Abstract
Background
Data regarding lacosamide treatment as an adjunctive therapy in patients representative of a focal-onset epilepsy population including those with and without intellectual/developmental disorders (IDDs) are limited.
Purpose
To evaluate the retention rates of lacosamide in focal-onset epilepsy patients with and without IDD.
Patients and methods
We retrospectively reviewed all consecutive electronic and paper medical records of patients diagnosed with focal-onset epilepsy who were treated with lacosamide in two tertiary epilepsy centers.
Results
One hundred and thirty-six patients who met the inclusion criteria were studied. Number of patients with IDD was 46 (33.8%). Median lacosamide dose was 300 mg/day. A total of 39 patients (28.7%) experienced side effects, and 22 of them (16.2%) discontinued lacosamide. The 1-, 2-, and 3-year retention rates of lacosamide in patients with IDD were 68%, 62%, and 53%, respectively. Kaplan–Meier survival analysis showed that the retention rates were significantly lower in patients with IDD when compared to patients without IDD (P=0.04). Cox regression analysis showed that concomitant use of sodium channel blocker antiepileptic drugs (AEDs) was the only independent predictor of retention rate of lacosamide treatment (P=0.03). In the subgroup of patients with IDD, the analysis was performed again and the number of background AEDs was the only predictor for the retention rate of lacosamide (P=0.04).
Conclusion
When compared to patients without IDD, retention rates of lacosamide adjunctive therapy were lower in patients with IDD. However, these rates were higher than the rates suggested with previously registered AEDs including lamotrigine, levetiracetam, and topiramate. Therefore, irrespective of having comorbid IDD, we might suggest that lacosamide is a well-retained drug with a high efficacy profile in patients with focal-onset epilepsy.
Keywords: lacosamide, focal-onset epilepsy, intellectual/developmental disorders
Introduction
Intellectual/developmental disorder (IDD) is defined as the condition of arrested or incomplete development of the mind, which is especially characterized by impairment of skills manifested during the developmental period that contribute to the overall level of intelligence.1
The prevalence of epilepsy in people with IDD is higher than in the population without IDD, which varies between 26% and 40%. However, treatment of these patients is usually challenging due to frequent antiepileptic drug (AED)-related side effects caused by polypharmacy and a more refractory course.2
Lacosamide is a third-generation AED with a proven efficacy for focal-onset epilepsies. It has a unique mechanism of action. It selectively enhances the slow activation of voltage-gated Na channels by altering their voltage dependence, but without affecting the fast channels.3,4 This is in contrast to that seen in traditional sodium channel blocking AEDs. The efficacy of lacosamide has been demonstrated in large clinical studies, but data regarding the use of lacosamide in patients representative of a general epilepsy population including those with IDD are limited. Patients with IDD are usually excluded from randomized studies due to several ethical concerns. Lack of capacity to give consent, restricted reporting of adverse events due to communication problems, in addition to incompliance to drugs are among the main concerns.5 Therefore, in our study, we aimed to assess the retention rates of adjunctive lacosamide treatment, particularly in patients with comorbid IDD in a real-world setting.
Patients and methods
We retrospectively reviewed all the medical records of patients diagnosed with epilepsy who were treated with lacosamide in two large tertiary epilepsy centers over a 3-year period.
Patients aged ≥16 years were included only if they had a diagnosis of focal-onset epilepsy. Subjects were excluded if the available information was inadequate and/or if the dose(s) of other AED(s) were increased simultaneously with lacosamide initiation.
Patients were divided into two subgroups: patients with IDD and patients without IDD. IDD was graded in concordance with the Diagnostic and Statistical Manual of Mental Disorders, fifth edition criteria.6,7 As a general approach in both clinics, a basal electrocardiogram was obtained for all patients with IDD. PR, QT intervals, and QRS durations were analyzed before lacosamide treatment.
In both centers, epilepsy outpatient clinics are being directed by the epilepsy specialists (EAD in Akdeniz University and BOG in Necmettin Erbakan University) and all appointments are arranged only with the legal representatives of the patients with IDD.
The study was performed in compliance with the Declaration of Helsinki, and the study protocol was approved by the Akdeniz University School of Medicine Ethics Committee. As per the institutional review board guidelines, patients’ personal information and confidentialities were searched only after obtaining local committee’s approvals of Akdeniz and Necmettin Erbakan universities. As approved by the Akdeniz University School of Medicine Ethics Committee, individual patient informed consent was exempted, since the personal information was encrypted.
Data were systematically recorded based on age, gender, duration of epilepsy, and severity of IDD. In accordance with the International League against Epilepsy (ILAE) guidelines, seizures were classified by the mechanism of onset. Etiology of epilepsy, duration of lacosamide treatment, concomitant behavioral medication, adverse events, and number of previous and current AEDs were recorded.
Due to inadequate reimbursement for care management and cost factors, the etiologic factor(s) could not be elucidated in the whole of the patients with IDD. In these patients, we could not exclude perinatal infections or genetically inherited diseases, which precludes us from asserting a definite diagnosis. Therefore, patients with a diagnosis of “hypoxic birth” were classified under the heading “patients with presumed birth-related complications and/or genetically inherited diseases”.
