D. L. (2015). Regulation of ozone-induced lung inflammation and injury by the beta- galactoside-binding lectin galectin-3. Toxicol. Appl. Pharmacol. 284, 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunil V. R., Patel-Vayas K., Shen J., Laskin J. D., Laskin D. L. (2012). Classical and alternative macrophage activation in the lung following ozone-induced oxidative stress. Toxicol. Appl. Pharmacol. 263, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirski F. K., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P., Figueiredo J. L., Kohler R. H., Chudnovskiy A., Waterman P., et al. (2009). Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe R. M., Li Z., Potts E. N., Frush S., Liu N., Gunn M. D., Foster W. M., Noble P. W., Hollingsworth J. W. (2011). Ozone inhalation promotes CX3CR1-dependent maturation of resident lung macrophages that limit oxidative stress and inflammation. J. Immunol. 187, 4800–4808. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Tripp R. A., Topham D. J., Watson S. R., Doherty P. C. (1997). Bone marrow can function as a lymphoid organ during a primary immune response under conditions of disrupted lymphocyte trafficking. J. Immunol. 158, 3716–3720. [PubMed] [Google Scholar]
- Vallyathan V., Shi X. (1997). The role of oxygen free radicals in occupational and environmental lung diseases. Environ. Health Perspect. 105 Suppl 1, 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T. A., Chawla A., Pollard J. W. (2013). Macrophage biology in development, homeostasis and disease. Nature 496, 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn J. I., Nagaraj S., Collazo M., Gabrilovich D. I. (2008). Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 181, 5791–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaynagetdinov R., Sherrill T. P., Kendall P. L., Segal B. H., Weller K. P., Tighe R. M., Blackwell T. S. (2013). Identification of myeloid cell subsets in murine lungs using flow cytometry. Am. J. Respir. Cell Mol. Biol. 49, 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Lei G. S., Shao S., Jung H. W., Durant P. J., Lee C. H. (2012). Accumulation of myeloid-derived suppressor cells in the lungs during Pneumocystis pneumonia. Infect. Immun. 80, 3634–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.