Supplemental Digital Content is available in the text.
Keywords: blood pressure, hypertension, pre-eclampsia, pregnancy, white coat hypertension
Abstract
Hypertensive disorders during pregnancy result in substantial maternal morbidity and are a leading cause of maternal deaths worldwide. Self-monitoring of blood pressure (BP) might improve the detection and management of hypertensive disorders of pregnancy, but few data are available, including regarding appropriate thresholds. This systematic review and individual patient data analysis aimed to assess the current evidence on differences between clinic and self-monitored BP through pregnancy. MEDLINE and 10 other electronic databases were searched for articles published up to and including July 2016 using a strategy designed to capture all the literature on self-monitoring of BP during pregnancy. Investigators of included studies were contacted requesting individual patient data: self-monitored and clinic BP and demographic data. Twenty-one studies that utilized self-monitoring of BP during pregnancy were identified. Individual patient data from self-monitored and clinic readings were available from 7 plus 1 unpublished articles (8 studies; n=758) and 2 further studies published summary data. Analysis revealed a mean self-monitoring clinic difference of ≤1.2 mm Hg systolic BP throughout pregnancy although there was significant heterogeneity (difference in means, I2 >80% throughout pregnancy). Although the overall population difference was small, levels of white coat hypertension were high, particularly toward the end of pregnancy. The available literature includes no evidence of a systematic difference between self and clinic readings, suggesting that appropriate treatment and diagnostic thresholds for self-monitoring during pregnancy would be equivalent to standard clinic thresholds.
Worldwide, ≈10% of women have high blood pressure (BP) during pregnancy.1 Hypertensive disorders in pregnancy (HDP), including preeclampsia, are one of the commonest causes of maternal mortality2,3 and are a leading cause of direct maternal deaths in the United Kingdom.4,5
Hypertension is commonly defined as systolic BP of ≥140 mm Hg or diastolic BP ≥90 mm Hg. If hypertension presents after 20 weeks gestation, it is known as gestational hypertension, and when combined with the presence of maternal organ dysfunction, uteroplacental dysfunction, or significant proteinuria (>300 mg proteinuria/24 hours or >30 mg/mmol creatinine [protein-creatinine ratio]), it is known as preeclampsia.6–11 Preeclampsia complicates 2% to 8% of all pregnancies and up to 25% of pregnancies in women with chronic hypertension.12
Current guidelines identify risk factors for preeclampsia based on medical and family histories.6,13 The UK guidelines recommend BP monitoring with increased frequency in those at higher risk of preeclampsia.6 Despite this, women still develop preeclampsia in the interval between antenatal visits, and a significant proportion of subsequent deaths occur after HDP, which developed after apparently normal antenatal visits.14 Reliance on intermittent clinic measurements may lead to both false-positive (white coat hypertension [WCH]) and -negative (masked hypertension) interpretations. Enhanced identification of developing hypertension among higher risk women could improve outcomes.
Self-monitoring of BP (SMBP), where individuals measure their own BP in a home setting, allows multiple measurements for several days with little disturbance of lifestyle and is now commonplace in adults with hypertension.15–17 Regular SMBP might improve detection of hypertension in the pregnant population while reducing the time, money, and inconvenience of frequent appointments, without compromising the ability to detect and monitor a potentially serious disease. Potential advantages of SMBP include more frequent BP monitoring, improved accuracy, and increased acceptability to pregnant women.18,19
The importance and potential of self-monitored BP measurement has been noted in both a joint statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association and the recent European Society of Hypertension guidelines.20 Consequently, in some countries, SMBP during pregnancy is becoming common: a Canadian pilot survey found >60% of women diagnosed with nonproteinuric hypertension in pregnancy were already SMBP.21 A subsequent survey, of Canadian obstetricians and primary care physicians, found that most used SMBP to check for WCH (where BP is higher in clinic than at home) rather than ambulatory BP monitoring.22
Thresholds for SMBP in the hypertensive nonpregnant population are generally lower than when using clinic monitoring, but the clinic−home difference varies dependent on the population,23,24 and general populations have been shown to have minimal differences between home and clinic readings.25,26 Recent guidelines from the society of Obstetrician and Gynaecologists of Canada suggest a self-monitoring threshold of 135/85 mm Hg.27 However, there are limited data on SMBP monitoring during pregnancy to guide such recommendations. This study aimed to systematically review the available evidence for differences between self-monitored and clinic BP during pregnancy and investigate this using individual patient data (IPD) where available.
Methods
Data Availability
Data were obtained from third parties for this project. Several of the participating studies required specific data sharing agreements from their host institution. Current agreements are for the purposes of this analysis alone, and as such new approval would be required. Requests for Data Sharing should be directed to information.guardian@phc.ox.ac.uk. Such requests will be considered by all data holders involved in this collaboration. There were no specific study materials.
Search Strategy for Identification of Studies
The searches were run from database inception to July 2016, using a combination of title/abstract keywords and subject headings for self-measurement, BP, and pregnancy (Figure S1 in the online-only Data Supplement). No date or publication date limits were applied. We searched the Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effectiveness, Cochrane Central Register of Controlled Trials (The Cochrane Library, Wiley), Medline (OvidSP; 1946–present), Embase (OvidSP; 1974–present), CINAHL (EbscoHOST; 1980–present), PsycINFO (OvidSP; 1967–present), Science Citation Index Expanded, Social Sciences Citation Index, Conference Proceedings Citation Index-Science & Conference Proceedings Citation Index-Social Science & Humanities (Web of Knowledge; 1945–present).
Inclusion Criteria
All primary studies of any study design that included SMBP during pregnancy in a sample of multiple women were included. Each corresponding author was contacted requesting IPD or summary data for self-monitored and clinic BP. Studies that provided clinic and self-monitored data or published sufficient data to allow comparison were included in the analysis. This study was registered on Prospero: CRD42016050528.
Data Extraction
Two reviewers (K.T. and J.H.) independently reviewed the titles and abstracts of identified articles and, after assessment of the full text of potentially relevant papers, extracted data (Figure S2). Data extraction included a methodological quality assessment adapted from the Quality Assessment of Diagnostic Accuracy Studies checklist28—clear selection criteria for participants, response rate, validation of sphygmomanometer, validation of self-monitored readings, and blinding of those performing clinic measurements. Data extractions were performed in duplicate and disagreements resolved by consensus.
