Abstract
BACKGROUND AND OBJECTIVES
Toxoplasmosis, caused by Toxoplasma gondii, is diagnosed mainly by serological methods that are hindered by insufficient sensitivity. When it fails, it becomes necessary to rely on either direct detection of the parasite or DNA detection by polymerase chain reaction (PCR). We aimed to establish molecular tools for toxoplasmosis research in the country by using PCR targeting the B1 gene and compare it with ELISA results.
DESIGN AND SETTING
Conducted at the College of Science, King Khalid University, Abha, Saudi Arabia between January 2009 and April 2010 on Saudi pregnant women attending three major hospitals in the Aseer region.
PATIENTS AND METHODS
Peripheral blood samples (n=137) were collected from patients. DNA was extracted and the B1 T gondii gene was amplified by PCR. The amplicons were visualized and sequenced, and the results were analyzed. For comparison, sera were tested for anti-T gondii IgG and IgM by enzyme-linked immunosorbent assay (ELISA).
RESULTS
Of the 137 samples tested, the B1 gene could be amplified in 56 cases (41%) by PCR. DNA sequencing confirmed these results. IgM-ELISA assay detected 9 (6.5%) of these cases. The results of immunoglobulin G detection were positive in 53 (38.6%) of the patients.
CONCLUSION
The present study showed the need for PCR as a confirmatory assay in addition to serological assays to detect recent infection. We recommend national implementation of these molecular diagnostic tools.
Toxoplasma gondii is an obligate intracellular protozoan parasite with a global distribution in humans and other warm-blooded animals. It is a coccidian parasite of cats as final hosts, and all non-feline warm-blooded animals (including humans) as intermediate hosts.1 About one-third of the world’s human population is infected with the parasite.2 Intermediate hosts, including humans, acquire T gondii by ingesting either tissues of infected animals, food and drink contaminated with sporulated oocysts from cat feces or soil, tissue cysts contained in undercooked meat or meat byproducts containing the cysts.3,4 Although Toxoplasma infection is often benign, congenital toxoplasmosis can lead to severe sequelae for the fetus and newborn.5,6 The infection may cause miscarriage, death in utero, or severe neurological lesions, whereas fetal infection occurring later in pregnancy may result in either congenital disease or subclinical infection.
Globally, the prevalence and incidence of infection vary with the population group and geographic location. 7 This may be related to several factors, including culture, nutritional habits, age, and hygiene habits.8,9 In Saudi Arabia, there is no national systemic serological screening program for pregnant women. The diagnosis of a recent infection is determined by serological results obtained from a single serum sample, in contrast to other countries (eg, France), where sera for testing are obtained at regular intervals throughout gestation. Several studies have shown variation in the seroprevalence in different regions of the country. These studies showed that the prevalence of IgG was as low as 25% and as high as 42.1%.10–15 Most, if not all, studies on toxoplasmosis in Saudi Arabia have been serology-based studies.
Serological diagnosis represents the most widely used approach to define the stage of infection, whether current, recent, or past.16 However, despite its high sensitivity, these tests can provide ambiguous results. In such cases, direct detection of the parasite is necessary for a definitive diagnosis, which can be achieved classically by intraperitoneal inoculation of laboratory animals and inoculation of cells in culture.17 These methods, however, are time consuming and expensive. Where serological assays are unreliable or when the clinical diagnosis is doubted, PCR-based techniques can be performed.18 Detection of T gondii DNA using PCR minimizes the problems faced when using serology-based or cultured-based assays. It saves time and labor, offering the advantages of high sensitivity and specificity. PCR has been used to demonstrate the presence of Toxoplasma in various clinical samples: brain,19 whole blood,20 amniotic fluid, CSF,19 aqueous humor, and lymph nodes.20 PCR is of utmost importance in diagnosing Toxoplasma infection in cases of immunosuppressive therapy or in patients with AIDS.21
Several approaches based on PCR have been developed and offer a significant improvement in diagnosis, especially for congenital toxoplasmosis. The current Toxoplasma PCR assays essentially targets two main loci. The first is the 35-repeat B1 gene.22 Several groups have designed different sets of primers to different locations on the gene.23,24 Another widely used target is the single-copy gene (P30), which codes for the major surface antigen P30.21 Here again, different sets of primers have been designed.19,25 As introduction of molecular diagnostic techniques is expected to improve the toxoplasmosis diagnosis, the present study aimed to determine the incidence of toxoplasmosis among pregnant women in the Aseer region by PCR.
