Abstract
BACKGROUND AND OBJECTIVES
Methicillin-resistant Staphylococcus aureus (MRSA) emerged in 1960 and was a problem confined largely to the healthcare setting, or hospital-associated MRSA (HA-MRSA). In the 1990s, community-associated MRSA (CA-MRSA) infections appeared. In Saudi Arabia, the prevalence of MRSA has increased in the past ten years and severe community-acquired infection has been reported. Our objective was to investigate the prevalence of MRSA and their antibiotic susceptibilities in the western region of Saudi Arabia.
DESIGN AND SETTING
A retrospective review of the medical records of 186 S aureus infected patients diagnosed from November 2009 through October 2010.
METHODS
S aureus was Identified based on Gram stain, catalase and coagulase tests. Susceptibility testing was performed using antibiotic discs and the VITEK 2 system.
RESULTS
MRSA was isolated in 39.5% of the specimens. The isolates were commonly associated with wound, skin, and soft tissue infections (87.3%). The prevalence of MRSA was highest among patients who were 56 years old or older (52.2%). CA-MRSA infections represented 31.5% of community S aureus infections, while HA-MRSA accounted 52.6% of hospital S aureus (P=.0029). All MRSA isolates in our study were susceptible to vancomycin, linozolid and teicoplanin. However, multi-resistance was observed in 29.1% of the isolates and was significantly higher among HA-MRSA (P=.03).
CONCLUSIONS
The prevalence of MRSA was 39.5%, and infection was commonly associated with wound, skin, and soft tissue infections. MRSA was more prevalent in hospitals and among older patients. All MRSA susceptible to vancomycin, linozolid and teicoplanin.
Staphylococcus aureus infections are caused by methicillin-susceptible (MSSA) or methicillin-resistant (MRSA) strains.1,2 MRSA emerged in 1960 and was a problem confined largely to the healthcare setting, known as hospital-associated MRSA (HA-MRSA). In the late 1990s community-associated (acquired) MRSA (CA-MRSA) infections appeared in the United States and then emerged worldwide. CA-MRSA infections involve predominantly skin and soft tissue; however, severe infections have been described. 3–7 In Saudi Arabia, the prevalence MRSA has been increasing and severe community-acquired infections has been reported in the past 10 years.8–12 In the current study we retrospectively analysed the staphylococcus isolates in the western region of Saudi Arabia.
METHODS
The study was conducted at a university hospital in the western part of Saudi Arabia. Retrospectively, the medical records of patients who were diagnosed with S aureus infections were reviewed and data were extracted. The analysis included all isolates of S aureus collected from both outpatients and hospitalized patients. Patient-specific characteristics were collected, including age, sex, gender, a documented history of MRSA infection within the previous year, and hospitalization within the previous year.
The records of 186 S aureus infected patients diagnosed in the period between 1 November 2009 through 31 October 2010 were included in the study.
The microbiology laboratory had performed identification of S aureus based on colony morphology, Gram stain, catalase and coagulase tests. Susceptibility to oxacillin and cefoxitin was performed using oxacillin 1 μg disk and cefoxitin 30 μg disk according to the performance standards of the Clinical and Laboratory Standards Institute (CLSI).13 Susceptibility to trimethoprim/sulfmethoxazole, rifampicin, levofloxacin, erythromycin, clindamycin, tetracycline, vancomycin, linozolid, and teicoplanin of the isolates were conducted according to the CLSI standards by the clinical microbiology laboratory utilizing VITEK 2 (bio-Mérieux, Durham, North Carolina, United States).13 The chi-square test was used to evaluate the statistical significance of the observed differences in prevalence and susceptibility patterns of CA-MRSA compared HA-MRSA.
RESULTS
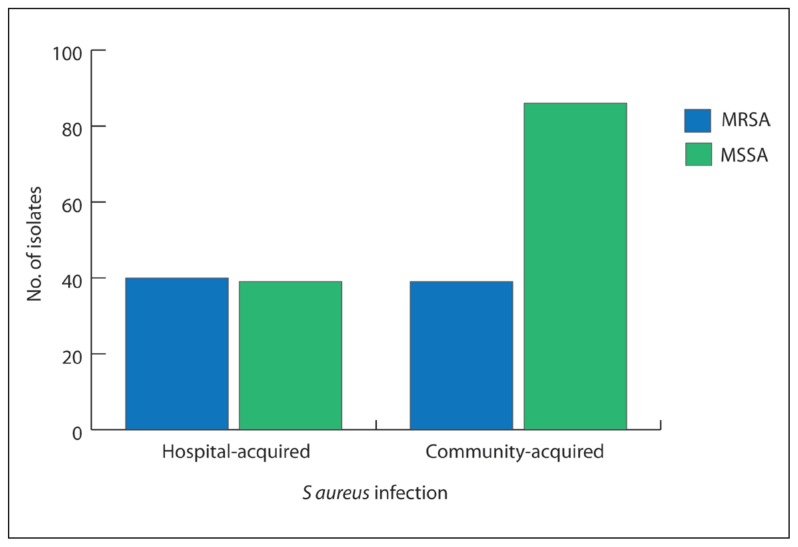
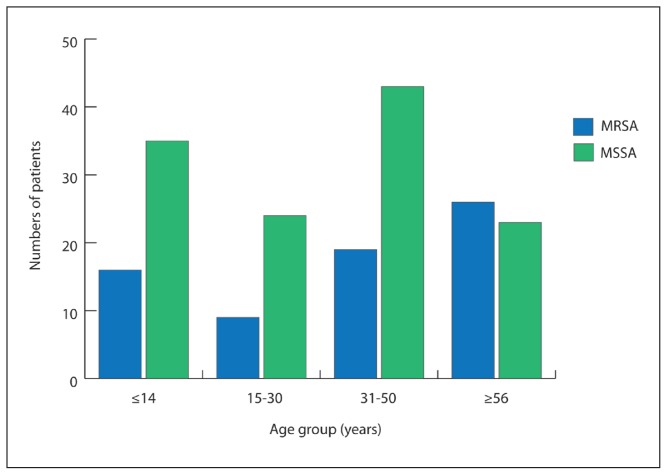
Two hundred S aureus isolates from 186 patients in the study period were analysed. The demographic and clinical data of the patients were evaluated to assess the significance of different risk factors underlying the acquisition of MRSA both in the community and hospital. The patients included 95 males and 91 females. MSSA was isolated in 121 specimens from 121 patients. Of MSSA infections, 85 were community-acquired and 36 were acquired in hospitals. Seventy-nine MRSA isolates isolated from 65 patients were registered during the study period. Of the 65 MRSA isolates, 39 were community-acquired and 40 were acquired in hospital in patients with no history of hospitalization in the previous year. The total S aureus infections acquired in the community were 124 of which 39 were MRSA (31.5%), while the total hospital isolates were 76; of these 40 were MRSA (52.6%). The proportion of MRSA infection out of the total S aureus infection was found to be significantly higher in the hospital (P=.0029) (Figure 1). Patients from different age groups were diagnosed during the study period. The prevalence of MRSA was significantly higher among patients who were 56 years old or older (52.2%) (Figure 2).