AEDs were classified according to whether they are sodium channel blockers (phenytoin, carbamazepine, lamotrigine, oxcarbazepine) or non-sodium channel blockers.8
In both centers, lacosamide dose was initiated and up-titrated to the optimal dose according to the treating physician’s usual practice. Hospital charts included all clinically relevant parameters which had been captured at regular time intervals in the first year (baseline, after a month, and every 3 months). However, due to the retrospective design of the study, no exact visit windows could be defined after the first year and the visit schedules were up to the physician’s discretion and the patients’ health conditions.
Statistical analyses
Statistical analysis was carried out using SPSS 18 Software (SPSS Inc., Chicago, IL, USA). Continuous data were characterized by median and range and mean±SD. Categorical data were characterized by number and percentage. Significances of the differences between continuous variables were tested by Mann–Whitney U test. Chi-squared test was used for categorical comparisons of nominal values. Kaplan–Meier analysis was conducted to estimate the retention rates of lacosamide treatment. Log-rank test was used to compare the retention rates of the patients with and without IDD. Patients still using lacosamide at their last follow-up were regarded as censored in the survival analysis. In order to assess the retention rates in concordance with the literature, 1-, 2-, and 3-year time periods were chosen.
Cox regression analysis was performed to identify the predictors of retention rate and the efficacy of lacosamide treatment. The level of significance was set at 0.05.
Results
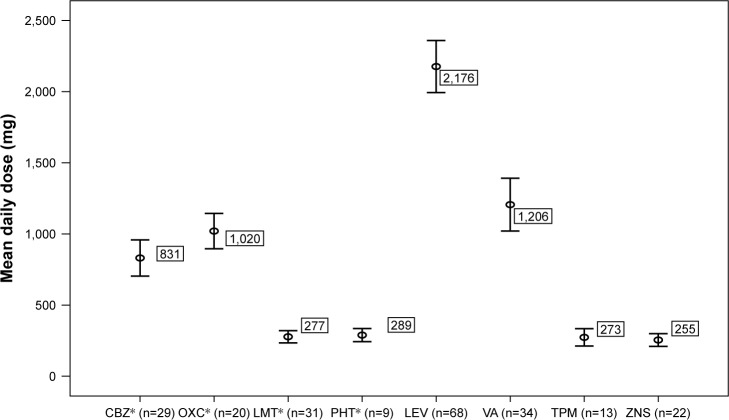
Demographic and clinical characteristics of the patients are displayed in Table 1. One hundred and thirty-six patients (70 male, 66 female) were identified who met the inclusion criteria with a median age of 30. Median age of onset for epilepsy was 14 years, and median duration of epilepsy was 11 years. Median and mean follow-up after initiation of lacosamide was 11.5 (range: 1–38) and 15.3±11.7 months, respectively. Median lacosamide dose was 300 mg/day. In 17 patients, lacosamide was not escalated to 400 mg, despite the absence of efficacy and any side effect. On average, 1.8±0.8 (median 2) AEDs were used alongside lacosamide. Mean daily doses of background AEDs are shown in Figure 1.
Table 1.
Demographic characteristics of the patients
| Value | |
|---|---|
| Age (years) | 30 (16–79); 34±14.5a |
| Gender, n (%) | |
| Male | 70 (51.5%)b |
| Female | 66 (48.5%)b |
| Epilepsy onset (age) | 14 (1–65); 19.7±17.7a |
| Epilepsy duration (years) | 11 (1–52); 14.3±11.0a |
| Lacosamide dose (mg/day) | 300 (200–400); 323.5±66.9a |
| Follow-up duration (months) | 11.5 (1–38); 15.3±11.7 |
| Severity of IDDc (n) | 46 |
| Mild to moderate | 10 (21.7%)d |
| Severe | 28 (60.9%)d |
| Profound | 8 (17.4%)d |
| Background AEDs (n) | |
| Valproic acid | 34 (25.0%)b |
| Carbamazepine | 29 (21.3%)b |
| Oxcarbazepine | 20 (14.7%)b |
| Lamotrigine | 31 (22.8%)b |
| Levetiracetam | 68 (50.0%)b |
| Topiramate | 13 (9.6%)b |
| Epdantoin | 9 (6.6%)b |
| Zonisamide | 22 (16.2%)b |
| Concomitant antipsychotic medication (n) | 17 (12.5%)b |
Notes:
Median (minimum–maximum); mean±SD.
Number (percentage).
According to the DSM-V criteria.
Percentages were calculated according to the total number of patients with IDD.
Abbreviations: AED, antiepileptic drug; DSM-V, Diagnostic and Statistical Manual of Mental Disorders, fifth edition; IDD, intellectual/developmental disorder.
Figure 1.
Daily mean doses of background AEDs in the study population.
Note: *Sodium channel blockers: carbamazepine, lamotrigine, oxcarbazepine, phenytoin.
Abbreviations: AED, antiepileptic drug; CBZ, carbamazepine; LEV, levetiracetam; LMT, lamotrigine; OXC, oxcarbazepine; PHT, phenytoin; TPM, topiramate; VA, valproic acid; ZNS, zonisamide.