PATIENTS AND METHODS
Aseer is a province in the southwest region of Saudi Arabia. This study was carried out at Abha General Hospital, the Maternal and Child Private Hospital and King Faisal Armed Forces Hospital located in Khamis Mushayt. This study was approved was by the Ethical Committee of King Khalid University. All women gave their written consent. For each patient (n=137), a 1 to 2 mL venous blood sample was collected in EDTA tubes that were transported to the molecular biology laboratory. The positive control was obtained from the genomic DNA extracted from the brains of mice experimentally infected with RH strain. The negative control was a PCR reaction without DNA template that was always included when performing PCR reactions. Patients were considered acutely infected on the basis of immunological status like the presence of IgM antibody titers or a four-fold rise in IgG antibody titers when 2-fold dilutions of both acute and convalescent serum were tested simultaneously by enzyme-linked immunosorbent assay (ELISA) or immune-fluorescent methods.
DNA isolation and PCR of the T gondii B1 gene
DNA was isolated using a commercial purification system (Qiagen, Valencia, California, United States), following the manufacturer’s instructions for DNA purification from blood. Final DNA pellets were resuspended in 30 μL of TBE buffer (10 mM Tris, 1 mM EDTA, pH 7.2). The PCR method used here was developed by Burg and colleagues in 1989, using the B1 gene as a target.22 Primers were manufactured by Gibco BRL Life Technologies (Basel, Switzerland). The following PCR mixtures. For the first and second round of PCR, the following were assembled: 0.5 μM primers, 200 μM dNTPs, 1.5 mM MgCl2, 1.5 units Amplitaq polymerase, and 10 μL DNA template were used, with a total volume of 50 μL. 10 μL DNA template from the from first round were used, with a total volume of 50 μL. The primer sequences, expected size of PCR products, and PCR conditions for amplification of the B1 gene are shown in Table 1.
Table 1.
Primers expected molecular weight of PCR products and PCR conditions for B1 gene.
| Primers | Sequence (5′-3′) | Size (bp) | PCR conditions |
|---|---|---|---|
|
| |||
| First round | |||
| OB1/F | 5′-GGAACTGCATCCGTTCATGAG-3′ | 200 bp | 950°C for 5 mins followed by 40 cycles (950°C for 30 secs, 560°C for 30 secs, 720°C for 30 secs. |
| OB1/R | 5′-TCTTTAAAGCGTTCGTGGTC-3′ | ||
| Second round | |||
| IB1/F | 5′-TGCATAGGTTGCAGTCACTG-3′ | 100 bp | 950°C for 5 mins followed by 35 cycles (950°C for 30 secs, 560°C for 30 secs, 720°C for 30 secs. |
| IB1/R | 5′-GGCGACCAATCTGCGAATACACC-3′ | ||
PCR amplification of genomic DNA from human, fungal, bacterial, and from pregnant women using T gondii B1-specific primers
Primer specificity was tested. Outer and nested B1 amplification reactions were carried out using genomic DNA from human samples and from different fungal and bacterial samples, including Candida albicans, Aspergillus fumigatus, Staphylococcus epidermidis, and Escherichia coli. The positive control consisted of genomic T gondii DNA. PCR was also carried out with blood samples (n=137) obtained from study participants. Gel electrophoresis was performed in 1X Trisborate-EDTA running buffer on a 2% agarose gel. After cooling, ethidium bromide was added to a final concentration of 0.5 μg/mL. DNA was mixed with 6X DNA loading buffer and loaded into the submerged gel. Electrophoresis was carried out in TBE at 80 to 120 volts. Fluorescence from DNA-bound ethidium bromide was visualized by short wavelength UV light and photographed.
