Abstract
Our ears are remarkable sensory organs, providing the important senses of balance and hearing. The complex structure of the inner ear, or ‘labyrinth’, along with the assorted neuroepithelia, has evolved to detect head movements and sounds with impressive sensitivity. The rub is that the inner ear is highly vulnerable to genetic lesions and environmental insults. According to National Institute of Health estimates, hearing loss is one of the most commonly inherited or acquired sensorineural diseases. To understand the causes of deafness and balance disorders, it is imperative to understand the underlying biology of the inner ear, especially the inner workings of the sensory receptors. These receptors, which are termed hair cells, are particularly susceptible to genetic mutations--more than two dozen genes are associated with defects in this cell type in humans. Over the past decade, a substantial amount of progress has been made in working out the molecular basis of hair-cell function using vertebrate animal models. Given the transparency of the inner ear and the genetic tools that are available, zebrafish have become an increasingly popular animal model for the study of deafness and vestibular dysfunction. Mutagenesis screens for larval defects in hearing and balance have been fruitful in finding key components, many of which have been implicated in human deafness. This review will focus on the genes that are required for hair-cell function in zebrafish, with a particular emphasis on mechanotransduction. In addition, the generation of new tools available for the characterization of zebrafish hair-cell mutants will be discussed.
Keywords: hair cell, inner ear, lateral line organ, deafness gene, zebrafish, mechanotransduction, hair bundle
Introduction
In vetebrates, head movements and sounds are sensed by the inner ear. The inner ear contains multiple groups of neuroepithelia that are specialized for diverse functions. Sensory patches are housed in uniquely shaped compartments dedicated to either one sense or the other, and sometimes a combination of both.
For sensing gravity and rotation of the head, all vertebrates rely on a vestibular apparatus. The anatomy of this apparatus is relatively conserved among species, with some prominent exceptions in jaw-less vertebrates such as the hagfish and lamprey (Beisel et al., 2005). Though the structures and endorgans may vary in terms of size, shape, and number, the vestibular portion of the ear in many jawed vertebrates largely includes three semicircular canals, positioned in different orientations in space (Fritzsch et al., 2002; Whitfield and Hammond, 2007). The semicircular canals participate in the detection of angular acceleration of the head. There are also macular endorgans, such as the utricle and saccule, and in some cases, the lagena. These pouch-like structures contain a neuroepithelium coupled to calcium carbonate crystals. The crystals either aggregate into one massive structure called an otolith (Figure 1A), or in amniotes they may be small and numerous, and are known as otoconia. Macular endorgans mainly detect linear acceleration and gravity, but also occupy different orientations in space. Either type of vestibular structure relies on displacement of either the fluid in the canals, or the dense crystal structures during head movements to induce excitation of the neuroepithelia.
Figure 1.
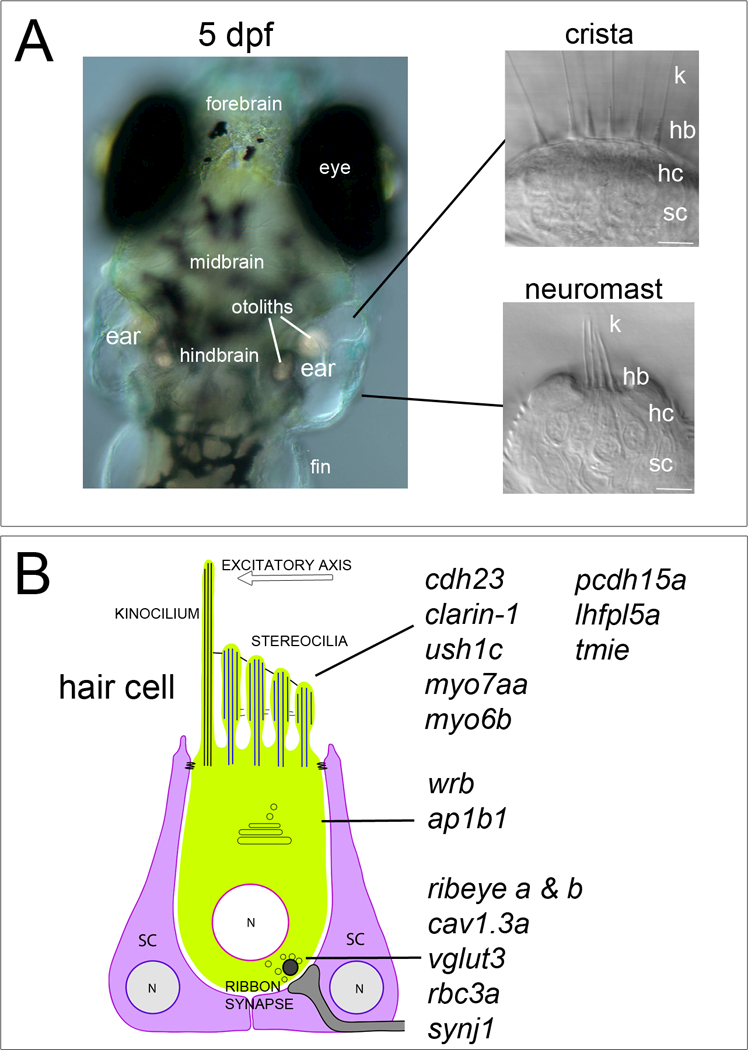
Genes required for hair-cell function in larval zebrafish. A, Dorsal view of the head region of a 5 dpf larva. The focal plane is centered on the two transparent inner ears, which contain two macular endorgans associated with otoliths, known as the anterior and posterior maculae (destined to become the utricle and saccule, respectively), and three developing semi-circular canals. Right panels, example of a cluster of hair cells in a crista positioned within a semi-circular canal, and a superficial neuromast of the lateral line organ. At this stage, approximately 50 neuromasts are present at the surface of the skin. B, Genes implicated in deafness and/or vestibular dysfunction in zebrafish. Mutations were generated by chemical (ethylnitrosourea) or retroviral mutagenesis for large-scale screens, or by gene editing technology. Three general categories are shown: genes that are required for (i) mechanotransduction, with protein products localized to the hair bundle (top group), or genes required for (ii) the function of the secretory pathway (middle group), and genes required for (iii) normal synaptic transmission (lower group). The excitatory axis of the hair bundle is indicated by the white arrow. k, kinocilium; hb, hair bundle (stereocilia); hc, hair cell; sc, supporting cell. Scale bars, 5 μm.
For sound detection, further structures have evolved in amniotes as an outgrowth from the saccule or as an elongation of the lagenar pouch (Fritzsch and Straka, 2014). The enlongated endorgans in amphibians, reptiles and birds, and the snail-shaped cochlea in mammals, are dedicated to hearing, and they have developed a greater sensitivity to sound intensity and can detect a wider range of frequencies. In fish and to some extent in amphibians, the saccule and potentially the lagena are used for hearing (Khorevin, 2008). Sound causes the body of the fish to move, but because of the greater density of the otoliths, the otoliths lag behind the movement of the surrounding tissue. The inertia of the otolith with respect to the neuroepithelium can cause excitation of the sensory cells (see below). Fish that rely solely on this type of stimulation are usually limited in hearing ability. Some fish such as the zebrafish, however, have a specialization that increases their sensitivity to sound. This specialization consists of a series of bones known as the Weberian ossicles that connect a fluid filled sac in contact with the saccule to the gas-filled swimbladder. Sound traveling through the fish’s body sets the swimbladder into motion via compression of the gas inside, and this oscillation amplifies the sound delivered to the saccule by these bony elements (Fritzsch and Straka, 2014). The ossicles appear later in development during the juvenile stage, but it is still the case that the saccule (posterior macula) is sufficient for hearing even in zebrafish larvae as young as 5 days post fertilization (dpf) (Mo et al., 2010).
Aquatic vertebrates such as fish and amphibians also possess a lateral line organ. At the larval stage, this organ consists of numerous small patches of neuroepithelia at the surface of the skin called neuromasts (Figure 1A). Later on, some species develop long, narrow canals under the skin with openings at either end. The canals encapsulate the neuroepithelia. Both the superficial and canal elements of the lateral line organ detect water movements, allowing aquatic animals to have a sense of ‘distant touch’ of their local environment (Bleckmann, 2008). This sense is important for detecting prey or predators, schooling behavior, and orientation in moving water.