Individual Patient Data
Authors from all eligible studies were approached to provide anonymized IPD. The data were considered at 4 time periods (5–14, 15–22, 23–32, and 33–42 weeks’ gestation), and these were chosen to enable the inclusion and analysis of relevant comparative data from included studies throughout pregnancy. A trimester-based comparison would have resulted in loss of data or inappropriate comparisons.
Data Analysis and Statistical Methods
The absolute differences in BP between clinic and self-measurements were calculated (means and SDs) across all studies and in subgroups according to gestational stage. These differences were calculated using the nearest (closest in time, within 3 days either side) clinic and self-monitored readings for each woman in each comparison period, including only one comparison per women per stage (5–14, 15–22, 23–32, and 33–42 weeks) to avoid bias toward women who completed more readings than others.
Meta-analysis was undertaken in Stata13 (StataCorp, LP) to create forest plots, and the I2 statistic was used to estimate statistical heterogeneity for each outcome. IPD data were used to calculate the means of differences. A subgroup analysis of normotensive and hypertensive participants (based on clinic BP ≥140/90) was completed to examine differences between the groups. To compare with aggregate data, the difference in means was also calculated. Where substantial heterogeneity was detected, the direction of effect was assessed, and potential reasons for heterogeneity were considered. A random-effects analysis was used, and differences between self-monitored and clinic readings were assessed using scatter and Bland-Altman plots.
Definitions
There was no evidence before this work to guide the definitions of hypertension on the basis of home readings. In general unselected populations, there is little difference between home and clinic readings.25,26 The results from the review, which included mixed populations of both normotensive and hypertensive women, suggested little overall difference between home and clinic readings. Current UK and international guidelines use a clinic threshold of 140/90 mm Hg for diagnosing hypertension during pregnancy. For the purposes of this work, true hypertension was considered present when self-monitored and clinic readings both had systolic BP ≥140 and diastolic BP ≥90 mm Hg in the comparison period. Masked hypertension was defined as normotensive clinic BP <140/90 mm Hg with self-monitored readings of systolic BP ≥140 or diastolic BP ≥90 mm Hg. Finally, WCH was defined as clinic systolic BP ≥140 mm Hg and diastolic BP ≥90 mm Hg with normotensive self-monitored readings (<140/90 mm Hg). Recognizing that home monitoring thresholds for hypertension suggested for the nonpregnant hypertensive population are lower (135/85 mm Hg), a sensitivity analysis was performed using a home threshold for hypertension of 135/85 mm Hg.
Results
Findings From the Systematic Review
From 2654 journal articles identified, 121 were assessed in full. Twenty-one articles included SMBP during pregnancy in a sample of multiple women. Corresponding authors were contacted requesting IPD or summary data for self-monitored and clinic BP: there was no reply from 4 and a further 10 were unable to provide IPD, leaving 7 studies (6 authors) where investigators provided BP data from both self-monitoring and clinic BP during pregnancy (n=596; Figure 1).29–34 One further study undertaken by our group and not published in full at the time was included (n=162; total 758).35 Of the 10 studies unable to provide data, 2 had published summary data of self-monitored-clinic comparison (Table 1).36,37
Figure 1.
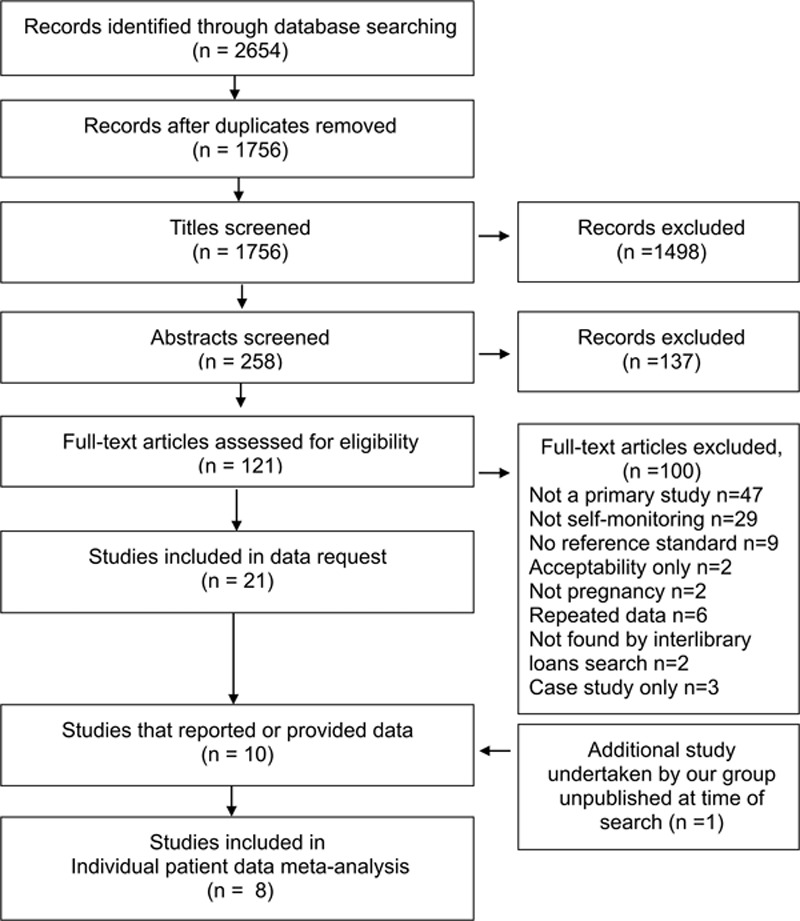
Flow diagram of the systematic review. Studies included in the analysis of clinic and self-monitored blood pressure during pregnancy.
Table 1.