Presumed birth-related complications and/or genetically inherited diseases constituted the major part of the study population (n=47; 34.6%). Thirty-three patients (24.3%) had brain tumor documented on brain magnetic resonance imaging and/or computed tomography. Eighteen patients (13.2%) had mesial temporal sclerosis. Cerebrovascular or degenerative diseases were the cause in eight patients (5.9%). Etiologic classification of the patient population is presented in Table 2.
Table 2.
Etiologic classification of the patient population
| Etiology | n |
|---|---|
| Birth related and/or genetica | 47 (34.6%) |
| Brain tumor | 33 (24.3%) |
| Mesial temporal sclerosis | 18 (13.2%) |
| Trauma | 11 (8.1%) |
| Cerebrovascular/degenerative disease | 8 (5.9%) |
| Cortical dysplasia | 6 (4.4%) |
| Meningitis sequelae | 6 (4.4%) |
| Tuberous sclerosis | 5 (3.7%) |
| Arteriovenous malformation | 1 (0.7%) |
| Paraneoplastic limbic encephalitis | 1 (0.7%) |
Note:
Presumed birth-related complications and/or genetically inherited diseases.
Demographic and clinical characteristics of patients with and without IDD are demonstrated in Table 3. Gender rates, mean follow-up, and mean lacosamide dose did not differ significantly. Average age was lower (P<0.001) and the number of background AEDs was significantly higher in patients with IDD (P=0.006).
Table 3.
Comparison of patients with and without IDD
| IDD (+), n=46 | IDD (−), n=90 | P-valueb | |
|---|---|---|---|
| Age (years)a | 26.8±11.0; 23 (16–66) | 37.7±14.7; 33.5 (16–79) | <0.001 |
| Gender (male) | 22 (47.8%) | 48 (53.3%) | 0.59 |
| Follow-upa | 15.3±12.9; 11 (1–38) | 15.3±11.1; 12 (1–38) | 0.67 |
| LCM dosea | 337.0±67.0; 325 (200–400) | 316.7±66.2; 300 (200–400) | 0.07 |
| Use of concomitant SCB | 30 (65.2%) | 48 (53.3%) | 0.20 |
| Background AED, na | 2.07±0.90; 2 (1–4) | 1.62±0.57; 2 (1–3) | 0.006 |
| Side effect | 16 (34.8%) | 23 (25.6%) | 0.32 |
Notes:
Continuous variables are defined as mean ± SD and median (minimum–maximum), respectively.
Mann–Whitney U test was used for the comparison of continuous variables.
Abbreviations: Background AED nr, number of initial antiepileptic drugs; IDD, intellectual/developmental disorders; LCM, lacosamide; concomitant SCB, concomitant traditional sodium channel blocking agent.
Lacosamide was reported to be effective in 66.2% (n=90) of the patients in reducing seizure frequency, severity, or both. In 41 patients (30.1%), there was no effect on seizures. In 5 patients (3.7%), increased seizure activity was observed.
Kaplan–Meier survival analysis was conducted to estimate and compare the retention rates of lacosamide in patients with and without IDD. The 1-, 2-, and 3-year retention rates of lacosamide in patients with IDD were 68%, 62%, and 53%, respectively. The 1-, 2-, and 3-year retention rates of lacosamide in patients without IDD were 88%, 73%, and 33%, respectively (Table 4). Kaplan–Meier survival analysis showed that the retention rates were significantly lower in patients with IDD when compared to those in patients without IDD (P=0.04; Figure 2).
Table 4.
Retention rates of LCM treatment during follow-up in patients with and without IDD
| Time period (months) | 0 | 6 | 12 | 18 | 24 | 30 | 36 |
|---|---|---|---|---|---|---|---|
| Patients at risk (on LCM), n | |||||||
| IDD − | 90 | 70 | 46 | 34 | 22 | 17 | 3 |
| IDD + | 46 | 31 | 22 | 20 | 14 | 9 | 4 |
| Discontinuation, n | |||||||
| IDD − | 7 | 1 | 1 | 1 | 3 | 1 | 1 |
| IDD + | 12 | 2 | 0 | 0 | 1 | 1 | 0 |
| Censored patients, n | |||||||
| IDD − | 13 | 23 | 11 | 11 | 2 | 13 | 2 |
| IDD + | 3 | 7 | 2 | 6 | 4 | 4 | 4 |
| Retention rate, % | |||||||
| IDD − | 92 | 90 | 88 | 85 | 73 | 66 | 33 |
| IDD + | 73 | 68 | 68 | 68 | 62 | 53 | 53 |
Abbreviations: IDD, intellectual/developmental disorder; LCM, lacosamide.
Figure 2.