Despite the seemingly different nature of head movements and sound, the inner ear employs the same type of receptor cell, the hair cell, to sense both vestibular and auditory stimuli. Hair cells are secondary receptors in that they do not possess axons, but rather are innervated by afferent neurons that transmit signals to the brain. Though their evolutionary origin is unclear, auditory/vestibular hair cells share remarkable morphological and functional similarities to mechanosensory coronal cells in sea squirts (Burighel et al., 2011; Fritzsch and Straka, 2014). In addition, hair cells are quite similar to certain types of electroreceptors that may have evolved as a modification of lateral-line hair cells (Baker et al., 2013). Like most sensory receptors, hair cells have a highly specialized structure at their apical surfaces. These apical structures consist of a bundle of villi-like structures called stereocilia, collectively referred to as a hair bundle. Stereocilia are arranged in rows of increasing height in an asymmetric fashion (Figure 1B). Unlike a conventional villus, each stereocilium has a densely packed core of actin filaments and rather than bending when deflected, stereocilia pivot about their insertion site, providing an unusual stiffness to the hair bundle (Crawford and Fettiplace, 1985; Howard and Ashmore, 1986). This stiffness is key to the sensory capabilities of the hair cell. Stiffness tends to be proportional to the number of stereocilia (Howard and Ashmore, 1986), and lateral extracellular filaments and horizontal top connectors ensure that hair bundles move as a unit (Kozlov et al., 2007). When a sound wave or turning of the head causes the stereocilia to pivot about their insertion site, then mechanosensitive channels open and the hair cell depolarizes. The conversion of mechanical energy to an electrical signal within the cell is called mechanotransduction. Excitation happens mainly in one direction, namely when the bundle is deflected towards the taller stereocilia, or the tubulin-based kinocilium if it is present (Figure 1B). Kinocilia are asymmetrically located and are interconnected with the tallest stereocilia. The first actual demonstration of directional sensitivity of hair cells was made with recordings from lateral-line hair cells (Flock and Wersall, 1962). Running along the excitatory axis of the hair bundle are fine, usually singular filaments that interconnect the stereocilia at their tips (Figure 1B; Figure 2A-B). These filaments are known as tip links and deflection of hair bundles along the excitatory axis increases tension on tip links. This increase in tension results in the opening of mechanotransduction channels at the tips of the stereocilia (Basu et al., 2016; Corey and Hudspeth, 1983a). In contrast, deflections toward the shorter stereocilia are inhibitory and cause hyperpolarization of the cell. The extracellular fluid bathing the hair bundles (known as endolymph) is unusually rich in K+ ions, and because of a difference in electrical potential across the epithelium, K+ flows into the hair cell during excitatory deflections. Influx of K+ through the transduction channels results in depolarization of the soma. Depolarization, however, does not cause an action potential to fire, but rather results in graded changes in membrane potential. Subsequently the change in membrane voltage causes calcium channels at hair cell synapses to open, leading to the fusion of synaptic vesicles and the release of neurotransmitter.
Figure 2.
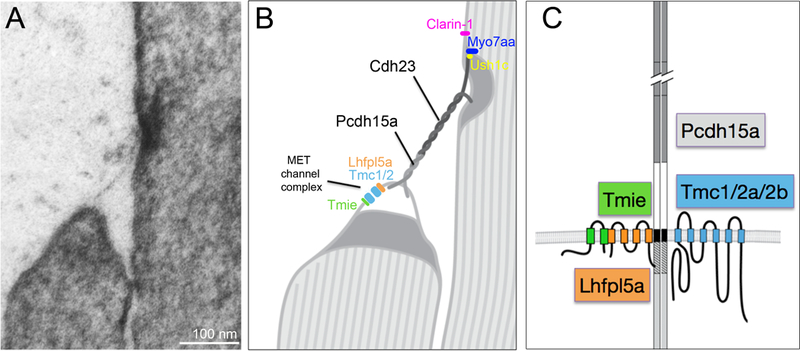
Working model of the mechanotransduction machinery in zebrafish hair cells. A, Transmission electron micrograph of a tip link of a zebrafish hair cell (anterior macula). Note the tenting of the lower tip, suggesting that tension on the membrane was present. Also present is the upper density or plaque in the taller stereocilium. B, Schematic of the transduction components, including the presumptive orientation of the novel cadherins, Pcdh15a and Cdh23, and the position of the transduction channel at the lower link. C, Integral membrane proteins associated with Pcdh15a that are critical for mechanotransduction in zebrafish. The requirement for the Tmc proteins, which are currently the best candidates for the mechanotransduction channel in mammalian hair cells, has yet to be demonstrated in zebrafish. However, Pcdh15a physically associates with Tmc2a (see Maeda et al., 2014).
Sensory hair cells differ morphologically among the different endorgans and even within an endorgan, likely reflecting some specialization to the particular type of stimuli detected (Xue and Peterson, 2006). For example, hair cells in semi-circular canals (known as ampullary hair cells) usually contain several rows of stereocilia that vary greatly in height, giving rise to a steep staircase or highly tapered edge to the bundle (Peterson et al., 1996). This configuration results in a tall, yet narrow and conically shaped hair bundle (Figure 1A, top panel). Often the asymmetrically localized kinocilia are three-fold longer than the tallest stereocilia. Compared to other hair cell types, this type of bundle architecture is likely to be more compliant, and due to the large differences in height, forces from the kinocilium to the stereocilia may propagate less well (Peterson et al., 1996; Pickles, 1993; Silber et al., 2004). Presumably their bundle architecture makes ampullary hair cells ideal for sensing low frequency rotations of the head. Rather than free standing, the long kinocilia of ampullary hair cells are embedded in a gelatinous covering called a cupula that extends all the way across the canal. These jelly-like structures enhance the detection of fluid displacements in the canals. The embedded kinocilia couple the movements of the cupula to the shorter bundles of stereocilia. Neuromasts of the lateral line organ also have a cupula covering the neuroepithelium in a similar manner. In the case of macular organs, kinocilia are attached to a gelatinous structure, the overlying otolithic membrane, which in turn is coupled to the otoliths (or otoconia). As mentioned above, amniotes have additional organs for detecting sound and their receptors such as the inner and outer hair cells in mammals have taken on diversified roles in audition. Additional specializations of the vestibular hair cells have also evolved, such as type 1 versus type 2 hair cells. These cell types are defined by their innervation patterns. Type 1 hair cells have an unusual calyx formed by the afferent neuron that completely envelopes the cell body, whereas type 2 cells have discrete afferent and efferent boutons that create multiple contacts with the basolateral membrane. In zebrafish, the inner ear and lateral-line hair cells most closely resemble type 2 vestibular hair cells in terms of morphology. The variability among zebrafish hair cells occurs mainly in the architecture of the hair bundles (Figure 1A, right panels). A potential exception to this classification is that the afferents fibers in zebrafish typically have very broad contacts to the basolateral membrane with some calyx-like features (see (Nicolson, 2015) for review).
Over the past couple of decades, many zebrafish mutants with auditory/vestibular deficits have been isolated in large-scale mutagenesis screens, with the largest group of mutants being identified in the Nuesslein-Volhard laboratory (Granato et al., 1996; Nicolson et al., 1998). Most forward genetic screens have made simple assessments of swimming behavior and response to tapping on the petri dish, thereby eliciting the acoustic startle reflex. Other studies have identified genetic forms of deafness in human patients and then sought to create zebrafish models of the human disease. To date, more than 14 zebrafish genes have been implicated in hair-cell function using mutagenesis or genome editing methods (Figure 1B; for knockdown studies of auditory/vestibular function using morpholinos, the reader is referred to a recent review on fish models by Blanco-Sanchez et al. (2016)). These genes can be separated into two general classes: 1) those that are required for mechanotransduction, or 2) those required for normal synaptic transmission. A third class disrupts protein processing and sorting that affects both processes. Due to space limitations, this review will focus on those genes that affect mechanotransduction. The genes required for normal synaptic transmission are listed in Figure 1B, however, the reader is referred to a recent review on mutations or gene knockdown experiments affecting ribbon synapses in zebrafish (Nicolson, 2015). In addition, for more information on the topics of development and regeneration, the reader is referred to recent reviews on the morphogenesis of the zebrafish inner ear and development of the sensory epithelia (Alsina and Whitfield, 2016), or hair-cell regeneration in the lateral line organ (Kniss et al., 2016).