Studies Included in Analysis
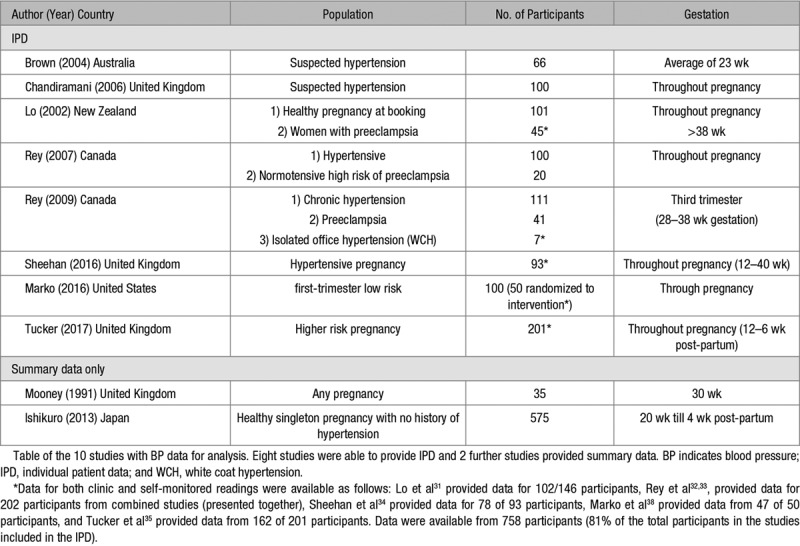
Characteristics of Studies Included in the IPD Analysis
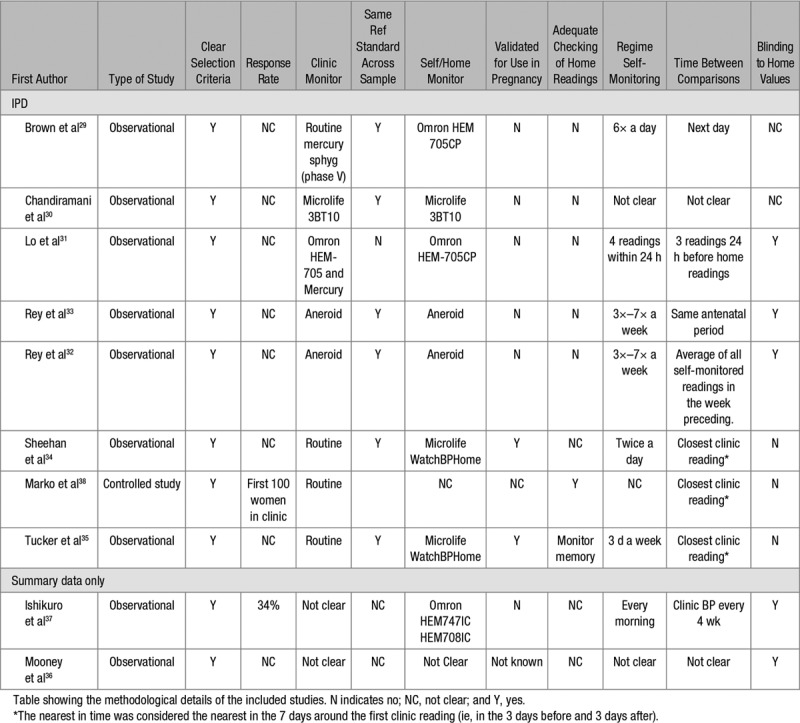
Included studies were performed between 2002 and 2017 on a range of populations, including women with a healthy pregnancy,31,38 women with suspected hypertension,29,30 and those with known gestational hypertension or preeclampsia.31,32,34 Considerable variation was seen in both monitoring schedules and the gestation period when the readings were taken. Only 2 studies used a monitor that had passed validation testing in pregnancy (Table 2).34,35
Table 2.
Methodological Details of the Included Studies
Difference Between Self-Monitored and Clinic Readings Though Pregnancy
IPD from the 8 included studies (n=758),29–35,38 contained BP readings throughout pregnancy (Figure 2). The overall mean differences between home and clinic readings appeared small throughout pregnancy (Table 3), with mean of differences between 1.5 to 2.2 mm Hg systolic (Figure 2) and 0.7 to 1.5 mm Hg diastolic BP (Figure S3). However, there was significant heterogeneity between studies (I2 >80 at all gestational stages for systolic BP), which was only slightly improved by removal of single studies.
Figure 2.
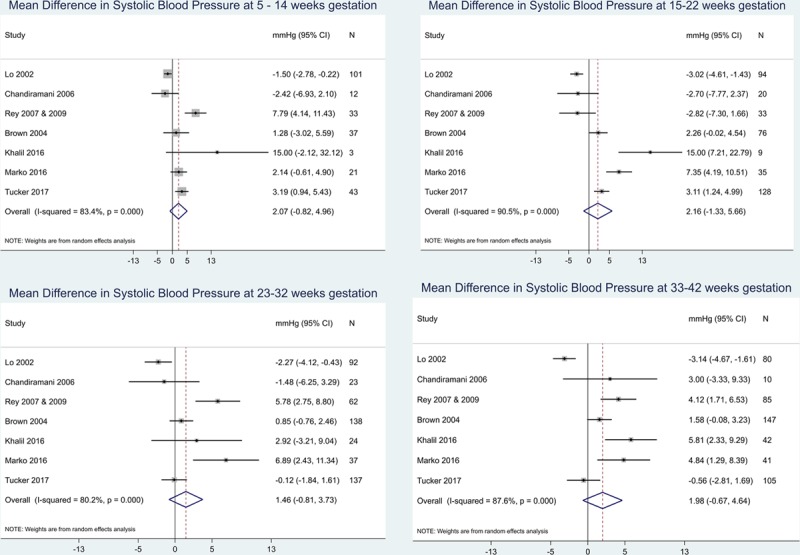
Comparison of clinic and self-monitored systolic blood pressure (BP). Forest plots were constructed to examine the difference in mean BP (clinic–home) by the type of monitoring and study group. Data were analyzed as continuous variables and presented here in mm Hg, plotted by gestational stage. CI indicates confidence interval.
Table 3.
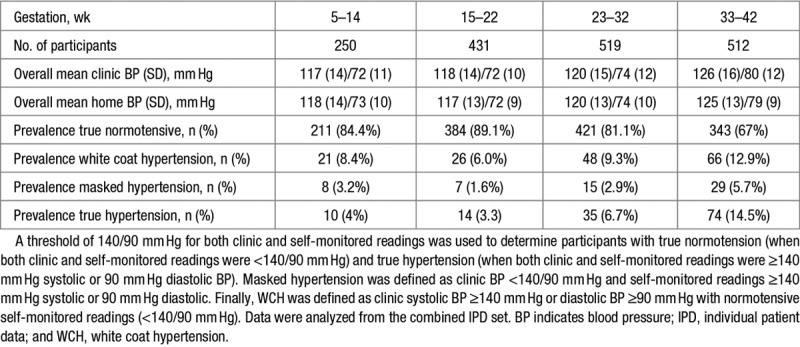
Overall Blood Pressure by Gestation Plus Prevalence of True, Masked, and White Coat Hypertension
Subgroup analysis of normotensive and hypertensive groups showed that normotensive pregnant women have little differences between home and clinic readings with mean differences between −0.6 and 1.2 mm Hg systolic (Figures S9 and S10) and −0.6 and 0.7 mm Hg diastolic. Hypertensive women, however, had much larger differences between home and clinic (Figures S7 and S8). The heterogeneity remained high, and the number of hypertensive participants included at the early stages of pregnancy was low.