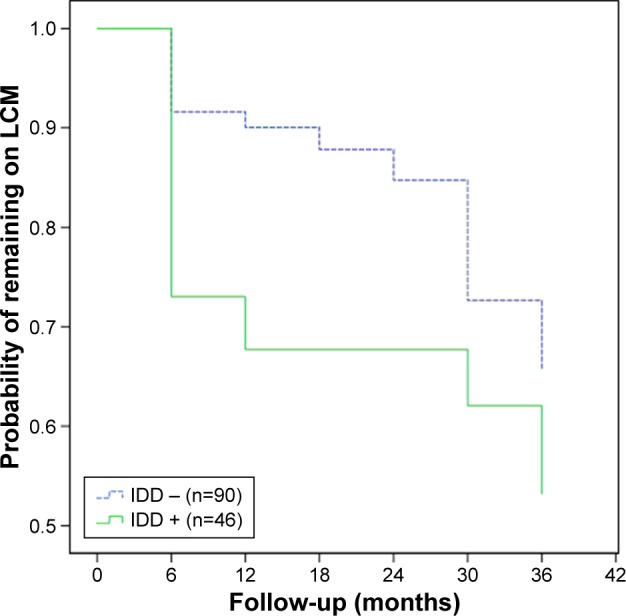
Kaplan–Meier survival analysis showed that the retention rates were significantly lower in patients with IDD when compared to those in patients without IDD (P=0.04).
Abbreviations: IDD, intellectual/developmental disorder; LCM, lacosamide.
In our study population, the number of patients with brain tumor was high (24.3%); therefore, to prevent a bias regarding the severity of epilepsy associated with brain tumor, a distinct assessment was provided excluding this subgroup and the statistical significances were recalculated. The statistical significance was still present after excluding the patients with brain tumor (P=0.03).
A total of 39 patients (28.7%) experienced side effects. While 22 of them (16.2%) discontinued lacosamide (Table 5), 17 (12.5%) continued the drug despite experiencing side effects. Eight patients (5.9%) discontinued lacosamide due to lack of efficacy. One of the patients discontinued lacosamide due to an unplanned pregnancy (Table 5).
Table 5.
Daily dosage scheme of LCM and other AEDs at the time of discontinuation and the reasons for discontinuing LCM are shown
| No. | Patient no. | Gender | Age (years) | Etiology | Discontinuation reason | Follow-up (months) | Lacosamide dose (mg) | Other AED, dosage (mg) |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | M | 32 | Birth related and/or genetica | Behavioral disorder | 1b | 300 | LEV3,000, OXC900 |
| 2 | 7 | M | 44 | Birth related and/or genetica | GI intolerance | 6 | 300 | OXC1,200 |
| 3 | 12 | F | 34 | Brain tumor | Nausea/vomitingc | 3 | 300 | LAM300, CRB1,200 |
| 4 | 15 | M | 36 | Birth related and/or genetica | Behavioral disorder | 1b | 400 | LEV3,000 |
| 5 | 16 | F | 22 | Mesial temporal sclerosis | Lack of efficacy | 27 | 400 | ZNS300, PHN300 |
| 6 | 22 | M | 19 | Birth related and/or genetica | Behavioral disorder | 1b | 400 | LEV2,000, PHN300 |
| 7 | 23 | F | 37 | Brain tumor | Lack of efficacy | 16 | 200 | VAL1,500, PHN300 |
| 8 | 27 | F | 25 | Cortical dysplasia | Pregnancy | 37 | 400 | LEV3,000, ZNS200 |
| 9 | 30 | M | 28 | Birth related and/or genetica | Behavioral disorder | 1b | 350 | LEV2,000, VAL1,500, OXC600 |
| 10 | 31 | M | 20 | Meningitis sequelae | Palpitation | 1 | 300 | LAM300, PHN300 |
| 11 | 35 | M | 36 | Birth related and/or genetica | Nausea/vomiting | 3 | 300 | LEV2,000, VAL2,000, LAM200 |
| 12 | 45 | F | 50 | Birth related and/or genetica | Behavioral disorder | 1b | 200 | LEV2,000, VAL1,000 |
| 13 | 46 | F | 16 | Birth related and/or genetica | Lack of efficacy | 34 | 300 | LAM300, OXC1,200, VAL2,000, ZNS200 |
| 14 | 49 | M | 21 | Post-traumatic | Nausea/vomiting | 1 | 300 | LAM400, CRB1,200 |
| 15 | 51 | F | 20 | Birth related and/or genetica | Behavioral disorder | 1b | 300 | LEV1,500 |
| 16 | 54 | F | 22 | Birth related and/or genetica | Lack of efficacy | 2 | 200 | CRB1,200, ZNS200 |
| 17 | 67 | F | 16 | Birth related and/or genetica | Allergic reaction | 2 | 300 | LAM200, OXC1,200 |
| 18 | 69 | M | 29 | Birth related and/or genetica | Palpitation | 4 | 400 | LEV3,000, VAL3,000, CRB1,200 |
| 19 | 75 | M | 33 | Mesial temporal sclerosis | Suicidal thoughts | 10 | 300 | LEV2,000, PHN200 |
| 20 | 77 | F | 27 | Post-traumatic | Behavioral disorder | 1b | 300 | LEV2,000, OXC1,200 |
| 21 | 78 | M | 52 | Brain tumor | Lack of efficacy | 28 | 300 | LEV3,000, CRB1,000 |
| 22 | 83 | F | 43 | Birth related and/or genetica | Palpitation | 2 | 400 | LAM350, PHN400 |
| 23 | 86 | F | 28 | Post-traumatic | Lack of efficacy | 19 | 300 | LEV2,000 |
| 24 | 91 | M | 52 | Brain tumor | Lack of efficacy | 26 | 300 | LEV1,000, CRB400 |
| 25 | 92 | F | 18 | Birth related and/or genetica | Nausea/vomitingd | 1 | 300 | VAL750, CRB300 |
| 26 | 113 | F | 29 | Mesial temporal sclerosis | Nausea/vomitingc | 1 | 200 | VAL1,500, OXC1,200 |
| 27 | 117 | F | 18 | Birth related and/or genetica | Rash | 11 | 400 | LEV3,000, LAM300, CRB600, ZNS200 |
| 28 | 124 | F | 58 | Post-traumatic | Fatigue | 30 | 300 | LEV3,000, CRB400 |
| 29 | 128 | M | 36 | Brain tumor | Nausea | 4 | 200 | LEV1,500, PHN300 |
| 30 | 129 | F | 57 | Cerebrovascular disease | Nausea/vomiting | 2 | 300 | LEV3,000, OXC900 |
| 31 | 136 | M | 27 | Birth related and/or genetic | Lack of efficacy | 25 | 300 | LEV1,000, CRB1,200, VAL1,000 |
Notes:
Presumed birth-related complications and/or genetically inherited diseases.