Onset and evaluation of hair-cell function in zebrafish
Recently, a number of new methods have been developed or adapted to zebrafish to assess changes in behavior, physiology, and gene expression in larval auditory/vestibular mutants. These tools have been important for describing the level of dysfunction in mutants and gaining insights into the biology of hair-cell function.
Behavioral tests that are helpful for quantifying deficits are centered on robust reflexes that exist at the larval stage. For example, rotation of the head of a larva around an earth horizontal axis (in dark conditions) will induce reflexive eye movements as early as 72 hours postfertilization (hpf) (Mo et al., 2010). In mutants lacking the anterior otolith with the posterior otolith still present, the reflex is absent. This observation suggests that there already is a division of function at this early stage in development, and that the anterior macula is important for detecting linear acceleration and maintaining balance. The utricle in other species also detects linear acceleration of the head, indicating that this function is highly conserved among vertebrates. Differences in the robustness of the vestibular-induced eye movements in mutants are readily detectable using this method.
Measurement of neuronal responses evoked by acoustic stimuli has been reported in adult zebrafish. These experiments include recordings of auditory brainstem responses. Interestingly, a recent report found that zebrafish are sensitive to a broader range of frequencies than originally thought (Wang et al., 2015). Previous studies showed that adult fish were responsive up to 4 kHz. In the report by Wang et al., they observed reliably evoked potentials up to 8 kHz, and if provided at a higher intensity of 155 dB (re 1 μ Pascal), responses to 12 kHz tone bursts were detectable. In larvae, such recordings are not routine and most experiments rather focus on the acoustic startle reflex as a measure of auditory function. The acoustic startle reflex is quite robust at 4–5 dpf, the stage at which the larvae are free swimming and lethality (due to a mutation) is usually not an issue. At this stage, acoustic startle reflexes can be evoked with frequencies of 100 – 1200 Hz (Zeddies, 2005).
One concern with relying on the acoustic startle reflex is that genetic defects may disrupt the function of neurons or cells required for producing a startle reflex, e.g. the Mauthner cell, motor neurons, and so on. In addition, the acoustic startle reflex is not the most sensitive measure of hearing, i.e. a weak stimulus may be detected by the inner ear but may not result in a movement of the larva’s trunk. A more sensitive assay for hearing in larvae involves assessing prepulse inhibition (PPI) of the acoustic startle reflex (Bhandiwad et al., 2013). Presumably the presentation of a weak stimulus before a strong stimulus will dampen the response of the larvae to the stronger stimulus. Larvae at 5 dpf have lower response thresholds for stimuli between 100 and 1 kHz when a prepulse is provided; the greatest differences between the acoustic startle reflex and the PPI assay were observed at low frequencies (100–300 Hz). Still, the PPI method relies on observing a startle reflex and seeing a reduction of the reflex if a prepulse tone can be heard. Thus, studies of hearing deficits in larvae would benefit from other, more direct measures of hair-cell activity to assess the function of a particular gene.
With respect to characterizing physiological properties of zebrafish hair cells, the least invasive method is the measurement of microphonic potentials. This type of recording measures external voltage changes that occur in the vicinity of hair bundle when cations flow through the transduction channel and subsequently activate voltage-gated and other assorted channels in the basolateral membrane (Corey and Hudspeth, 1983b). As the neuromast hair cells are conveniently superficial, it is possible to nestle a recording pipette close to the hair bundles and collectively stimulate the cluster of hair cells with a water jet (Trapani and Nicolson, 2010). Such recordings of extracellular potentials have been done for many decades on fish and amphibians and have proven valuable in assessing whether the sum of currents (mechanotransduction plus basolateral currents) is affected in mutants. This method was also recently adapted to measuring the microphonics of inner ear hair cells. Recordings of the hair cells of the posterior macula (saccule) show that microphonic potentials can be detected as early as 23 hpf (Tanimoto et al., 2011; Yao et al., 2016). Remarkably, already within 28 hpf, mechanical stimulation of macular hair cells can elicit postsynaptic currents in Mauthner cells in the hindbrain, which receive inputs from auditory afferent neurons (Tanimoto et al., 2009). These studies attest to how quickly nascent hair cells become functional and how early the circuitry to the brain is operational.
Despite the relative ease of microphonic recordings, the presence of a change in the summation of currents in a mutant does not indicate which types of currents are affected. Thus, another type of recording was recently established in zebrafish larvae: whole-cell recordings of lateral-line hair cells (Olt et al., 2014, 2016a, 2016b; Ricci et al., 2013). The technique is challenging and to date, direct measurements of mechanotransduction currents are sparse, but further use and development of this method will be critical for characterizing different types of currents in various mutants. This method is also useful for assessing exocytosis at hair cell synapses by measuring capacitance changes. Largely due to the relatively small size of neuromast hair cells, the duration of the stimulus needed to see a change in capacitance is long (1 second) in comparison to other preparations and suggests that detecting differences in the kinetics of exocytosis may be challenging.
Another useful recording is a loose patch recording of the afferent neurons that innervate the hair cells (Obholzer et al., 2008; Trapani and Nicolson, 2010). The number of spikes or spike rate in the afferent neurons is indicative of the level of synaptic transmission, and one can assess phase locking, which is the ability of innervating neurons to fire in phase with the deflection of the hair bundles. Also, this type of recording provides information about ‘run down’ or depression of neurotransmitter release. One drawback to the method is that each neuron innervates many hair cells, and the recording is thus a readout of many synapses (at 5 dpf, > 15 synapses per neuron), so it is difficult to say what is happening at individual synapses.
Recording microphonic potentials is relatively straightforward, whereas whole cell recordings can be time consuming due to the need to remove skin cells and supporting cells with a suction pipette before patching onto hair cells (Olt et al., 2016). Neither method is amenable to high-throughput experiments. As an alternative to performing recordings of transduction currents in zebrafish hair cells, one can use labeling with vital dyes such as the styryl dye FM1–43 as a proxy for mechanotransduction. Labeling with FM1–43 was first reported in frog lateral-line hair cells (Nishikawa and Sasaki, 1996) and then later shown to correlate with the decrease in microphonics in various zebrafish mutants (Seiler and Nicolson, 1999). The loading of FM1–43 dye in hair cells is extremely fast, unlike conventional endocytosis. Voltage clamp experiments using mouse cochlear hair cells demonstrated that FM1–43 acts as a permeant blocker of transduction channels, explaining the fast kinetics of labeling (Gale et al., 2001). Other vital dyes such as DASPEI appear to work in the same way. Due to its convenience and robust fluorescent signal, the use of FM1–43 is widespread in the field for assessing mechanotransduction defects in auditory and vestibular hair cells in a variety of species.
Another boon to the analysis of zebrafish hair cells has been the introduction of genetically encoded calcium indicators (Kindt et al., 2012a; Zhang et al., 2016). Such sensors allow one to analyze individual cells, or even the activity of subcellular regions within a hair cell. In line with the early onset of function of inner ear hair cells, calcium imaging of individual lateral-line hair cells followed by scanning electron microscopy revealed that lateral-line hair cells respond to mechanical stimuli before their hair bundles are mature (Kindt et al., 2012b). In fact, one row of stereocilia (as opposed to five or more rows of stereocilia in mature bundles) is sufficient to mediate mechanically evoked responses. Interestingly, the response to mechanical stimuli is initially in the wrong direction, i.e. upon deflection along the inhibitory axis. Gradually the nascent hair cells become responsive to both inhibitory and excitatory deflections, and this pattern eventually gives way to only the excitatory response in more mature hair cells. The basis for these initial responses is not fully understood, but mechanosensitivity does require tip links and is sensitive to known mechanotransduction blockers (Kindt et al., 2012b). A bidirectional response was also reported in immature cochlear hair cells (Waguespack et al., 2007), suggesting that such developmental steps may occur in other hair-cell types. Another genetic tool that has been adapted to zebrafish hair cells is the expression of Channel-rhodopsin (Monesson-Olson et al., 2014). Although less precise in terms of phase-locking to afferent spiking, this tool allows one to activate hair cells with a pulse of light.