Differences in means (office and home) were plotted for both the IPD data and summary data from other studies where possible (Figures S4 and S5).36,37 Reported differences in means were higher from studies for which IPD data were not available (4.06 mm Hg at 15–22/40 and 2.9 mm Hg at 23–32/40).36,37
Influence of Absolute BP on Mean Difference Between Self-Monitored and Clinic Readings
Bland-Altman and scatter plots show good agreement between self-monitored and clinic BP across the range of both systolic and diastolic pressures (Figure 3; Figure S6). At later gestation, the number of readings above the limits of agreement were higher than in early pregnancy (Figure 3).
Figure 3.
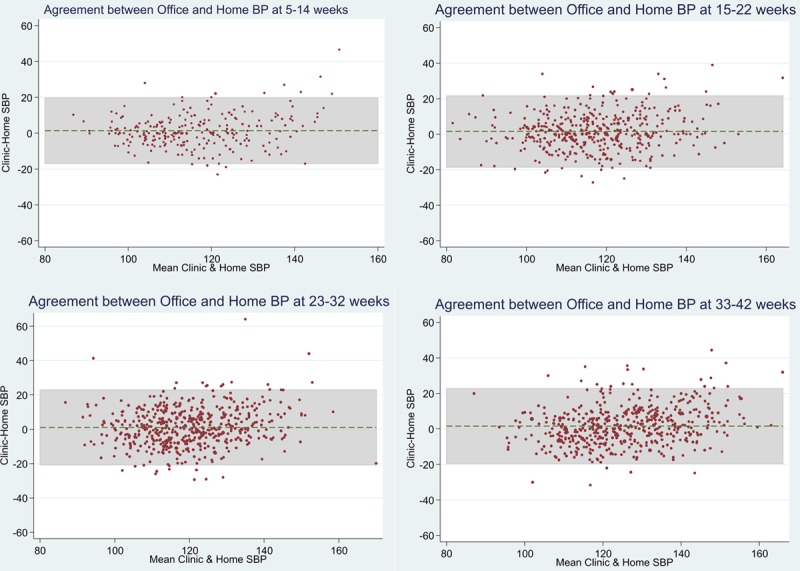
Agreement between clinic and self-monitored blood pressure (BP) readings during pregnancy. Bland-Altman plots were used to examine the influence of mean BP on the clinic−self difference. The mean clinic and self-monitored readings were plotted against clinic−self monitored readings (complete cases). At 5 to 14 wk, there was a mean difference of 1.403, 6.8% (17 of 250) readings were outside limits of agreement, and 95% limits of agreement were −16.943, 19.750. At 15 to 22 wk, a mean difference of 1.550 was observed, 6.26% (27 of 431) readings were outside limits of agreement, and the 95% limits of agreement were −18.576, 21.677. At 23 to 32 wk gestation, there was a mean difference of 1.067, 4.82% (25 of 519) readings were outside limits of agreement, and the 95% limits of agreement were −20.736, 22.871. At 33 to 42 wk gestation, there was a mean difference of 1.494, 4.66% (22 of 472) readings were outside limits of agreement, and 95% limits of agreement were −19.429, 22.417. Diastolic plots are shown in Figure S6.
Prevalence of True and WCH by Gestation
As gestation increased, mean clinic BP initially remained low, and then began to rise from 33 weeks gestation. Using a threshold of ≥140/90 mm Hg for hypertension for both clinic and self-monitored BP, the prevalence of true hypertension and WCH increased with gestation. At 33 to 42 weeks, the prevalence of WCH and true hypertension was similar, indicating women with hypertension on clinic measurements were as likely to have normal BP when self-monitoring as they were to have true hypertension (Table 3). A sensitivity analysis of a self-monitored 135/85 mm Hg threshold is shown in Table S1.
Discussion
This systematic literature review identified 21 studies that included SMBP during pregnancy. IPD were available for both self-monitored and clinic BP readings from 8 studies (758 patients).29–35,38 Comparison of self-monitored and clinic readings revealed no clinically relevant difference throughout pregnancy, with significant heterogeneity between studies that may be accounted for by the varying populations. Interestingly, although overall differences between self-monitored and clinic pressure were small, the prevalence of WCH increased reaching 13% toward the end of pregnancy (33–42 weeks gestation). By this stage, WCH was as prevalent as true hypertension.
Strengths and Weaknesses
This study’s strengths include the systematic capture of all of the current literature and the inclusion of IPD data from 8 studies and >750 women. To our knowledge, this is the first comparison of self-monitored and office readings using data drawn from multiple studies, settings, and countries. Weaknesses include that 11 of the 21 studies may have had additional data on self-monitored and clinic readings but did not publish or provide it.18,39–48 In addition, IPD from the largest study to date were not available.37
All studies had some degree of methodological flaw (or lack of clarity), and only 2 studies used a self-monitoring device validated in the pregnant population. The validation of monitors in pregnancy and preeclampsia is important as pregnancy causes changes to the vasculature meaning that many generally validated monitors are inaccurate.49 Furthermore, the development of preeclampsia leads to significant and widespread hemodynamic disturbances, which have been shown to affect the accuracy of some BP monitors that are accurate in normal pregnancy.50–52 Only 5 monitors have been validated for home use in pregnancy.41,53–56
The selection of participants by contributing studies was generally clear although details of pregnancy stage were sometimes lacking and only 1 study was randomized. Attrition reporting was not always clear, and checking of self-monitored readings was not reported in any of the studies. Reporting of results was generally adequate (Table 2).
Studies were statistically and clinically heterogenous reflecting diverse populations (health state, gestation of pregnancy) and clinical contexts (date and country of study). Many included studies were relatively small and published for a long time period (1991–2017), during which time there have been advances in BP monitoring technology. Most studies included in this review used monitors that have not been validated in pregnancy. The monitor used by both Brown et al,29 and Lo et al31 subsequently failed validation in the preeclamptic population. However, removing these studies from our analysis did not remove the observed heterogeneity. It is clear that further validation of BP monitors in the pregnant population is needed including individuals with preeclampsia and obesity.