Symptoms appeared in the first 2 weeks.
Accompanied by vertiginous symptoms.
Weight loss was observed.
Abbreviations: AED, antiepileptic drug; CRB, carbamazepine; GI, gastrointestinal; LAM, lamotrigine; LCM, lacosamide; LEV, levetiracetam; OXC, oxcarbazepine; PHN, phenytoin; VAL, valproic acid; ZNS, zonisamide.
In the whole study population (with and without IDD), reasons for discontinuation were nausea/vomiting (n=7) and behavioral disorders [(n=7); all of these patients were receiving levetiracetam treatment before initiation of lacosamide, whereas six of them had IDD], insufficient benefit (n=8) and palpitation (n=3).
Cox regression analysis showed that the concomitant use of a sodium channel blocker AED was the only independent predictor of retention rate of lacosamide treatment (P=0.03), as shown in Table 6. In the subgroup of patients with IDD, the analysis was performed again and the number of background AEDs was found to be the only predictor for the retention rate of lacosamide (P=0.04).
Table 6.
Cox regression analysis showed that use of traditional sodium channel blockers was the only independent predictor of retention rate of LCM treatment
| Odds ratio | 95% CI | P-value | |
|---|---|---|---|
| Age (years) | 1.01 | 0.98–1.04 | 0.43 |
| Gender (male) | 1.65 | 0.79–3.43 | 0.18 |
| Presence of IDD | 0.58 | 0.25–1.32 | 0.19 |
| Use of concomitant SCB | 0.31 | 0.11–0.89 | 0.03 |
| Background AEDs, n | 1.51 | 0.94–2.45 | 0.09 |
Note: Background AED, initial antiepileptic drug.
Abbreviations: AED, antiepileptic drug; IDD, intellectual/developmental disorder; LCM, lacosamide; SCB, sodium channel blocker.
Discussion
In this two-centered retrospectively designed study, we found the following: 1) the retention rates of lacosamide for the whole study population at 1, 2, and 3 years were 68%, 62%, and 53% respectively; 2) according to Kaplan–Meier survival analysis, the retention rate of lacosamide was significantly lower in patients with IDD when compared to patients without IDD (P=0.04); 3) behavioral problems (anxiety, unsteadiness, and/or aggressive behavior) were observed in seven patients, who were receiving levetiracetam as the co-medication; and 4) while the predictor of retention rate for the whole study population was on a sodium channel blocker AED, the predictor of retention for the subgroup of IDD patients was the number of background AEDs.
Currently, there are only three studies in which the retention rates of lacosamide were assessed specifically in IDD patients.9–11 One of these studies was reported by Brenner et al,9 in which 132 patients with IDD were evaluated. In this study, the estimated 1-, 2-, and 3-year retention rates of lacosamide were found as 64%, 57%, and 56%, respectively. Our findings regarding the retention rates in the IDD subgroup are in line with this study. They have also reported that behavioral side effects were noted in a high proportion of patients (24.2%). In our study population, the behavioral side effects were encountered in 13.0% of patients with IDD. The other study was reported by Böttcher et al.10 They analyzed 136 patients with IDD. Different from our study, they analyzed the retention rate of lacosamide both in children and adults, and both the patients with focal-onset epilepsy and with other types of seizures were included. Our study comprises only those patients with focal-onset epilepsy as is indicated by US Food and Drug Administration. In this study, long-term retention rates were reported as 62.0% at 1 year, 43.7% at 2 years, and 29.1% at 3 and 4 years. Regarding the retention rate in patients with IDD for the first year, our results are also concordant with this study. However, when compared with this study, we found higher rates of retention for lacosamide at 2 and 3 years. This might be explained by the high doses in their study (100–800 mg/day). In our study, none of the patients received lacosamide >400 mg/day. This finding is in line with a recent meta-analysis reported by Zaccara et al.12 In this meta-analysis, it is suggested that drug withdrawals are more common if AEDs are used at higher doses than recommended, and that the adverse events of an AED are clearly and significantly dose related.12
The other major finding reported by Böttcher et al10 was that concomitant sodium channel blocking AED was not a predictor for a lower retention rate. Our results support this finding; the concomitant sodium channel blocking AED was not a predictor for a lower retention rate in patients with IDD. In concordance with their results, we also suggest that the sole determinant of retention in patients with IDD is the number of background AEDs.