ELISA assay
Anti-Toxoplasma IgM and IgG antibodies were detected in the patients’ sera using commercially available ELISA kits (Human GMBH, Wiesbaden, Germany), according to the manufacturer’s instructions. The optical densities (OD) were measured at dual wavelengths of 450 nm and 630 nm using an ELISA plate reader (Humareader, Germany). The quantity of anti-Toxoplasma IgM and IgG in patient sera were calculated from a standard curve, using the ELISA plate reader built-in software in the point-to-point mode.
RESULTS
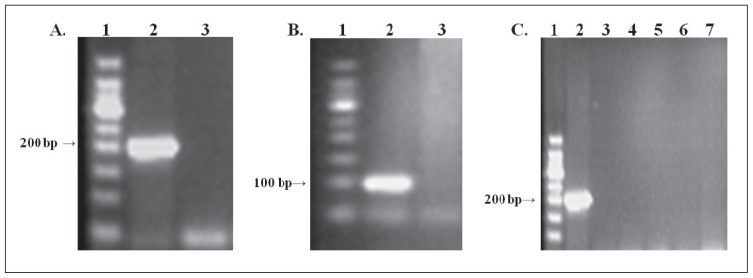
As shown in Figure 1A, the positive control yielded an expected band of about 200 bp, with no band in the negative control lane after the first round of PCR. The second round of PCR yielded the expected band (about 100 bp), as shown in Figure 1B. Specificity testing revealed that only the genomic DNA isolated from Toxoplasma was amplified while DNA from human, fungal and bacterial sources were not amplified. Only the positive control (T gondii DNA) was amplified (Figure 1C). The presence of human DNA in the sample did not inhibit amplification of the 200 bp B1 PCR product from T gondii DNA.
Figure 1.
Amplification of the T gondii B1 gene by PCR. A) First round of PCR using the outer pair of primers targeted to the B1 gene. B) Second round of PCR using the inner pair of primers targeted to the B1 gene. C) Gel showing a band for the positive control, but no bands from the DNA samples. Lane 1, molecular size marker; lane 2, positive control with the expected molecular weight; lane 3, human gDNA; lane 4, Candida albicans DNA; lane 5, Aspergillus fumigatus DNA; lane 6, Staphylococcus epidermidis DNA; lane 7, Escherichia coli DNA.
Detection of Toxoplasma DNA amongst pregnant women by PCR
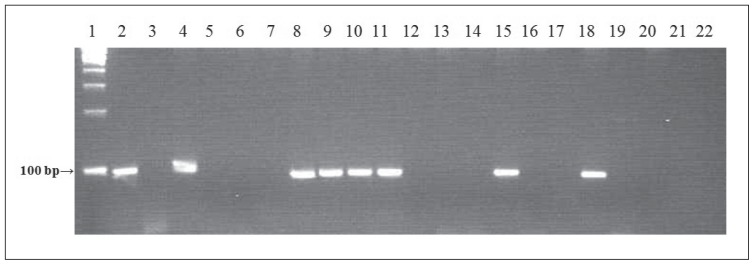
Positive samples gave bands of the expected molecular size (100 bp) after the second round of PCR (Figure 2). PCR results showed that Toxoplasma DNA was found in 41% of patient samples (56 of 137). When blasted against the Toxoplasma database (www.toxodb.org) and the National Center for Biotechnology Information (NCBI) database, all sequences were shown to be of Toxoplasma B1 gene origin. Confirmatory procedures for PCR-positive cases were performed in King Faisal Specialist Hospital and Research Center (KFSHRC), Riyadh, Saudi Arabia. DNA sequencing was also carried out as an additional confirmatory procedure at the DNA sequencing core facility at the KFSHRC Research Center.