A further potential cause of the observed heterogeneity could be differences in the populations studied. For example, both Lo et al31 and Ishikuro et al37 (Figures S4 and S5) observed normotensive healthy pregnant populations, and both Brown et al29 and Chandiramani et al30 (Figure 2; Figures S3–S5) selected women who had suspected hypertension; however, the clinic−self monitored differences in these study groups were opposing (Figure 2; Figures S3–S5). It may be that the monitor used, schedule of monitoring, or some other factor was more influential.
Overall, the data were representative of populations that may use home monitoring, either to detect hypertension in pregnancy (normotensive pregnancies31,35), differentiate between true and WCH (suspected but not confirmed hypertension29,30), or manage hypertension (women with gestational hypertension or preeclampsia30–32).
Comparison With the Previous Literature
Clear SMBP thresholds for hypertension in pregnancy have not been established in the United Kingdom.23 Canadian guidelines recommend a self-monitored threshold of 135/85 mm Hg although it is unclear on what this is based, presumably the nonpregnant population.17,27 A sensitivity analysis using a threshold for hypertension of 135/85 mm Hg for home monitoring showed a slightly higher level of discordance with diagnosis based on clinic readings. However, subgroup analysis of women with hypertension (defined by clinic readings) showed that this group had a much larger home-office difference, suggesting that this group was selecting for a larger white coat effect and therefore lower thresholds may be appropriate.
Previous reports have suggested that the prevalence of WCH in pregnancy may be considerable.43,57 In the general population, the amount of white coat effect is higher in hypertensive groups compared with normotensive groups.24 This is thought to be because the higher average BP is more likely to result in variations above threshold and because this population may be more anxious about clinic results. This increase in white coat effect in hypertensive groups seems also to be true in pregnancy as several studies show high prevalence of WCH in hypertensive pregnancy.33,37,43,57 Some data suggest that women with WCH are at low risk of complications, but others that WCH reflects increased variability and hence risk.38 Further work is required to understand the true prognosis of WCH in pregnancy.
Perspectives
Current UK guidelines recommend more frequent BP measurements should be considered in those at higher risk of preeclampsia.6 It is for these women that SMBP is likely to be most effective in terms of diagnosing higher BP in pregnancy. SMBP could also be beneficial to identify WCH in those presenting with high clinic pressures and to improve the monitoring of women with true chronic or gestational hypertension.43
If SMBP were to become commonplace in antenatal care, reference thresholds for self-monitored readings would be needed. Previous studies have examined the normal range of out-of-office BP during healthy pregnancy (predominantly using ambulatory BP monitoring) with varying conclusions.42,58 This is the first study to compare self-monitored and office readings across multiple studies. We found that self-monitored and clinic readings had little overall difference, suggesting that a self-monitored threshold equivalent to clinic threshold (currently 140/90 mm Hg) would be appropriate during pregnancy for normotensive women. Confirmation of this would require a study relating home BP to outcome. In addition, although we found little variation in mean differences, some women had clinically important differences between home and clinic readings, and therefore the prognostic significance of WCH and masked hypertension in pregnancy should also be considered. Subgroup analysis revealed that hypertensive pregnant women tended to have much larger home–office difference than normotensive pregnant women.
The clinical context of home monitoring is an important consideration. Whether the self-monitoring was additional to standard antenatal clinic visits, or being used as a replacement is relevant, as would which group of women are involved; those at risk of HDP, or those with HDP (ie, is the monitoring for diagnosis or management of hypertension?). For both groups, it would be reasonable to suggest that women with home readings of 140/90 mm Hg should be seen in clinic for safety. As current guidelines suggest starting medication for hypertension at 150/100 mm Hg, additional appointments in clinic may not be justified if clinic readings are likely to be <140/90 mm Hg. Hypertensive pregnant women defined on clinic pressures, however, may require a threshold similar to the hypertensive population outside pregnancy (135/85 mm Hg); previous studies have reported a high prevalence of WCH, and our subgroup analysis would support this.
Conclusions
This IPD analysis provides the best evidence to date on suitable thresholds for the diagnosis of HDP. Although showing heterogeneity between studies, overall there seems to be little difference between self-monitored and clinic readings. This evidence needs to be tempered by the knowledge that most monitors used in included studies were not validated and that monitoring schedules varied between studies. However, a finding of broad equivalence of clinic and home BP is in line with general population studies outside of pregnancy, unlike those with participants included on the basis of raised BP in the clinic. In turn, it suggests that home BP thresholds used for decision making in an unselected population in pregnancy should be equivalent to those currently used in clinic. SMBP, therefore, seems to have a role in ruling out WCH in those presenting with high clinic pressures. Understanding the true place of self-monitored BP in pregnancy will require further studies with validated monitors to confirm the equivalence of thresholds, including careful linkage to outcomes for women and their offspring. In addition, outcome studies are needed to better understand the prognosis of both WCH and masked hypertension in pregnancy.
Acknowledgments
We thank Dr Thierry Denolle, Dr Paul Mooney, Prof Kevin Dalton, Dr Ross-McGill, Prof William Rayburn, Dr Zuspan, Prof Jim Thornton, and Dr Janet Hirst who have provided advice and support and Carla Betts for administration support.
Sources of Funding
This article represents independent research commissioned by the National Institute for Health Research (NIHR) School of Primary Care Research (SPCR; SPCR project no. 171). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health. R.J. McManus received support from an NIHR Professorship (NIHR-RP-02-12-015) and the NIHR Oxford Collaboration for Leadership in Applied Health Research and Care. C. Bankhead is supported by the NIHR Biomedical Research Centre, Oxford. The study was conceived by R.J. McManus who in collaboration with K.L. Tucker, C. Heneghan, J. Hodgkinson, and L. Mackillop gained the funding. The protocol was developed by K.L. Tucker, R.J. McManus, J. Hodgkinson, C. Bankhead, and R. Stevens with the advice and support of all authors. Literature searches were performed by N. Roberts, and review process completed by J. Hodgkinson and K.L. Tucker. Data were provided by E. Rey, C. Lo, K.S. Taylor, R.A. North, M. Chandiramani, R.S. Taylor, M. Brown, A. Khalil, K. Marko, and J. Waugh. Data cleaning was performed by K.L. Tucker, C. Bankhead, and K.S. Taylor, and statistical analysis was performed by K.L. Tucker and C. Bankhead with advice from R. Stevens. The first draft of the article was written by K.L. Tucker with R.J. McManus and subsequently edited and approved by all coauthors. All authors have read and approved the final manuscript.