There is another study in which lacosamide use is described.13 This study comprises only pediatric patients. In this study also, McGinnis and Kessler used retention time as the primary outcome in their large cohort. They concluded that simultaneous use of another sodium channel blocking AED increases the treatment failure by 85%.
Several other studies have also shown that concomitant use of sodium channel blocking AEDs and lacosamide is associated with greater side effects.14–17 Our results are in line with these studies. We also suggest that simultaneous use of sodium channel blocking AEDs with lacosamide is associated with a lower retention rate. However, regarding the discontinuation rates with the combination treatment of lacosamide and sodium blocking AEDs, the results are complicated.18
Primary outcome measures of AED trials include percent seizure reduction, responder rate (≥%50 seizure reduction), retention rate, and compliance.19 Both percent seizure reduction and responder rate require a “prospective baseline”, whereas compliance is regarded as a complex and impractical measure. On the other hand, retention rate has gained acceptance as a naturalistic functional endpoint encompassing efficacy, tolerability, and safety, in which no prospective data are required. Retention rate is calculated by measuring the time to treatment failure/study withdrawal for any reason and has grown in use as a primary measurement in AED studies. In 1998, an ILAE report defining outcome measures appropriate for AED clinical trials indicated that retention was a relevant endpoint. The advantage of using the retention time is that it can be applied readily to everyday practice, which measures a patient’s willingness to take a drug.20
Therefore, we assessed the retention rate in our study. However, as the retention rate does not measure the actual changes in seizure frequency, we also reviewed our data based on three categories: 1) decrease in seizure frequency; 2) increase in seizure frequency; and 3) no change. We found that lacosamide was effective in 66.2% of the patients in reducing seizure frequency, severity, or both. In 30.1% of the patients, there was no effect on seizures, whereas seizure activity was increased in 3.7% of the patients.
In our study population, behavioral problems were reported in seven (six of them had IDD) patients after lacosamide initiation. These patients were sharing two features: 1) they had frequent seizures before lacosamide treatment as was reported by caregivers and 2) they were on levetiracetam co-medication before lacosamide treatment. In all of these patients, behavioral problems were encountered after seizures remitted and despite remission, they discontinued lacosamide. We tried to have a possible explanation for the high efficacy of lacosamide and the emergence of behavioral problems. The only explanation we could find was the phenomenon described as “forced normalization” (FN), which was firstly described by Landolt in 1953 in a group of patients with poorly controlled epilepsy who had psychotic episodes associated with remission of seizures and disappearance of epileptiform activity on their electroencephalographs.21 The primary and supportive diagnostic criteria of FN have been outlined in Table 7. According to these criteria (electroencephalograph is not required if other supportive data are available), we hypothesized that FN was the most likely diagnosis in our patient population, because the behavioral changes occurred in an acute/subacute fashion, there were no seizures for more than a week (corroborated by a relative/a caregiver), and symptoms emerged within the recent change of the drug regimen (lacosamide was initiated within 15 days in all patients presenting with behavioral problems).
Table 7.
Proposed criteria for forced normalization
| Primary (essential) criteria |
| 1. Established diagnosis of epilepsy based on clinical history, EEG, and imaging |
| 2. Presence of a behavioral disturbance of acute or subacute onset characterized by one or more of the following: • Psychosis with thought disorder, delusions, hallucinations • Significant mood change, hypomania or mania, or depression • Anxiety with depersonalization, derealization • Hysteria: motor, sensory, aphasia |
| 3A. Reduction in the total number of spikes counted in a 60-minute waking-state electroencephalographic recording with a 16-channel machine, using standard 10–20 electrode placement, by over 50% compared with a similar recording performed during a normal state of behavior |
| OR |
| 3B. Report of complete cessation of seizures for at least 1 week, corroborated by a relative or a carer Supportive criteria Recent change (within 30 days) of pharmacotherapeutic regimen Report of similar episodes of seizure cessation and behavioral disturbance in the past, from a close relative or a carer, or general practitioner, or documentation of this in hospital records with or without electroencephalographic evidence. This may or may not be linked with an anticonvulsant drug |
| To make the diagnosis |
| Primary criteria 1, 2, and 3A |
| OR |
| Primary criteria 1, 2, and 3B and one supportive criterion |
In observations from a prospective audit, it has been reported that patients with learning disabilities were more prone to develop intolerable psychiatric problems compared to those without learning disabilities.22 In this report, it has been suggested that untangling psychiatric comorbidities from behavioral and other issues in patients with IDD can be challenging, and that misdiagnoses might be more common than in general population. There are several other studies supporting this observation.23,24
On the other hand, albeit our finding regarding the emergence of symptoms which are compatible with FN, it is not possible to relate these findings directly to IDD or only to the mechanisms of the drug. This finding may only provide a framework which may help clinicians dealing with patients with IDD as psychotic disorder, hallucinations, agitation, and aggression have been reported in patients receiving lacosamide treatment in preapproval clinical trials and in unpublished postmarketing data by the US Food and Drug Administration in 2014.25–31
In our study population, lacosamide was not escalated to 400 mg despite the absence of efficacy and any side effect (n=17). Median dose of lacosamide was 300 mg in this subgroup. The explanation for this was the lack of any other suitable AED options in patients with drug-resistant epilepsy in our country. Due to an acceptable side effect profile, caregivers/patients sometimes opt to use this dose of lacosamide despite there being no significant efficacy. However, we chose to include these patients as we wanted to conduct a study representing the real world; there are patients who will go on using AEDs despite the absence of any significant efficacy in clinical practice. Additionally, this condition meets the definition of retention rate as well as “the willingness of a patient to take the drug”.