Figure 2.
Gel showing a band for the positive control (lane 1), but not the negative control (lane 2). Lanes 3 to 22 show representative samples obtained from pregnant women in the study. Positive samples yielded bands of the expected molecular size (100 bp) after the second round of PCR. The PCR products (15 μL) were resolved on 2% agarose/TBE gels and visualized under UV illumination after ethidium bromide staining.
PCR, IgM and IgG results
IgM ELISA assay detected 9 (6.5%) of these cases to be immunoglobulin M positives with 128 (93.5%) to be negatives. The results of immunoglobulin G detection were positive in 53 (38.6%) of the patients and negative in 84 cases (61.4%). The comparisons between PCR and ELISA assays reveled that 8 and 32 blood samples were found to be positive for both PCR and IgM and IgG ELISA with consensus of (91% and 58%, respectively). On the other hand, PCR detected 27 (49%) positives that could not be detected by ELISA against IgG (Table 2).
DISCUSSION
Infection with T gondii during pregnancy in humans can result in abortion, stillbirth, or congenital toxoplasmosis. 26 In the present study, PCR was compared with the detection of immunoglobulins M and G to Toxoplasma by ELISA. Of 137 tested cases, T gondii DNA in blood was found in 56 (41%) cases. IgM ELISA assay detected 9 (6.5%) of these cases to be immunoglobulin M positives. The results of immunoglobulin G detection were positive in 53 (38.6%) of the patients. When resent toxoplasmosis is suspected, the serum should be investigated for IgG and IgM antibodies that are sometimes unreliable. Since severe immune system dysfunction results in a lack of production of antibodies, advanced molecular methods should be sought. PCR can be performed on amniotic fluid which can be helpful in determining fetal infection following acute acquired infection of the mother (http://www.cdc.gov/parasites/toxoplasmosis).
Few studies have been conducted to study the various aspects of toxoplasmosis on pregnant Saudi women in Saudi Arabia. The vast majority of studies for the various aspects of toxoplasmosis on pregnant Saudi women in Saudi Arabia were serology-based and did not utilize molecular tools for diagnosis. These assays provide high sensitivity, but the specificity varies depending on the test and kit used. Traditional diagnostic techniques may fail to detect anti-Toxoplasma IgG or IgM during the acute infection in cases of immunosuppressive therapy and in AIDS patients. This failure could also be attributed to low titers of the antibody in recently acquired infections, such that the antibody levels are below the level of detection by routine tests. In these cases, it becomes necessary to rely on direct detection of the parasite by PCR, which has been shown to be a potentially powerful diagnostic method with high sensitivity (capable of detecting a single tachyzoite) compared to culture, which is insensitive and time-consuming. 27
Use of PCR significantly changed the diagnostic means for prenatal diagnosis.28 In the current study, positive PCR results were found among 41% (56/137) of pregnant women in the Aseer region. Previous studies have documented that PCR can detect the parasite DNA in blood samples of women before or during pregnancy.29 The presence of Toxoplasma DNA in maternal blood probably indicates a recent infection or apparent parasitemia, which is likely to be clinically significant.30 PCR is the only method that can detect low levels of the T gondii organism and even destroyed parasites.19