Disclosures
The authors declare the following conflicts of interest: A. Khalil has the Copyright for An App and Software that enables pregnant women to monitor blood pressure at home. This was developed as part of an Innovation Grant funded by the Health Foundation. L. Mackillop receives consultancy fees from Drayson Technologies. The other authors report no conflicts.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.118.10917.
Novelty and Significance
What Is New?
This study combined the currently available evidence on clinic and self-monitored blood pressure during pregnancy to consider thresholds for home monitoring.
What Is Relevant?
There was little overall difference between self-monitored and clinic blood pressure readings, suggesting that appropriate thresholds for self-monitoring would be equivalent to clinic thresholds.
White coat hypertension may be common toward the end of pregnancy, and more research is needed to assess the impact of this.
More studies using monitors validated in pregnancy are needed.
Summary
Data from >750 pregnant women showed that there was little over all difference between self-monitored and clinic blood pressure readings. However, there were high levels of white coat hypertension toward the end of pregnancy.
References
- 1.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130–137. doi: 10.1053/j.semperi.2009.02.010. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, Gülmezoglu AM, Temmerman M, Alkema L. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–e333. doi: 10.1016/S2214-109X(14)70227-X. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 3.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074. doi: 10.1016/S0140-6736(06)68397-9. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 4.Knight M KS, Brocklehurst P, Neilson J, Shakespeare J, Kurinczuk JJ, editors. on Behalf of MBRRACEUK. Saving Lives, Improving Mothers’ Care Lessons Learned to Inform Future Maternity Care From the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009–2012. Maternal, Newborn and Infant Clinical Outcome Review Programme. Oxford, UK: National Perinatal Epidemiology Unit, University of Oxford; 2014. [Google Scholar]
- 5.Cantwell R, Clutton-Brock T, Cooper G, et al. Saving Mothers’ Lives: reviewing maternal deaths to make motherhood safer: 2006-2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(suppl 1):1–203. doi: 10.1111/j.1471-0528.2010.02847.x. doi: 10.1111/j.1471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 6.NICE. NICE Clinical Guideline 62. Antenatal Care. Published: March 2008; updated Jan 2017. https://www.nice.org.uk/guidance/cg62/resources/antenatal-care-for-uncomplicated-pregnancies-pdf-975564597445.
- 7.Milne F, Redman C, Walker J, Baker P, Black R, Blincowe J, Cooper C, Fletcher G, Jokinen M, Moran PA, Nelson-Piercy C, Robson S, Shennan A, Tuffnell A, Waugh J PRECOG II Group. Assessing the onset of pre-eclampsia in the hospital day unit: summary of the pre-eclampsia guideline (PRECOG II). BMJ. 2009;339:b3129. doi: 10.1136/bmj.b3129. [DOI] [PubMed] [Google Scholar]
- 8.National Collaborating Centre for Women’s and Children's Health (UK) Hypertension in pregnancy: the management of hypertensive disorders during pregnancy. London, UK: RCOG Press; 2011. NICE Clinical Guideline 107. [PubMed] [Google Scholar]
- 9.World Health Organization. WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia. Geneva, Switzerland: World Health Organization; 2011. [PubMed] [Google Scholar]
- 10.Lindheimer MD, Taler SJ, Cunningham FG. Hypertension in pregnancy. J Am Soc Hypertens. 2008;2:484–494. doi: 10.1016/j.jash.2008.10.001. doi: 10.1016/j.jash.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, et al. ESC guidelines on the management of cardiovascular diseases during pregnancy: the task force on the management of cardiovascular diseases during pregnancy of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:3147–3197. doi: 10.1093/eurheartj/ehr218. [DOI] [PubMed] [Google Scholar]
- 12.Creasy RK, Robert R, Iams J, Lockwood C, Moore T, editors. Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice. 6th ed. Philadelphia, PA: Saunders Elsevier; 2009. [Google Scholar]
- 13.American College of Obstetricians and Gynecologists. Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. ACOG Guidelines. 2013. https://www.acog.org/Clinical-Guidance-and-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy. [DOI] [PubMed]
- 14.Department of Health, Welsh Office, Scottish Home and Health Department, and Department of Health and Social Services, Northern Ireland. Report on Confidential Enquiries into Maternal Deaths in the United Kingtom 1991–1993. London, UK: HMSO; 1996. [Google Scholar]
- 15.Baral-Grant S, Haque MS, Nouwen A, Greenfield SM, McManus RJ. Self-monitoring of blood pressure in hypertension: a UK primary care survey. Int J Hypertens. 2012;2012:582068. doi: 10.1155/2012/582068. doi: 10.1155/2012/582068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bray EP, Holder R, Mant J, McManus RJ. Does self-monitoring reduce blood pressure? Meta-analysis with meta-regression of randomized controlled trials. Ann Med. 2010;42:371–386. doi: 10.3109/07853890.2010.489567. doi: 10.3109/07853890.2010.489567. [DOI] [PubMed] [Google Scholar]
- 17.National Clinical Guideline Centre (UK) The Clinical Management of Primary Hypertension in Adults. London, UK: Royal College of Physicians (UK); 2011. NICE Clinical Guidelines, No. 127. [PubMed] [Google Scholar]
- 18.Naef RW, III, Perry KG, Jr, Magann EF, McLaughlin BN, Chauhan SP, Morrison JC. Home blood pressure monitoring for pregnant patients with hypertension. J Perinatol. 1998;18:226–229. [PubMed] [Google Scholar]