Limitations of our study
While our study has supplied information from a clinic-centered perspective, it has some limitations which have to be pointed out. First, the retrospective nature of the study precludes us from making a definitive conclusion on seizure frequency after lacosamide. To overcome this limitation, we used retention rate in accordance with recent data and ILAE reports. Retention rate is considered as a measure of clinical effectiveness which combines both efficacy and tolerability. In light of these data, we not only also used the retention rate as a primary outcome in our study, but also added our data to represent the seizure frequency changes (defined as reduction, increase, or no change) after lacosamide treatment. Another limitation is the relatively small patient population. Therefore, we used comparative data and analyzed the patient population according to the existence of IDD.
Conclusion
Our study shows that the retention rate for adjunctive treatment with lacosamide in a real-world setting is comparable with recently published studies. Given the paucity of studies among patients with epilepsy and IDD comorbidity, we believe further studies might shed light on this important area.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organisation . Tenth Revision. Anonymous International Statistical Classification of Diseases and Health Related Problems. Geneva: 1992. International Statistical Classification of Diseases and Related Health Problems. [Google Scholar]
- 2.Mcgrother CW, Bhaumik S, Thorp CF, Hauck A, Branford D, Watson JM. Epilepsy in adults with intellectual disabilities: prevalence, associations and service implications. Seizure. 2006;15(6):376–386. doi: 10.1016/j.seizure.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Cawello W. Clinical pharmacokinetic and pharmacodynamic profile of lacosamide. Clin Pharmacokinet. 2015;54(9):901–914. doi: 10.1007/s40262-015-0276-0. [DOI] [PubMed] [Google Scholar]
- 4.Kellinghaus C. Lacosamide as treatment for partial epilepsy: mechanisms of action, pharmacology, effects, and safety. Ther Clin Risk Manag. 2009;5:757–766. doi: 10.2147/tcrm.s5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lennox N, Taylor M, Rey-Conde T, Bain C, Purdie DM, Boyle F. Beating the barriers: recruitment of people with intellectual disability to participate in research. J Intellect Disabil Res. 2005;49(Pt 4):296–305. doi: 10.1111/j.1365-2788.2005.00618.x. [DOI] [PubMed] [Google Scholar]
- 6.AAIDD (American Association on Intellectual Developmental Disabilities) Intellectual disability: Definition, classification, and systems of supports. Washington, DC: AAIDD; 2010. [Google Scholar]
- 7.APA (American Psychiatric Association) 2013. Diagnostic and statistical manual of mental disorders, fifth edition. Diagnostic and Statistical Manual of Mental Disorders Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC Washington: APADC; 2013. [Google Scholar]
- 8.Brodie MJ. Sodium Channel Blockers in the Treatment of Epilepsy. CNS Drugs. 2017;31(7):527–534. doi: 10.1007/s40263-017-0441-0. [DOI] [PubMed] [Google Scholar]
- 9.Brenner J, Majoie HJM, van Beek S, Carpay JA. The retention of lacosamide in patients with epilepsy and intellectual disability in three specialised institutions. Seizure. 2017;52:123–130. doi: 10.1016/j.seizure.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Böttcher S, Lutz MT, Mayer T. Lacosamide in the treatment of patients with epilepsy and intellectual disabilities: A long-term study of 136 patients. Epilepsia. 2017;58(10):1749–1754. doi: 10.1111/epi.13869. [DOI] [PubMed] [Google Scholar]
- 11.Mcginty RN, Costello DJ. Long-term lacosamide retention-Real-world experience at a tertiary epilepsy center in Ireland. Epilepsy Behav. 2017;68:141–145. doi: 10.1016/j.yebeh.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Zaccara G, Giovannelli F, Maratea D, Fadda V, Verrotti A. Neurological adverse events of new generation sodium blocker antiepileptic drugs. Meta-analysis of randomized, double-blinded studies with eslicarbazepine acetate, lacosamide and oxcarbazepine. Seizure. 2013;22(7):528–536. doi: 10.1016/j.seizure.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Mcginnis E, Kessler SK. Lacosamide use in children with epilepsy: Retention rate and effect of concomitant sodium channel blockers in a large cohort. Epilepsia. 2016;57(9):1416–1425. doi: 10.1111/epi.13466. [DOI] [PubMed] [Google Scholar]