Components of the mechanotransduction complex
It has been long recognized that the top of the apical hair bundle is the site of mechanotransduction (Hudspeth, 1982). Initially the mechanism of transduction was not clear. The discovery of tip links running parallel to the excitatory axis was a key finding (Pickles et al., 1984), and their requirement for mechanotransduction narrowed the focus of the field to this region of the hair bundle (Assad et al., 1991). Much progress has been made in identifying the molecular composition of the tip links and the proteins that are associated with them (Figure 2). One of the first components of the tip links to be identified was a novel FAT-like Cadherin, Cadherin 23 (Cdh23)(Siemens et al., 2004; Söllner et al., 2004). This discovery occurred in parallel in studies of zebrafish and mouse mutants, and in the zebrafish mutant, the weak allele of cdh23 did not have an obvious effect on the integrity of hair bundles, thus providing genetic evidence for a direct role in mechanotransduction. Another component was a protocadherin, PCDH15, that was shown to interact with CDH23 (Kazmierczak et al., 2007). This interaction was demonstrated in an in vitro preparation of the mouse proteins using scanning electron microscopy, and by immunogold labeling of mouse stereocilia. Although it was not known at the time to be part of the tip link, a study on Pcdh15a in zebrafish had similar findings to the above study on Cdh23, that is, evidence in the fish mutants suggested that Pcdh15 had a functional role in mechanotransduction, rather than just serving as an adhesion molecule that interconnected stereocilia (Seiler et al., 2005). In earlier studies, both CDH23 and PCDH15 were implicated in human deafness and the phenotype in the corresponding mouse Cdh23 and Pcdh15 mutants was severe splaying and disorganization of bundles (see review in (Brown et al., 2008)). This splaying phenotype in mice suggested that these cadherins were critical for keeping the bundle together, however the allelic series of these cadherins in zebrafish (as well as the salsa allele of Cdh23 in mice (Schwander et al., 2009)) showed otherwise, and were important for deducing a role in mechanotransduction.
As observed in mammals (Ahmed et al., 2006; Webb et al., 2011), fish express alternative splice variants of the cytoplasmic domain (CD) of pcdh15a (Maeda et al., 2014). The splice variants pcdh15a-CD1 and pcdh15a-CD3 encode different variations of the C-terminus. Interestingly, a CD2 isoform doesn’t exist in zebrafish, yet this isoform is critical for mechanotransduction in mature auditory hair cells in the cochlea (Pepermans et al., 2014). In the inner ear or lateral line organ of zebrafish, transgenic expression of either CD-1 or CD-3 isoform is sufficient for restoring auditory and vestibular function to protein null pcdh15a mutants and transgenic fish are consequently adult viable (Maeda et al., 2017). Surprisingly, a survey of the domains of Pcdh15a that are required for function revealed that the isoform specific regions are dispensable for hair-cell function. In contrast, a region near the transmembrane domain that is present in both isoforms (hatched region in Figure 2C) is critical for the function of Pcdh15a. Further analysis showed a role for the transmembrane domain as well. Both the transmembrane and common region domains of Pcdh15 were found to mediate interactions between fish and mouse Pcdh15 and Transmembrane channel-like (TMC) proteins in heterologous systems (Maeda et al., 2014). The structure-function study of Pcdh15a provides further evidence that these domains are critical for function in vivo.
Like the cadherin genes, mutations in TMC1 are causative for deafness in humans (Kurima et al., 2002), and recent experiments analyzing mice carrying a double knock out of Tmc1 and a closely related paralogue, Tmc2, showed that mechanotransduction is completely absent in vestibular and cochlear hair cells (Kawashima et al., 2011). TMC1 and TMC2 are multispanning membrane proteins that belong to a family of membrane proteins with eight members. Although topology prediction programs predict up to 10 transmembrane domains, experiments assaying the accessibility of various regions of heterologously expressed TMC1 suggests that it has six transmembrane domains, which is similar to potassium channels (Labay et al., 2010). The precise nature of the role of the TMC1 and TMC2 in mechanotransduction is still unresolved, but so far the TMCs are the best candidates for the pore forming subunits of the mechanotransduction channel (Pan and Holt, 2015). Several studies of the mouse knock outs support the idea that these two proteins are critical for tip-link mediated mechanotransduction, however, there is some controversy as to whether they are pore-forming or rather ancillary subunits (see opposing viewpoints by (Corey and Holt, 2016) and (Wu and Müller, 2016)). The role of the Tmc orthologues in the zebrafish has yet to be explored. However, in an unbiased molecular screen, Tmc2a was identified as a protein that interacts with Pcdh15a (Maeda et al., 2014). This interaction is essential in vivo for normal hair-cell activity as overexpression the Pcdh15a-binding fragment of Tmc2a disrupts mechanically-evoked calcium transients in lateral-line hair cells. Interaction of both the zebrafish and mouse proteins demonstrate the conservation of this complex (Beurg et al., 2015). Such an interaction places the Tmcs into a central position within the transduction complex, that is, anchored or attached to the tip link. The dominant negative effect in zebrafish hair cells by the N-terminus of Tmc2a suggests that the Tmcs are likely to be critical for mechanotransduction, however, due to a gene duplication of tmc2 in zebrafish, a triple knock out may be required to draw any conclusions about the role of Tmc1 and Tmc2a and 2b in zebrafish hair cells.
Another membrane protein, Transmembrane inner ear protein (Tmie), was first shown in zebrafish as being required for mechanotransduction in hair cells of the larval inner ear (Gleason et al., 2009). Although the inner ear hair cells appear to be grossly normal, electron microscopy of lateral-line hair cells showed that kinocilia were abnormally short and that tip links were absent in tmie mutants. This study suggests that Tmie is required for normal maturation of hair bundles and localization of the mechanotransduction machinery. In a later study in mice, it was found that hair bundles including tip links were normal in Tmie mutant cochlear hair cells, yet transduction was absent (Zhao et al., 2014). TMIE also interacts with the CD2 isoform of PCDH15, but this interaction appears to be functionally relevant only in cochlear hair cells. These different phenotypes and interactions suggest that Tmie’s precise role in hair cells may be dependent on context, that is, the protein-protein interactions may differ in various types of hair cell.
As a fourth member of the membrane complex, the tetraspan membrane protein LHFPL5 (Lipoma HMGIC Fusion Partner-Like 5) has been implicated in trafficking of PCDH15 to the hair bundle, and may serve as an ancillary subunit of the transduction channel (Xiong et al., 2012). Interestingly, if PCDH15 is overexpressed in mouse Lhfpl5−/− cochlear hair cells, then rescue of transduction occurs, suggesting that LHFPL5 is not absolutely required for mechanotransduction. A mutation in the zebrafish orthologue lhfpl5a was identified as causative for deafness and balance in larvae (Obholzer et al., 2012). Like the mouse mutant, Lhfpl5a is required for proper localization of Pcdh15a in zebrafish hair bundles (Maeda et al., 2017). Thus, the interaction between these two transduction components appears to be conserved among vertebrates.
The mechanotransduction proteins mentioned thus far are either filamentous components of the tip link, or localize to the lower end of the tip link. The membrane deformation or ‘tenting’ of the stereociliary tip at this end suggests that the plasma membrane is compliant or elastic (Powers et al., 2012). In mouse cochlear hair cells, experiments using high-resolution imaging suggest that calcium influx takes place at this lower site (Beurg et al., 2009). The function of the upper end of the link may be to provide tension on the tip link. Unlike the tenting of the plasma membrane at the lower link, electron micrographs show that an electron density is present at the site of insertion and its appearance is often concave, as if the membrane is being drawn inward (Figure 2A). A different collection of proteins is required at upper end of the tip link based on the collective studies in vertebrates. The proteins in zebrafish include an unconventional myosin, Myo7aa, a scaffolding protein, Ush1c (also known as Harmonin), and a tetraspan membrane protein, Clarin-1 (Figure 2B; (Ernest et al., 2000; Gopal et al., 2015; Phillips et al., 2011)). All three of the genes have been implicated in Usher syndrome in humans, which involves sensory deficits in both the inner ear and retina. In zebrafish, myo7aa was the first gene identified as a deafness gene and the phenotype is quite similar to that of the Myo7a (also known as shaker-1) mouse mutants (Ernest et al., 2000). Both animal models display severe splaying of hair bundles in mutants expressing strong alleles. Notably, this phenotype of splayed or disorganized hair bundles is observed in the majority of zebrafish mechanotransduction mutants expressing strong alleles (Blanco-Sanchez et al., 2014; Gopal et al., 2015; Seiler et al., 2004, 2005; Söllner et al., 2004). A study of mechanotransduction in cochlear hair cells of the Myo7a mouse mutant, suggests that Myo7a may be involved in adaptation of transduction currents (Kros et al., 2002). Upon prolonged deflection, the tension in the tip links is thought to readjust, and the readjustment at the upper link may be accomplished by repositioning of the insertion site. As a motor molecule at the upper end of the tip link, Myo7a would be the right type of molecule to carry out such a task. With regard to a potential protein complex of the upper link components, Myo7aa was found to interact with Cdh23 and a scaffolding protein, Ush1c, in the secretory pathway using proximity labeling in zebrafish hair cells (Blanco-Sanchez et al., 2014). This data suggests that a multimeric complex is assembled in the endoplasmic reticulum (ER). Mutations in cdh23 or ush1c cause an increase in the expression of the heat shock protein Hspa5, an indicator of ER stress. The stress reaction is presumably due to exposed or unfolded proteins that accumulate and overwhelm the unfolded protein response in this organelle.