Several PCR-based assays have been developed for the detection of T gondii DNA with B1 repetitive sequences, which has been proven to be more sensitive when compared to other targets (eg, P30 and rDNA).31 This is probably because B1 is a repeated DNA sequence with a higher copy number than the single-copy P30 gene.29 Additionally, the short DNA sequence of the B1 multicopy gene often provides a better PCR amplification target than the longer P30 gene. Recently, the AF146527 sequence (a sequence that has 200–300 copies in the T gondii genome) has been used. The same gene was then successfully used by other groups.32,33 This method is considered to be very sensitive and specific. Many authors recommended PCR over most serologic techniques.33 The primers used in this study did not produce any PCR amplicons when the template genomic DNA was derived from human genomic DNA, or bacterial or fungal DNA, despite the report by Kompalic-Cristo and colleagues that these primers can amplify parts of the human genome.34
The high prevalence of Toxoplasma in this region (Aseer region) of the country could be attributed to several factors. This region has a high population of cats (the final host of the parasite), so there is a high risk of infection to intermediate hosts (including humans).35 These cats live near human dwellings, businesses, and restaurants. Living in close contact with host animals (cats and dogs) and vehicles of oocyst transmission (flies and ants) are important risk factors for the infection. 36,37 Other intermediate hosts of T gondii infections are sheep and chickens, which represent another important risk factor in toxoplasmosis distribution, as these are the major meat sources of this region. In a recent study in sheep, PCR testing using the B1 gene was reported to yield positive results in 50% of tested samples.38 Recent studies have identified water contaminated with sporulated oocysts as a potential source of infection. Consumption of contaminated shellfish can also lead to infection.5 Circumstantial evidence suggests that oocyst-induced infections in humans are clinically more severe than tissue cyst-acquired infections.37 Other modes of transmission to humans include transplacental transmission, and through organ transplantation or blood transfusion. The results presented by Burg and colleagues showed that a single T gondii parasite could be detected by PCR.22 Another explanation for the high rate of positive test results by PCR is that the amplification of B1 could represent samples containing the parasite DNA but no viable pathogens, as the PCR test does not rely on live parasites to show a positive result.38
The results of this study show that the PCR test, when used as a confirmatory procedure along with routine serological tests in pregnant women, is of great usefulness. This study will contribute to the development of new strategies for therapeutic intervention against toxoplasmosis in Saudi Arabia. Our present research is focused and restricted to the diagnosis of toxoplasmosis in pregnancy. This was the initial commitment made at the proposal and approval of the protocol. The future direction for this research should extend to study neonatal outcome in positive PCR pregnant women.
REFERENCES
- 1.Ajioka JW, Soldati D. Toxoplasma-molecular and cellular biology. Norfolk, UK: Horizon Bioscience; 2007. pp. 37–58. [Google Scholar]
- 2.Kim K, Weiss LM. Toxoplasma: the next 100 years. Microbes Infect. 2008;10(9):978–984. doi: 10.1016/j.micinf.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 1998;11:267–299. doi: 10.1128/cmr.11.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hany EM, Azab MS, Abousamra NK, Rahbar MH, Elghannam M, Raafat D. Seroprevalence of and risk factors for Toxoplasma gondii antibodies among asymptomatic blood donors. Egypt Parasitology Research. 2009;(104):1471–147. doi: 10.1007/s00436-009-1350-z. [DOI] [PubMed] [Google Scholar]