- 14.Kamel JT, Degruyter MA, D’Souza WJ, Cook MJ. Clinical experience with using lacosamide for the treatment of epilepsy in a tertiary centre. Acta Neurol Scand. 2013;127(3):149–153. doi: 10.1111/j.1600-0404.2012.01704.x. [DOI] [PubMed] [Google Scholar]
- 15.Novy J, Patsalos PN, Sander JW, Sisodiya SM. Lacosamide neurotoxicity associated with concomitant use of sodium channel-blocking antiepileptic drugs: a pharmacodynamic interaction? Epilepsy Behav. 2011;20(1):20–23. doi: 10.1016/j.yebeh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Sake JK, Hebert D, Isojärvi J, et al. A pooled analysis of lacosamide clinical trial data grouped by mechanism of action of concomitant antiepileptic drugs. CNS Drugs. 2010;24(12):1055–1068. doi: 10.2165/11587550-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Villanueva V, Garcés M, López-Gomáriz E, et al. Early add-on lacosamide in a real-life setting: results of the REALLY study. Clin Drug Investig. 2015;35(2):121–131. doi: 10.1007/s40261-014-0255-5. [DOI] [PubMed] [Google Scholar]
- 18.Neal A, D’Souza W, Hepworth G, Lawn N, Cook M, Nikpour A. Efficacy and tolerability of adjuvant lacosamide: The role of clinical characteristics and mechanisms of action of concomitant AEDs. Epilepsy Behav. 2018;80:25–32. doi: 10.1016/j.yebeh.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Menachem E, Sander JW, Privitera M, Gilliam F. Measuring outcomes of treatment with antiepileptic drugs in clinical trials. Epilepsy Behav. 2010;18(1–2):24–30. doi: 10.1016/j.yebeh.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 20.ILAE Commission on Antiepileptic Drugs Considerations on designing clinical trials to evaluate the place of new antiepileptic drugs in the treatment of newly diagnosed and chronic patients with epilepsy. Epilepsia. 1998;39(7):799–803. doi: 10.1111/j.1528-1157.1998.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 21.Landolt H. On various correlations between the electro-encephalogram and normal and pathological psychic processes. Schweiz Med Wochenschr. 1963;93:107–110. [PubMed] [Google Scholar]
- 22.Stephen LJ, Wishart A, Brodie MJ. Psychiatric side effects and anti-epileptic drugs: Observations from prospective audits. Epilepsy Behav. 2017;71(Pt A):73–78. doi: 10.1016/j.yebeh.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Espie CA, Watkins J, Curtice L, et al. Psychopathology in people with epilepsy and intellectual disability; an investigation of potential explanatory variables. J Neurol Neurosurg Psychiatry. 2003;74(11):1485–1492. doi: 10.1136/jnnp.74.11.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ring H, Zia A, Lindeman S, Himlok K. Interactions between seizure frequency, psychopathology, and severity of intellectual disability in a population with epilepsy and a learning disability. Epilepsy Behav. 2007;11(1):92–97. doi: 10.1016/j.yebeh.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Krishnamoorthy ES, Trimble MR, Sander JW, Kanner AM. Forced normalization at the interface between epilepsy and psychiatry. Epilepsy Behav. 2002;3(4):303–308. doi: 10.1016/s1525-5050(02)00052-5. [DOI] [PubMed] [Google Scholar]
- 26.Wolf P. In: Acute behavioral symptomatology at disappearance of epileptiform EEG abnormality: paradoxical or forced normalisation. Smith D, Trieman D, Trimble MR, editors. Vol. 55. New York, NY: Neurobehavioral problems in EpilepsyRaven Press; 1991. pp. 127–142. [PubMed] [Google Scholar]
- 27.Krishnamoorthy ES, Trimble MR, Blumer D. The classification of neuropsychiatric disorders in epilepsy: a proposal by the ILAE Commission on Psychobiology of Epilepsy. Epilepsy Behav. 2007;10(3):349–353. doi: 10.1016/j.yebeh.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Pollock DC. Models for understanding the antagonism between seizures and psychosis. Prog Neuropsychopharmacol Biol Psychiatry. 1987;11(4):483–504. doi: 10.1016/0278-5846(87)90017-0. [DOI] [PubMed] [Google Scholar]
- 29.Pinkhasov A, Lam T, Hayes D, Friedman M, Singh D, Cohen H. Lacosamide Induced Psychosis: Case Report, Review of Differential Diagnosis and Relevant Pharmacokinetics. Clin Neuropharmacol. 2015;38(5):198–200. doi: 10.1097/WNF.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 30.Abou Khaled K, Khoury J, Macaron G, Richa S. Forced normalization and psychosis following use of lacosamide. Seizure. 2016;41:96–99. doi: 10.1016/j.seizure.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Food and Drug Administration FDA Vimpat (lacosamide) Prescribing Information. 2014. [Google Scholar]