One protein that is not directly implicated in mechanotransduction is the unconventional motor protein myosin 6 (MYO6). This motor molecule is unusual in that it moves along actin filaments from the plus to minus ends, which is the opposite direction of conventional myosins (Wells et al., 1999). In Myo6 mouse mutants, adaptation of transduction currents is abnormal, but this effect is proposed to be due to abnormal maturation of hair cells such as a defect in the downregulation or trafficking of proteins such as CDH23 back out of the bundle during development (Marcotti et al., 2016). Like the corresponding mouse mutants, mutations in myo6b cause the disorganization and fusion of stereocilia (Avraham et al., 1995; Kappler et al., 2004; Seiler et al., 2004). The effects on mechanotransduction currents have not been explored in the zebrafish mutants.
Protein trafficking in hair cells
Assembly of protein complexes and trafficking of the essential components of the transduction machinery in hair cells is not well understood. As discussed above, some assembly of protein complexes takes place in the secretory pathway. Targeting of components to the hair bundle can be disrupted if the binding partners are absent. Such is the case for Pcdh15a when Lhfpl5a is mutated (Xiong et al., 2012, Maeda et al., 2017) or for the upper link complex (Blanco-Sanchez et al., 2014). In addition, proteins may be initially targeted to the hair bundle, but then, because of a lack of assembly or interaction with a binding partner within the stereocilia, the protein is unstable and not seen in mature hair bundles. In zebrafish, this effect is seen in the mature hair bundles of cdh23 and myo7aa mutants. In these two mutants, the tip link protein Pcdh15a is present in immature bundles but undetectable in mature bundles (Maeda et al., 2017).
Besides defective formation of protein complexes, shortcomings in protein processing and sorting in hair cells may affect mechanotransduction. For example, targeting of a protein to the hair bundle may fail at an early step in the secretory pathway. In pinball wizard mutants, so called because of a deafblindness phenotype, a subset of essential membrane components simply does not make it into the secretory pathway (Daniele et al., 2016; Lin et al., 2016). The gene that is mutated in pinball wizard encodes a tryptophan-rich basic protein (Wrb) that is required for the processing of ‘tail-anchored’ membrane proteins into the ER. The Wrb receptor specializes in the insertion of proteins with a transmembrane domain very close to the C-terminus. In wrb mutants, the microphonics and labeling of hair cells with vital dyes are reduced, suggesting that Wrb participates in the processing of proteins required for mechanotransduction. Reduction of synaptic proteins was also noted in this mutant, along with deficits in photoreceptors.
With respect to protein sorting, it is noteworthy that hair cells are polarized epithelial cells, and proper apical versus basal sorting of proteins is critical for function. Isolated in two independent screens, mutations in the beta subunit of the adaptor protein 1 complex (ap1b1) lead to missorting of proteins in zebrafish hair cells (Clemens Grisham et al., 2013). Missorting of proteins appears to be global in these mutants, as hair cells eventually degenerate. The defect in mechanotransduction in ap1b1 mutants is partly due to trafficking of a Na+/K+ pump into the hair bundles. Normally this pump is restricted to the basolateral membrane, as opposed to the apical compartment, and Na+ levels are increased in mutant hair cells. Presumably, this imbalance in Na+ has detrimental effects on several cellular processes including the resting membrane potential.
Synaptic transmission
As mentioned above, the genetics of synaptic transmission in zebrafish hair cells was recently covered in an extensive review (see Nicolson, 2015). In brief, the genes listed in Figure 1B affect presynaptic components of the glutamatergic synapse in hair cells. Three genes, vesicular glutamate transporter 3 (vglut3), rabconnectin 3 alpha (rbc3α) and synaptojanin 1 (synj) are essential for the normal function and recycling of synaptic vesicles. Rbc3α is important for stabilizing the proton pump on synaptic vesicles, presumably enabling the glutamate transporter Vglut3 to utilize the proton gradient for loading of vesicles with neurotransmitter, whereas Synj1 is required for efficient recycling of synaptic vesicles (Einhorn et al., 2012; Obholzer et al., 2008; Trapani et al., 2009). With respect to synaptic ribbons, the ribbon itself consists mainly of Ribeye a and b protein (Zenisek et al., 2004). A recent study using CRISPR to knock out both ribeye genes showed that Ribeye protein levels were greatly reduced in zebrafish hair cells (Lv et al., 2016). Oddly, photoreceptors still expressed Ribeye protein, suggesting that alternative splicing may occur. Perhaps even more surprising, ghost-like ribbons, or rings of vesicles without electron dense material were observed within the cytoplasm of hair cells. Remaining halos of synaptic vesicles suggests that other proteins are still capable of organizing vesicles. However, Cav1.3a calcium channels were greatly reduced in number in the active zones of hair cells (Lv et al., 2016). This finding is consistent with a study using morpholinos against ribeye a and b showing that Ribeye is necessary for clustering Cav1.3a calcium channels (Sheets et al., 2011). This L-type calcium channel is required for synaptic transmission in hair cells and evoked afferent responses are completely absent in zebrafish mutants (Sheets et al., 2012; Sidi et al., 2004; Trapani and Nicolson, 2011). Interestingly, the ribbons are initially enlarged, more numerous, and have abnormal morphology in cav1.3a mutant hair cells, suggesting that calcium influx plays a role in shaping ribbons (Sheets et al., 2012).
Overlapping function in the visual system
It is worth noting that of the genes listed in Figure 1B, many are also required for function of the retina. With respect to the group affecting mechanotransduction, these genes include ush1c, which is expressed in Müller glial cells and is important for the organization and function of photoreceptor synapses (Phillips et al., 2011). The gene duplicate of pcdh15a (pcdh15b) is also required for organization of the outer segments of photoreceptors and morpholino knockdown of pcdh15b leads to visual deficits (Seiler et al., 2005). In addition, mutation of myo7aa is associated with mild degeneration and a reduction in function of photoreceptors (Wasfy et al., 2014). The human orthologues of ush1c, pcdh15, and myo7aa are implicated in a particular subtype of Usher syndrome, which is a more severe form of deafblindness (reviewed in (Bonnet and El-Amraoui, 2012)). Mutations in USH1C, PCDH15, and MYO7A cause profound hearing loss in infants combined with vestibular deficits. These patients subsequently experience progressive visual impairment that sets in before puberty. For unknown reasons, the visual impairment in the corresponding mouse mutants has been very mild or absent. Thus, the zebrafish mutants offer an opportunity to explore the function of these genes in the retina. Besides the USH1 genes, mutations in wrb and synj1 also cause pronounced defects in vision (Daniele et al., 2016; Van Epps et al., 2004; Lin et al., 2016). In addition, rbc3α is robustly expressed in the inner nuclear layer and retinal ganglion cells, and rbc3α mutants have a common manifestation of visual dysfunction, namely expanded melanocytes in bright conditions (Einhorn et al., 2012). Normally larvae contract their melancytes upon exposure to a light background, and this response is not present in rbc3α mutants. With the exception of myo7aa and ush1c, many of these genes are also expressed in the brain and their roles in the CNS have yet to be explored.