- 5.Jones JL, Dubey JP. Waterborne toxoplasmosis--recent developments. Exp Parasitol. 2010 Jan;124(1):10–25. doi: 10.1016/j.exppara.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Varella IS, Canti IC, Santos BR, Coppini AZ, Argondizzo LC, Tonin, Wagner MB. Prevalence of acute toxoplasmosis infection among 41,112 pregnant women and the mother to child transmission rate in a public hospital in South Brazil. Mem Inst Oswaldo Cruz, Rio de Janeiro. 2009;104(2):383–388. doi: 10.1590/s0074-02762009000200037. [DOI] [PubMed] [Google Scholar]
- 7.Giannoulis C, Zournatzi B, Giomisi A, Diza E, Tzafettas I. Toxoplasmosis during pregnancy: a case report and review of literature. Hippokratia. 2008;12(3):139–143. [PMC free article] [PubMed] [Google Scholar]
- 8.Jones JL, Lopez A, Wilson M, Schulkin J, Gibbs R. Congenital toxoplasmosis: a review. Obstet Gynecol Surv. 2001;56:296–305. doi: 10.1097/00006254-200105000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 10.Abbas SA, Basalamah A, Serebour F, Alfonso M. The prevalence of Toxoplasma gondii antibodies in Saudi women and the outcome of congenital infection among newborns in Saudi Arabia. Saudi Med J. 1986;(7):346–54. [Google Scholar]
- 11.Al-Mulhim AA, Al Qurashi MA. Serprevalence of Toxoplasmosis in pregnant mothers and new born infants in Eastern Province. Saudi Arabia. Saudi Society of Family and Community Medicine. 2001;8(1):45–53. [PMC free article] [PubMed] [Google Scholar]
- 12.Tonkal A. PCR versus ELISA in diagnosis of human toxoplasmosis in Jeddah, Saudi Arabia. Journal of Egyptian Society of Parasitology. 2008;38(3):707–714. [PubMed] [Google Scholar]
- 13.Al Mohammad HI, Balaha MH, Amin TT, El Damarany EE, Dwedar A. The accuracy of IgG avidity for detection of acute toxoplasmosis among pregnant Saudi women. TAF Preventive medicine Bulletin. 2010;9(1):7–14. [Google Scholar]
- 14.Al-Harthi SA, Jamaoom MB, Ghazi HO. Seroprevalence of Toxoplasma gondii among pregnant women in Makkah, Saudi Arabia. Umm Al-Qura Univ J Sci Med Eng. 2006;18(2):217–227. [Google Scholar]
- 15.El-Hady HM. Toxoplasmosis among pregnant mothers in Abha, Saudi Arabia. J Egyptian Soc Parasit. 1991;21:811–816. [PubMed] [Google Scholar]
- 16.Nagaty IM, Ibrahim KM, Abdel-Tawab AH, Hassan AE. Diagnosis of Toxoplasma gondii by ELISA and PCR mothers and their infants. J Egypt Soc Parasitol. 2009 Aug;39(2):625–32. [PubMed] [Google Scholar]
- 17.Derouin F, Mazeron MC, Garin YJ. Comparative study of tissue culture and mouse inoculation methods for demonstration of Toxoplasma gondii. J Clin Microbiol. 1987;25:1597–600. doi: 10.1128/jcm.25.9.1597-1600.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bastien P. Molecular diagnosis of toxoplasmosis. Trans R Soc Trop Med Hyg. 2002;96(Sup1):205–215. doi: 10.1016/s0035-9203(02)90078-7. [DOI] [PubMed] [Google Scholar]
- 19.Savva D, Morris JC, Johnson JD, Holliman RE. Polymerase chain reaction for detection of Toxoplasma gondii. J Med Microbiol. 1990;32:25–31. doi: 10.1099/00222615-32-1-25. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JD, Butcher PD, Savva D, Holliman RE. Application of the polymerase chain reaction to the diagnosis of human toxoplasmosis. Journal of Infection. 1993;26(2):147–158. doi: 10.1016/0163-4453(93)92788-x. [DOI] [PubMed] [Google Scholar]
- 21.O’Driscoll JC, Holliman RE. Toxoplasmosis and bone marrow transplantation. Rev Med Microbiol. 1991;2:215–22. [Google Scholar]
- 22.Burg JL, Grover CM, Pouletty P, Boothroyd JC. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J Clin Microbiol. 1989;27:1787–1792. doi: 10.1128/jcm.27.8.1787-1792.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenum PA, Holberg-Petersen M, Melby KK, Stray-Pedersen B. Diagnosis of congenital Toxoplasma gondii infection by polymerase chain reaction (PCR) on amniotic fluid samples. AI’MIS. 1998;106:680–686. [PubMed] [Google Scholar]