- 19.Taylor RS, Freeman L, North RA. Evaluation of ambulatory and self-initiated blood pressure monitors by pregnant and postpartum women. Hypertens Pregnancy. 2001;20:25–33. doi: 10.3109/10641950109152639. doi: 10.3109/10641950109152639. [DOI] [PubMed] [Google Scholar]
- 20.Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D American Heart Association; American Society of Hypertension; Preventive Cardiovascular Nurses Association. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:10–29. doi: 10.1161/HYPERTENSIONAHA.107.189010. doi: 10.1161/HYPERTENSIONAHA.107.189010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magee LA, von Dadelszen P, Chan S, et al. Women’s views of their experiences in the CHIPS (Control of Hypertension in Pregnancy Study) Pilot Trial. Hypertens Pregnancy. 2007;26:371–387. doi: 10.1080/10641950701547549. doi: 10.1080/10641950701547549. [DOI] [PubMed] [Google Scholar]
- 22.Dehaeck U, Thurston J, Gibson P, Stephanson K, Ross S. Blood pressure measurement for hypertension in pregnancy. J Obstet Gynaecol Can. 2010;32:328–334. doi: 10.1016/S1701-2163(16)34476-0. doi: 10.1016/S1701-2163(16)34476-0. [DOI] [PubMed] [Google Scholar]
- 23.National Clinical Guideline Centre (UK) Hypertension: Clinical Management of Primary Hypertension in Adults. London, UK: Royal College of Physicians (UK); 2011. NICE Clinical Guidelines, No. 127. [Google Scholar]
- 24.Staessen JA, O’Brien ET, Amery AK, Atkins N, Baumgart P, De Cort P, Degaute JP, Dolenc P, De Gaudemaris R, Enström I. Ambulatory blood pressure in normotensive and hypertensive subjects: results from an international database. J Hypertens Suppl. 1994;12:S1–S12. [PubMed] [Google Scholar]
- 25.Stergiou GS, Zourbaki AS, Skeva II, Mountokalakis TD. White coat effect detected using self-monitoring of blood pressure at home: comparison with ambulatory blood pressure. Am J Hypertens. 1998;11:820–827. doi: 10.1016/s0895-7061(98)00038-7. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz JE, Burg MM, Shimbo D, Broderick JE, Stone AA, Ishikawa J, Sloan R, Yurgel T, Grossman S, Pickering TG. Clinic blood pressure underestimates ambulatory blood pressure in an untreated employer-based US population: results from the Masked Hypertension Study. Circulation. 2016;134:1794–1807. doi: 10.1161/CIRCULATIONAHA.116.023404. doi: 10.1161/CIRCULATIONAHA.116.023404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P SOGC Hypertension Guideline Committee. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36:575–576. doi: 10.1016/S1701-2163(15)30533-8. doi: 10.1016/S1701-2163(15)30533-8. [DOI] [PubMed] [Google Scholar]
- 28.Whiting P RA, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown MA, McHugh L, Mangos G, Davis G. Automated self-initiated blood pressure or 24-hour ambulatory blood pressure monitoring in pregnancy? BJOG. 2004;111:38–41. doi: 10.1046/j.1471-0528.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 30.Chandiramani M, Boyce T, Waugh JJS. Is self blood pressure monitoring equivalent to clinic blood pressure monitoring in the hypertensive pregnant population?. Hypertens Pregnancy. 2006;25:167–167. [Google Scholar]
- 31.Lo C, Taylor RS, Gamble G, McCowan L, North RA. Use of automated home blood pressure monitoring in pregnancy: is it safe? Am J Obstet Gynecol. 2002;187:1321–1328. doi: 10.1067/mob.2002.126847. [DOI] [PubMed] [Google Scholar]
- 32.Rey E, Morin F, Boudreault J, Pilon F, Vincent D, Ouellet D. Blood pressure assessments in different subtypes of hypertensive pregnant women: office versus home patient- or nurse-measured blood pressure. Hypertens Pregnancy. 2009;28:168–177. doi: 10.1080/10641950802233072. doi: 10.1080/10641950802233072. [DOI] [PubMed] [Google Scholar]
- 33.Rey E, Pilon F, Boudreault J. Home blood pressure levels in pregnant women with chronic hypertension. Hypertens Pregnancy. 2007;26:403–414. doi: 10.1080/10641950701548000. doi: 10.1080/10641950701548000. [DOI] [PubMed] [Google Scholar]
- 34.Sheehan E, Thilaganathan B, Bhide A, Khalil A. Evaluation of home monitoring of hypertension in pregnancy. BJOG. 2016;123(supp)(1):29–30. [Google Scholar]
- 35.Tucker KL, Taylor KS, Crawford C, et al. Blood pressure self-monitoring in pregnancy: examining feasibility in a prospective cohort study. BMC Pregnancy Childbirth. 2017;17:442. doi: 10.1186/s12884-017-1605-0. doi: 10.1186/s12884-017-1605-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mooney P, Jaspar A, Cartwright W, Swindells HE, Dalton KJ. No difference between home and clinic blood pressure measurements in pregnancy: a computerized telemetric study. J Perinat Med. 1991;19:133–139. doi: 10.1515/jpme.1991.19.1-2.133. [DOI] [PubMed] [Google Scholar]
- 37.Ishikuro M, Obara T, Metoki H, Ohkubo T, Yamamoto M, Akutsu K, Sakurai K, Iwama N, Katagiri M, Yagihashi K, Yaegashi N, Mori S, Suzuki M, Kuriyama S, Imai Y. Blood pressure measured in the clinic and at home during pregnancy among nulliparous and multiparous women: the BOSHI study. Am J Hypertens. 2013;26:141–148. doi: 10.1093/ajh/hps002. doi: 10.1093/ajh/hps002. [DOI] [PubMed] [Google Scholar]
- 38.Marko KI, Ganju N, Brown J, Benham J, Gaba ND. Remote prenatal care monitoring with digital health tools can reduce visit frequency while improving satisfaction. Obstet Gynecol. 2016;127(suppl 1) doi: 10.1097/01.AOG.0000483620.40988.df. [Google Scholar]
- 39.Aberg A. Diagnostic methods for pregnancy hypertension. Significance of standardized conditions. Int J Technol Assess Health Care. 1992;8(suppl 1):72–74. doi: 10.1017/s0266462300012939. [DOI] [PubMed] [Google Scholar]