Perspectives
Despite the large number of zebrafish genomes screened in large-scale screens for auditory/vestibular mutants (> 7000 genomes from Tuebingen I and II mutagenesis screens, (Haffter et al., 1996)and personal observation), the number of single alleles of deafness genes indicates that saturation mutagenesis has not been fully achieved. For example, only single alleles of tmie, lhfpl5a and vlgut3 have been reported thus far in zebrafish. There are likely to be many more genes required for hair-cell function. Indeed, the number of loci associated with non-syndromic forms of human deafness is more than 100. Of the cloned genes, more than two dozen are required in hair cells (Duman and Tekin, 2012; Raviv et al., 2010). One of the most frequently mutated human deafness genes is connexin 26, yet a connexin mutant in zebrafish has yet to emerge from a mutagenesis screen for balance and hearing defects. In some cases a duplication of the deafness gene can make redundancy an issue in a behavioral screen. Presumably this scenario explains why certain genes have not been identified in mutagenesis screens such as tmc2, ribeye, and otoferlin genes that have been duplicated in zebrafish and are expressed in an overlapping fashion in hair cells. Duplicated genes are not always problematic if expression occurs in non-overlapping patterns, which is the case for pcdh15a and pcdh15b. pcdh15a is expressed predominantly in hair cells, whereas pcdh15b is expressed in retinal cells (and both are expressed in the brain)(Maeda et al., 2017; Seiler et al., 2005). As a solution to redundancy and/or to create new animal models, gene editing methods such as CRISPR are being widely used in zebrafish (Li et al., 2016) and are valuable for creating single or double mutants. In addition, the Sanger Institute carried out a large-scale mutation project with the goal of creating knock outs in every gene, and currently over 35,000 nonsense and missense mutations are listed in the database (Kettleborough et al., 2013), see http://www.sanger.ac.uk/resources/zebrafish/zmp/).
As the use of proteomics and transcriptomics continues to grow, editing tools or mutant resources will become more important for future studies of function. Like forward genetic screens, ‘omic’ methods may reveal novel factors in a biological process. The transcriptome of zebrafish hair cells was first published using microarray analysis of RNA isolated from adult lagenae, which revealed novel transcripts (McDermott et al., 2007). Recently, the method of thio-uracil (TU) tagging of transcripts was adapted to zebrafish larvae, and selective labeling of hair-cell transcripts revealed yet another set of new genes that have highly enriched expression in both inner ear and/or lateral-line hair cells (Erickson and Nicolson, 2015). The majority of these genes have unknown roles in hair cells and whether they are required for function awaits further characterization. The study of hair-cell specific or enriched genes will be greatly accelerated by the use of genetic tools outlined above.
Footnotes
References
- Ahmed ZM, Goodyear R, Riazuddin S, Lagziel A, Legan PK, Behra M, Burgess SM, Lilley KS, Wilcox ER, Riazuddin S, et al. (2006). The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J. Neurosci. Off. J. Soc. Neurosci. 26, 7022–7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsina B, and Whitfield TT (2016). Sculpting the labyrinth: Morphogenesis of the developing inner ear. Semin. Cell Dev. Biol. [DOI] [PubMed] [Google Scholar]
- Avraham KB, Hasson T, Steel KP, Kingsley DM, Russell LB, Mooseker MS, Copeland NG, and Jenkins NA (1995). The mouse Snell’s waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat. Genet. 11, 369–375. [DOI] [PubMed] [Google Scholar]
- Baker CVH, Modrell MS, and Gillis JA (2013). The evolution and development of vertebrate lateral line electroreceptors. J. Exp. Biol. 216, 2515–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Lagier S, Vologodskaia M, Fabella BA, and Hudspeth A (2016). Direct mechanical stimulation of tip links in hair cells through DNA tethers. eLife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisel KW, Wang-Lundberg Y, Maklad A, and Fritzsch B (2005). Development and evolution of the vestibular sensory apparatus of the mammalian ear. J. Vestib. Res. Equilib. Orientat. 15, 225–241. [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Fettiplace R, Nam J-H, and Ricci AJ (2009). Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat. Neurosci. 12, 553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Xiong W, Zhao B, Müller U, and Fettiplace R (2015). Subunit determination of the conductance of hair-cell mechanotransducer channels. Proc. Natl. Acad. Sci. 112, 1589–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandiwad AA, Zeddies DG, Raible DW, Rubel EW, and Sisneros JA (2013). Auditory sensitivity of larval zebrafish (Danio rerio) measured using a behavioral prepulse inhibition assay. J. Exp. Biol. 216, 3504–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Sanchez B, Clement A, Fierro J, Washbourne P, and Westerfield M (2014). Complexes of Usher proteins preassemble at the endoplasmic reticulum and are required for trafficking and ER homeostasis. Dis. Model. Mech. 7, 547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleckmann H (2008). Peripheral and central processing of lateral line information. J. Comp. Physiol. A 194, 145–158. [DOI] [PubMed] [Google Scholar]
- Bonnet C, and El-Amraoui A (2012). Usher syndrome (sensorineural deafness and retinitis pigmentosa): pathogenesis, molecular diagnosis and therapeutic approaches. Curr. Opin. Neurol. 25, 42–49. [DOI] [PubMed] [Google Scholar]
- Brown SDM, Hardisty-Hughes RE, and Mburu P (2008). Quiet as a mouse: dissecting the molecular and genetic basis of hearing. Nat Rev Genet 9, 277–290. [DOI] [PubMed] [Google Scholar]
- Burighel P, Caicci F, and Manni L (2011). Hair cells in non-vertebrate models: Lower chordates and molluscs. Hear. Res. 273, 14–24. [DOI] [PubMed] [Google Scholar]
- Clemens Grisham R, Kindt K, Finger-Baier K, Schmid B, and Nicolson T (2013). Mutations in ap1b1 cause mistargeting of the Na(+)/K(+)-ATPase pump in sensory hair cells. PloS One 8, e60866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DP, and Holt JR (2016). Are TMCs the Mechanotransduction Channels of Vertebrate Hair Cells? J. Neurosci. Off. J. Soc. Neurosci. 36, 10921–10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DP, and Hudspeth AJ (1983a). Kinetics of the receptor current in bullfrog saccular hair cells. J. Neurosci. Off. J. Soc. Neurosci. 3, 962–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DP, and Hudspeth AJ (1983b). Analysis of the microphonic potential of the bullfrog’s sacculus. J. Neurosci. Off. J. Soc. Neurosci. 3, 942–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, and Fettiplace R (1985). The mechanical properties of ciliary bundles of turtle cochlear hair cells. J. Physiol. 364, 359–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele LL, Emran F, Lobo GP, Gaivin RJ, and Perkins BD (2016). Mutation of wrb, a Component of the Guided Entry of Tail-Anchored Protein Pathway, Disrupts Photoreceptor Synapse Structure and Function. Investig. Opthalmology Vis. Sci. 57, 2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman D, and Tekin M (2012). Autosomal recessive nonsyndromic deafness genes: a review. Front. Biosci. J. Virtual Libr. 17, 2213–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn Z, Trapani JG, Liu Q, and Nicolson T (2012). Rabconnectin3α promotes stable activity of the H+ pump on synaptic vesicles in hair cells. J. Neurosci. Off. J. Soc. Neurosci. 32, 11144–11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps HA, Hayashi M, Lucast L, Stearns GW, Hurley JB, De Camilli P, and Brockerhoff SE (2004). The zebrafish nrc mutant reveals a role for the polyphosphoinositide phosphatase synaptojanin 1 in cone photoreceptor ribbon anchoring. J. Neurosci. Off. J. Soc. Neurosci. 24, 8641–8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson T, and Nicolson T (2015). Identification of sensory hair-cell transcripts by thiouracil-tagging in zebrafish. BMC Genomics 16, 842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernest S, Rauch GJ, Haffter P, Geisler R, Petit C, and Nicolson T (2000). Mariner is defective in myosin VIIA: a zebrafish model for human hereditary deafness. Hum. Mol. Genet. 9, 2189–2196. [DOI] [PubMed] [Google Scholar]