- 24.Pujol-Rique M, Deruin F, Garcia-Quintanilla A, Valls ME, Miro JM, Jimenez de Anta MT. Design of a one tube hemi-nested PCR for the detection of Toxoplasma gondii and comparison of three DNA purification methods. J Med Microbiol. 1999;48(1999):857–862. doi: 10.1099/00222615-48-9-857. [DOI] [PubMed] [Google Scholar]
- 25.James GS, Sintchenko G, Dickeson DJ, Gilbert GL. Comparison of cell culture, mouse inoculation and PCR for detection of Toxoplasma gondii: effects of storage conditions on sensitivity. J Clin Microbiol. 1996;34:1572–1575. doi: 10.1128/jcm.34.6.1572-1575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: Global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. 2009;39:1385. doi: 10.1016/j.ijpara.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Eida O. Evaluation of polymerase chain reaction on amniotic fluid for diagnosis of congenital toxoplasmosis. J Egypt Soc Parasitol. 2009;39(2):541–50. [PubMed] [Google Scholar]
- 28.Su C, Shwab EK, Zhou P, Zhu XQ, Dubey JP. Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology. 2010;137(1):1–11. doi: 10.1017/S0031182009991065. [DOI] [PubMed] [Google Scholar]
- 29.Chabbert E, Lachaud L, Crobu L, Bastien P. Comparison of two widely used PCR primer systems for detection of Toxoplasma in amniotic fluid, blood and tissues. J Clin Microbiol. 2004;42:1719–1722. doi: 10.1128/JCM.42.4.1719-1722.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slawska H, Czuba B, Gola J, Mazurek U, Wloch A, Wilczok T, Kaminski K. Diagnostic difficulties of Toxoplasma gondii infection in pregnant women. Is it possible to explain doubts by polymerase chain reaction? Ginekol Pol. 2005;76:536–542. [PubMed] [Google Scholar]
- 31.Guy EC, Joynson DHM. Potential of the polymerase chain reaction in the diagnosis of active Toxoplasma infection by detection of parasite in blood. J Infect Dis. 1995;172:319–322. doi: 10.1093/infdis/172.1.319. [DOI] [PubMed] [Google Scholar]
- 32.Jones CD, Okhravi N, Adamson P, Tasker S, Lightman S. Comparison of PCR detection methods for B1, P30, and 18S rDNA genes of T gondii in aqueous humor. Investigative Ophthalmology & Visual Science. 2000;41(3):634. [PubMed] [Google Scholar]
- 33.Wahab T, Edvinsson B, Palm D, Lindh J. Comparison of the AF146527 and B1 repeated elements, two real-time PCR targets used for detection of Toxoplasma gondii. Journal of Clinical Microbiology. 2010;48(2):591. doi: 10.1128/JCM.01113-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kompalic-Cristo A, Nogueira SA, Guedes AL, Frota C, González CF, Brandão A, Amendoeira MR, Britto C, Fernandes O. Lack of technical specicity in the Molecular diagnosis of toxoplasmosis. Trans R Soc Trop Med Hyg. 2004;98:92–95. doi: 10.1016/s0035-9203(03)00012-9. [DOI] [PubMed] [Google Scholar]
- 35.Dubey JP. History of the discovery of the life cycle of Toxoplasma gondii. International Journal for Parasitology. 2009;39(8):877–882. doi: 10.1016/j.ijpara.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Mason S, Quinnell RJ, Smith JE. Detection of Toxoplasma gondii in lambs via PCR screening and serological follow-up. Vet Parasitol. 2010;11:258–63. doi: 10.1016/j.vetpar.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 37.Vaillant V, de Valk H, Baron E, Ancelle T, Colin P, Delmas MC, Dufour B, Pouillot R, Le Strat Y, Weinbreck P, Jougla E, Desenclos JC. Foodborne infections in France. Foodborne Pathog Dis. 2005;2:221–232.39. doi: 10.1089/fpd.2005.2.221. [DOI] [PubMed] [Google Scholar]
- 38.Wastling JM, Nicoll S, Buxton D. Comparison of two gene amplification methods for the detection of Toxoplasma gondii in experimentally infected sheep. J Med Microbiol. 1993;38:360–365. doi: 10.1099/00222615-38-5-360. [DOI] [PubMed] [Google Scholar]