- 40.Cartwright W, Dalton KJ, Swindells H, Rushant S, Mooney P. Objective measurement of anxiety in hypertensive pregnant women managed in hospital and in the community. Br J Obstet Gynaecol. 1992;99:182–185. doi: 10.1111/j.1471-0528.1992.tb14495.x. [DOI] [PubMed] [Google Scholar]
- 41.Chung Y, de Greeff A, Shennan A. Validation and compliance of a home monitoring device in pregnancy: microlife WatchBP home. Hypertens Pregnancy. 2009;28:348–359. doi: 10.1080/10641950802601286. doi: 10.1080/10641950802601286. [DOI] [PubMed] [Google Scholar]
- 42.Denolle T, Daniel JC, Calvez C, Ottavioli JN, Esnault V, Herpin D. Home blood pressure during normal pregnancy. Am J Hypertens. 2005;18(9)(pt 1):1178–1180. doi: 10.1016/j.amjhyper.2005.03.736. doi: 10.1016/j.amjhyper.2005.03.736. [DOI] [PubMed] [Google Scholar]
- 43.Denolle T, Weber JL, Calvez C, Getin Y, Daniel JC, Lurton O, Cheve MT, Marechaud M, Bessec P, Carbonne B, Razafintsalama T. Diagnosis of white coat hypertension in pregnant women with teletransmitted home blood pressure. Hypertens Pregnancy. 2008;27:305–313. doi: 10.1080/10641950802000950. doi: 10.1080/10641950802000950. [DOI] [PubMed] [Google Scholar]
- 44.Hon EH, Fukushima T, Berumen M, Vargas N. Apparatus for home monitoring of pregnant hypertensive patients. J Matern Fetal Med. 1996;5:45–49. doi: 10.1002/(SICI)1520-6661(199601/02)5:1<45::AID-MFM11>3.0.CO;2-M. doi: 10.1002/(SICI)1520-6661(199601/02)5:1<45::AID-MFM11>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 45.Dalton KJ, Manning K, Robarts PJ, Dripps JH, Currie JR. Computerized home telemetry of maternal blood pressure in hypertensive pregnancy. Int J Biomed Comput. 1987;21:175–187. doi: 10.1016/0020-7101(87)90085-7. [DOI] [PubMed] [Google Scholar]
- 46.Rayburn WF, Zuspan FP, Piehl EJ. Self-monitoring of blood pressure during pregnancy. Am J Obstet Gynecol. 1984;148:159–162. doi: 10.1016/s0002-9378(84)80168-4. [DOI] [PubMed] [Google Scholar]
- 47.Ross-McGill H, Hewison J, Hirst J, Dowswell T, Holt A, Brunskill P, Thornton JG. Antenatal home blood pressure monitoring: a pilot randomised controlled trial. BJOG. 2000;107:217–221. doi: 10.1111/j.1471-0528.2000.tb11692.x. [DOI] [PubMed] [Google Scholar]
- 48.Waugh J, Bosio P, Habiba M, Boyce T, Shennan A, Halligan A. Home monitoring of blood pressure in pregnancy at high risk of pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2001;99:109–111. doi: 10.1016/s0301-2115(01)00353-0. [DOI] [PubMed] [Google Scholar]
- 49.Hodgkinson JA, Tucker KL, Crawford C, Greenfield SM, Heneghan C, Hinton L, Khan K, Locock L, Mackillop L, McCourt C, Selwood M, McManus RJ. Is self monitoring of blood pressure in pregnancy safe and effective? BMJ. 2014;349:g6616. doi: 10.1136/bmj.g6616. [DOI] [PubMed] [Google Scholar]
- 50.Ganzevoort W, Rep A, Bonsel GJ, de Vries JI, Wolf H. Plasma volume and blood pressure regulation in hypertensive pregnancy. J Hypertens. 2004;22:1235–1242. doi: 10.1097/01.hjh.0000125436.28861.09. [DOI] [PubMed] [Google Scholar]
- 51.Reinders A, Cuckson AC, Jones CR, Poet R, O’Sullivan G, Shennan AH. Validation of the Welch Allyn ‘Vital Signs’ blood pressure measurement device in pregnancy and pre-eclampsia. BJOG. 2003;110:134–138. [PubMed] [Google Scholar]
- 52.Natarajan P, Shennan AH, Penny J, Halligan AW, de Swiet M, Anthony J. Comparison of auscultatory and oscillometric automated blood pressure monitors in the setting of preeclampsia. Am J Obstet Gynecol. 1999;181(5)(pt 1):1203–1210. doi: 10.1016/s0002-9378(99)70109-2. [DOI] [PubMed] [Google Scholar]
- 53.de Greeff A, Beg Z, Gangji Z, Dorney E, Shennan AH. Accuracy of inflationary versus deflationary oscillometry in pregnancy and preeclampsia: OMRON-MIT versus OMRON-M7. Blood Press Monit. 2009;14:37–40. doi: 10.1097/MBP.0b013e32831e305d. doi: 10.1097/MBP.0b013e32831e305d. [DOI] [PubMed] [Google Scholar]
- 54.De Greeff A, Reggiori F, Anthony J, Shennan AH. A validated algorithm for blood pressure measurement in pregnancy and pre-eclampsia: Microlife 3BTO-A vs microlife 3AC1. Hypertens Pregnancy. 2006;25:163. [Google Scholar]
- 55.Golara M, Benedict A, Jones C, Randhawa M, Poston L, Shennan AH. Inflationary oscillometry provides accurate measurement of blood pressure in pre-eclampsia. BJOG. 2002;109:1143–1147. doi: 10.1111/j.1471-0528.2002.01487.x. [DOI] [PubMed] [Google Scholar]
- 56.Reinders A, Cuckson AC, Lee JT, Shennan AH. An accurate automated blood pressure device for use in pregnancy and pre-eclampsia: the Microlife 3BTO-A. BJOG. 2005;112:915–920. doi: 10.1111/j.1471-0528.2005.00617.x. doi: 10.1111/j.1471-0528.2005.00617.x. [DOI] [PubMed] [Google Scholar]
- 57.Homuth V, Jüpner A, Busjahn A, Rückert E, Reichert M, Faulhaber HD, Luft FC. [Clinical aspects and differential therapy of mild hypertension in pregnancy]. Zentralbl Gynakol. 1994;116:267–270. [PubMed] [Google Scholar]
- 58.Churchill D, Beevers DG. Differences between office and 24-hour ambulatory blood pressure measurement during pregnancy. Obstet Gynecol. 1996;88:455–461. doi: 10.1016/0029-7844(96)00192-5. doi: 10.1016/0029-7844(96)00192-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data were obtained from third parties for this project. Several of the participating studies required specific data sharing agreements from their host institution. Current agreements are for the purposes of this analysis alone, and as such new approval would be required. Requests for Data Sharing should be directed to information.guardian@phc.ox.ac.uk. Such requests will be considered by all data holders involved in this collaboration. There were no specific study materials.