- Flock A, and Wersall J (1962). A study of the orientation of the sensory hairs of the receptor cells in the lateral line organ of fish, with special reference to the function of the receptors. J. Cell Biol. 15, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, and Straka H (2014). Evolution of vertebrate mechanosensory hair cells and inner ears: toward identifying stimuli that select mutation driven altered morphologies. J. Comp. Physiol. A 200, 5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Jones K, Fariñas I, Maklad A, Lee J, and Reichardt LF (2002). Development and evolution of inner ear sensory epithelia and their innervation: Ear Sensory Development. J. Neurobiol. 53, 143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale JE, Marcotti W, Kennedy HJ, Kros CJ, and Richardson GP (2001). FM1–43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J. Neurosci. Off. J. Soc. Neurosci. 21, 7013–7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason MR, Nagiel A, Jamet S, Vologodskaia M, López-Schier H, and Hudspeth AJ (2009). The transmembrane inner ear (Tmie) protein is essential for normal hearing and balance in the zebrafish. Proc. Natl. Acad. Sci. U. S. A. 106, 21347–21352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal SR, Chen DH-C, Chou S-W, Zang J, Neuhauss SCF, Stepanyan R, McDermott BM, and Alagramam KN (2015). Zebrafish Models for the Mechanosensory Hair Cell Dysfunction in Usher Syndrome 3 Reveal That Clarin-1 Is an Essential Hair Bundle Protein. J. Neurosci. 35, 10188–10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato M, van Eeden FJ, Schach U, Trowe T, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, et al. (1996). Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Dev. Camb. Engl. 123, 399–413. [DOI] [PubMed] [Google Scholar]
- Haffter P, Granato M, Brand M, Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, van Eeden FJ, Jiang YJ, Heisenberg CP, et al. (1996). The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Dev. Camb. Engl. 123, 1–36. [DOI] [PubMed] [Google Scholar]
- Howard J, and Ashmore JF (1986). Stiffness of sensory hair bundles in the sacculus of the frog. Hear. Res. 23, 93–104. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ (1982). Extracellular current flow and the site of transduction by vertebrate hair cells. J. Neurosci. Off. J. Soc. Neurosci. 2, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler JA, Starr CJ, Chan DK, Kollmar R, and Hudspeth AJ (2004). A nonsense mutation in the gene encoding a zebrafish myosin VI isoform causes defects in hair-cell mechanotransduction. Proc. Natl. Acad. Sci. U. S. A. 101, 13056–13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima Y, Géléoc GSG, Kurima K, Labay V, Lelli A, Asai Y, Makishima T, Wu DK, Della Santina CC, Holt JR, et al. (2011). Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J. Clin. Invest. 121, 4796–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Müller U, and Kachar B (2007). Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449, 87–91. [DOI] [PubMed] [Google Scholar]
- Kettleborough RNW, Busch-Nentwich EM, Harvey SA, Dooley CM, de Bruijn E, van Eeden F, Sealy I, White RJ, Herd C, Nijman IJ, et al. (2013). A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 496, 494–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorevin VI (2008). The lagena (the third otolith endorgan in vertebrates). Neurophysiology 40, 142–159. [Google Scholar]
- Kindt KS, Finch G, and Nicolson T (2012a). Kinocilia Mediate Mechanosensitivity in Developing Zebrafish Hair Cells. Dev. Cell 23, 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt KS, Finch G, and Nicolson T (2012b). Kinocilia mediate mechanosensitivity in developing zebrafish hair cells. Dev. Cell 23, 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniss JS, Jiang L, and Piotrowski T (2016). Insights into sensory hair cell regeneration from the zebrafish lateral line. Curr. Opin. Genet. Dev. 40, 32–40. [DOI] [PubMed] [Google Scholar]
- Kozlov AS, Risler T, and Hudspeth AJ (2007). Coherent motion of stereocilia assures the concerted gating of hair-cell transduction channels. Nat. Neurosci. 10, 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kros CJ, Marcotti W, van Netten SM, Self TJ, Libby RT, Brown SDM, Richardson GP, and Steel KP (2002). Reduced climbing and increased slipping adaptation in cochlear hair cells of mice with Myo7a mutations. Nat. Neurosci. 5, 41–47. [DOI] [PubMed] [Google Scholar]
- Kurima K, Peters LM, Yang Y, Riazuddin S, Ahmed ZM, Naz S, Arnaud D, Drury S, Mo J, Makishima T, et al. (2002). Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat. Genet. 30, 277–284. [DOI] [PubMed] [Google Scholar]
- Labay V, Weichert RM, Makishima T, and Griffith AJ (2010). Topology of transmembrane channel-like gene 1 protein. Biochemistry (Mosc.) 49, 8592–8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhao L, Page-McCaw PS, and Chen W (2016). Zebrafish Genome Engineering Using the CRISPR–Cas9 System. Trends Genet. 32, 815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S-Y, Vollrath MA, Mangosing S, Shen J, Cardenas E, and Corey DP (2016). The zebrafish pinball wizard gene encodes WRB, a tail-anchored-protein receptor essential for inner-ear hair cells and retinal photoreceptors: A zebrafish deafness/blindness gene encodes WRB. J. Physiol. 594, 895–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv C, Stewart WJ, Akanyeti O, Frederick C, Zhu J, Santos-Sacchi J, Sheets L, Liao JC, and Zenisek D (2016). Synaptic Ribbons Require Ribeye for Electron Density, Proper Synaptic Localization, and Recruitment of Calcium Channels. Cell Rep. 15, 2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda R, Kindt KS, Mo W, Morgan CP, Erickson T, Zhao H, Clemens-Grisham R, Barr-Gillespie PG, and Nicolson T (2014). Tip-link protein protocadherin 15 interacts with transmembrane channel-like proteins TMC1 and TMC2. Proc. Natl. Acad. Sci. U. S. A. 111, 12907–12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda R, Pacentine IV, Erickson T, and Nicolson T (2017). Functional analysis of the transmembrane and cytoplasmic domains of Pcdh15a in zebrafish hair cells. J. Neurosci. 2216–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotti W, Corns LF, Goodyear RJ, Rzadzinska AK, Avraham KB, Steel KP, Richardson GP, and Kros CJ (2016). The acquisition of mechano-electrical transducer current adaptation in auditory hair cells requires myosin VI. J. Physiol. 594, 3667–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott BM, Baucom JM, and Hudspeth AJ (2007). Analysis and functional evaluation of the hair-cell transcriptome. Proc. Natl. Acad. Sci. 104, 11820–11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo W, Chen F, Nechiporuk A, and Nicolson T (2010). Quantification of vestibular-induced eye movements in zebrafish larvae. BMC Neurosci. 11, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monesson-Olson BD, Browning-Kamins J, Aziz-Bose R, Kreines F, and Trapani JG (2014). Optical Stimulation of Zebrafish Hair Cells Expressing Channelrhodopsin-2. PLoS ONE 9, e96641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson T (2015). Ribbon synapses in zebrafish hair cells. Hear. Res. 330, 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson T, Rüsch A, Friedrich RW, Granato M, Ruppersberg JP, and Nüsslein-Volhard C (1998). Genetic analysis of vertebrate sensory hair cell mechanosensation: the zebrafish circler mutants. Neuron 20, 271–283. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, and Sasaki F (1996). Internalization of styryl dye FM1–43 in the hair cells of lateral line organs in Xenopus larvae. J. Histochem. Cytochem. Off. J. Histochem. Soc. 44, 733–741. [DOI] [PubMed] [Google Scholar]
- Obholzer N, Wolfson S, Trapani JG, Mo W, Nechiporuk A, Busch-Nentwich E, Seiler C, Sidi S, Söllner C, Duncan RN, et al. (2008). Vesicular glutamate transporter 3 is required for synaptic transmission in zebrafish hair cells. J. Neurosci. Off. J. Soc. Neurosci. 28, 2110–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obholzer N, Swinburne IA, Schwab E, Nechiporuk AV, Nicolson T, and Megason SG (2012). Rapid positional cloning of zebrafish mutations by linkage and homozygosity mapping using whole-genome sequencing. Dev. Camb. Engl. 139, 4280–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olt J, Johnson SL, and Marcotti W (2014). In vivo and in vitro biophysical properties of hair cells from the lateral line and inner ear of developing and adult zebrafish. J. Physiol. 592, 2041–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olt J, Allen CE, and Marcotti W (2016a). In vivo physiological recording from the lateral line of juvenile zebrafish. J. Physiol. 594, 5427–5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olt J, Ordoobadi AJ, Marcotti W, and Trapani JG (2016b). Physiological recordings from the zebrafish lateral line. Methods Cell Biol. 133, 253–279. [DOI] [PubMed] [Google Scholar]
- Pan B, and Holt JR (2015). The molecules that mediate sensory transduction in the mammalian inner ear. Curr. Opin. Neurobiol. 34, 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepermans E, Michel V, Goodyear R, Bonnet C, Abdi S, Dupont T, Gherbi S, Holder M, Makrelouf M, Hardelin J-P, et al. (2014). The CD2 isoform of protocadherin-15 is an essential component of the tip-link complex in mature auditory hair cells. EMBO Mol. Med. 6, 984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson EH, Cotton JR, and Grant JW (1996). Structural Variation in Ciliary Bundles of the Posterior Semicircular Canal.: Quantitative Anatomy and Computational Analysis. Ann. N. Y. Acad. Sci. 781, 85–102. [DOI] [PubMed] [Google Scholar]
- Phillips JB, Blanco-Sanchez B, Lentz JJ, Tallafuss A, Khanobdee K, Sampath S, Jacobs ZG, Han PF, Mishra M, Titus TA, et al. (2011). Harmonin (Ush1c) is required in zebrafish Muller glial cells for photoreceptor synaptic development and function. Dis. Model. Mech. 4, 786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles JO (1993). A model for the mechanics of the stereociliar bundle on acousticolateral hair cells. Hear. Res. 68, 159–172. [DOI] [PubMed] [Google Scholar]
- Pickles JO, Comis SD, and Osborne MP (1984). Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear. Res. 15, 103–112. [DOI] [PubMed] [Google Scholar]
- Powers RJ, Roy S, Atilgan E, Brownell WE, Sun SX, Gillespie PG, and Spector AA (2012). Stereocilia Membrane Deformation: Implications for the Gating Spring and Mechanotransduction Channel. Biophys. J. 102, 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviv D, Dror AA, and Avraham KB (2010). Hearing loss: a common disorder caused by many rare alleles: Hearing loss. Ann. N. Y. Acad. Sci. 1214, 168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Bai J-P, Song L, Lv C, Zenisek D, and Santos-Sacchi J (2013). Patch-clamp recordings from lateral line neuromast hair cells of the living zebrafish. J. Neurosci. Off. J. Soc. Neurosci. 33, 3131–3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander M, Xiong W, Tokita J, Lelli A, Elledge HM, Kazmierczak P, Sczaniecka A, Kolatkar A, Wiltshire T, Kuhn P, et al. (2009). A mouse model for nonsyndromic deafness (DFNB12) links hearing loss to defects in tip links of mechanosensory hair cells. Proc. Natl. Acad. Sci. 106, 5252–5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler C, and Nicolson T (1999). Defective calmodulin-dependent rapid apical endocytosis in zebrafish sensory hair cell mutants. J. Neurobiol. 41, 424–434. [PubMed] [Google Scholar]
- Seiler C, Ben-David O, Sidi S, Hendrich O, Rusch A, Burnside B, Avraham KB, and Nicolson T (2004). Myosin VI is required for structural integrity of the apical surface of sensory hair cells in zebrafish. Dev. Biol. 272, 328–338. [DOI] [PubMed] [Google Scholar]
- Seiler C, Finger-Baier KC, Rinner O, Makhankov YV, Schwarz H, Neuhauss SCF, and Nicolson T (2005). Duplicated genes with split functions: independent roles of protocadherin15 orthologues in zebrafish hearing and vision. Dev. Camb. Engl. 132, 615–623. [DOI] [PubMed] [Google Scholar]
- Sheets L, Trapani JG, Mo W, Obholzer N, and Nicolson T (2011). Ribeye is required for presynaptic Ca(V)1.3a channel localization and afferent innervation of sensory hair cells. Dev. Camb. Engl. 138, 1309–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets L, Kindt KS, and Nicolson T (2012). Presynaptic CaV1.3 channels regulate synaptic ribbon size and are required for synaptic maintenance in sensory hair cells. J. Neurosci. Off. J. Soc. Neurosci. 32, 17273–17286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidi S, Busch-Nentwich E, Friedrich R, Schoenberger U, and Nicolson T (2004). gemini encodes a zebrafish L-type calcium channel that localizes at sensory hair cell ribbon synapses. J. Neurosci. Off. J. Soc. Neurosci. 24, 4213–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemens J, Lillo C, Dumont RA, Reynolds A, Williams DS, Gillespie PG, and Müller U (2004). Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature 428, 950–955. [DOI] [PubMed] [Google Scholar]
- Silber J, Cotton J, Nam J-H, Peterson EH, and Grant W (2004). Computational models of hair cell bundle mechanics: III. 3-D utricular bundles. Hear. Res. 197, 112–130. [DOI] [PubMed] [Google Scholar]
- Söllner C, Rauch G-J, Siemens J, Geisler R, Schuster SC, Müller U, Nicolson T, and Tübingen 2000 Screen Consortium (2004). Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature 428, 955–959. [DOI] [PubMed] [Google Scholar]
- Tanimoto M, Ota Y, Horikawa K, and Oda Y (2009). Auditory Input to CNS Is Acquired Coincidentally with Development of Inner Ear after Formation of Functional Afferent Pathway in Zebrafish. J. Neurosci. 29, 2762–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto M, Ota Y, Inoue M, and Oda Y (2011). Origin of Inner Ear Hair Cells: Morphological and Functional Differentiation from Ciliary Cells into Hair Cells in Zebrafish Inner Ear. J. Neurosci. 31, 3784–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani JG, and Nicolson T (2010). Physiological Recordings from Zebrafish Lateral-Line Hair Cells and Afferent Neurons. In Methods in Cell Biology, (Elsevier), pp. 219–231. [DOI] [PubMed] [Google Scholar]
- Trapani JG, and Nicolson T (2011). Mechanism of spontaneous activity in afferent neurons of the zebrafish lateral-line organ. J. Neurosci. Off. J. Soc. Neurosci. 31, 1614–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani JG, Obholzer N, Mo W, Brockerhoff SE, and Nicolson T (2009). Synaptojanin1 is required for temporal fidelity of synaptic transmission in hair cells. PLoS Genet. 5, e1000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waguespack J, Salles FT, Kachar B, and Ricci AJ (2007). Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J. Neurosci. Off. J. Soc. Neurosci. 27, 13890–13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Song Q, Yu D, Yang G, Xia L, Su K, Shi H, Wang J, and Yin S (2015). Ontogenetic development of the auditory sensory organ in zebrafish (Danio rerio): changes in hearing sensitivity and related morphology. Sci. Rep. 5, 15943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasfy MM, Matsui JI, Miller J, Dowling JE, and Perkins BD (2014). myosin 7aa(−/−) mutant zebrafish show mild photoreceptor degeneration and reduced electroretinographic responses. Exp. Eye Res. 122, 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SW, Grillet N, Andrade LR, Xiong W, Swarthout L, Della Santina CC, Kachar B, and Müller U (2011). Regulation of PCDH15 function in mechanosensory hair cells by alternative splicing of the cytoplasmic domain. Dev. Camb. Engl. 138, 1607–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AL, Lin AW, Chen LQ, Safer D, Cain SM, Hasson T, Carragher BO, Milligan RA, and Sweeney HL (1999). Myosin VI is an actin-based motor that moves backwards. Nature 401, 505–508. [DOI] [PubMed] [Google Scholar]
- Whitfield TT, and Hammond KL (2007). Axial patterning in the developing vertebrate inner ear. Int. J. Dev. Biol. 51, 507–520. [DOI] [PubMed] [Google Scholar]
- Wu Z, and Müller U (2016). Molecular Identity of the Mechanotransduction Channel in Hair Cells: Not Quiet There Yet. J. Neurosci. Off. J. Soc. Neurosci. 36, 10927–10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Grillet N, Elledge HM, Wagner TFJ, Zhao B, Johnson KR, Kazmierczak P, and Müller U (2012). TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell 151, 1283–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, and Peterson EH (2006). Hair bundle heights in the utricle: differences between macular locations and hair cell types. J. Neurophysiol. 95, 171–186. [DOI] [PubMed] [Google Scholar]
- Yao Q, DeSmidt AA, Tekin M, Liu X, and Lu Z (2016). Hearing Assessment in Zebrafish During the First Week Postfertilization. Zebrafish 13, 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeddies DG (2005). Development of the acoustically evoked behavioral response in zebrafish to pure tones. J. Exp. Biol. 208, 1363–1372. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Horst NK, Merrifield C, Sterling P, and Matthews G (2004). Visualizing synaptic ribbons in the living cell. J. Neurosci. Off. J. Soc. Neurosci. 24, 9752–9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QX, He XJ, Wong HC, and Kindt KS (2016). Functional calcium imaging in zebrafish lateral-line hair cells. In Methods in Cell Biology, (Elsevier), pp. 229–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wu Z, Grillet N, Yan L, Xiong W, Harkins-Perry S, and Müller U (2014). TMIE is an essential component of the mechanotransduction machinery of cochlear hair cells. Neuron 84, 954–967. [DOI] [PMC free article] [PubMed] [Google Scholar]