Figure 1.
Prevalence of S aureus infection. The figure shows the number of methicillin susceptible and methicillin resistant isolates of both community and hospital isolates. MSSA: methicillin susceptible S aureus; MRSA: methicillin resistant S aureus; HA: hospital acquired; CA: community-acquired.
Figure 2.
Significantly higher prevalence of MRSA among patients who are 56-year-old or more (P .02–.03)
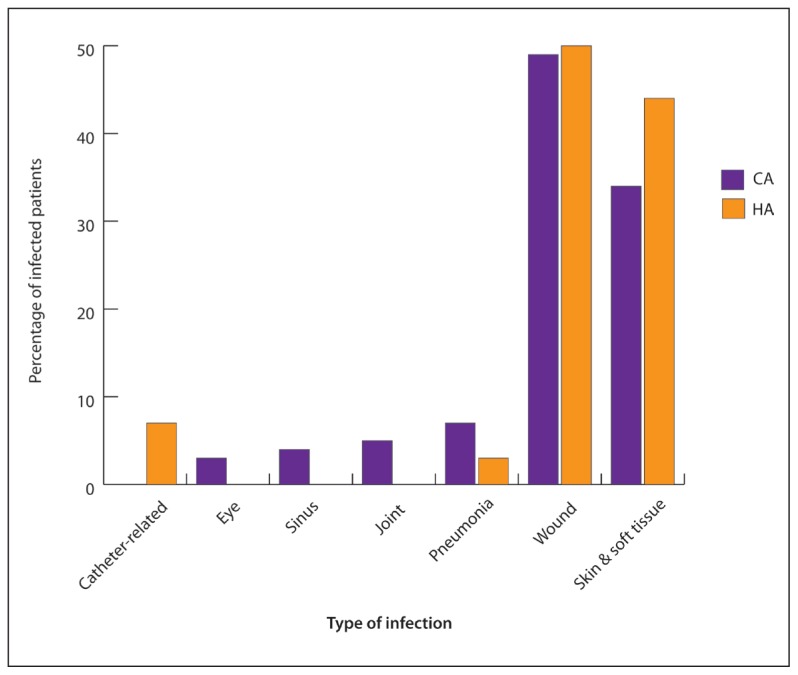
All MRSA isolates in our study were susceptible in vitro to vancomycin, linozolid and teicoplanin. Resistance to other tested antibiotics was variable with significantly higher resistance to levofloxacin, erythromycin, and clindamycin among HAMRSA isolates compared to CA-MRSA (Table 1). Resistance to four or more antibiotics was observed in 29.1% of the isolates and was significantly higher among HA-MRSA (P=.03). The most common infections caused by MRSA, both in hospitals and the community were wound, skin and soft tissue infections (87.3%), followed by pneumonia (5.1%) (Figure 3). Co-morbidity was documented in 37/121 MSSA, and 20/79 MRSA (P=.42). Diabetes mellitus was the most common documented co-morbidity (45.9% of MSSA, 50.0% of MRSA).
Table 1.
In vitro antibiotic susceptibilities of MRSA isolates.
| Variable | Total tested | Resistant isolates | Percent of resistant isolates (%) | Proportion | P |
|---|---|---|---|---|---|
|
| |||||
| Trimethoprim/sulfmethoxazole | |||||
| CA | 32 | 7 | 22 | 0.218 | 0.0711 |
| HA | 39 | 16 | 41 | 0.41 | |
| Refampicin | |||||
| CA | 27 | 5 | 18.5 | 0.185 | 0.0924 |
| HA | 35 | 13 | 37.1 | 0.371 | |
| Levofloxacin | |||||
| CA | 27 | 9 | 33.3 | 0.333 | 0.0118 |
| HA | 30 | 20 | 66.7 | 0.666 | |
| Erythromycin | |||||
| CA | 28 | 15 | 53.6 | 0.535 | 0.0259 |
| HA | 36 | 28 | 77.8 | 0.777 | |
| Clindamycin | |||||
| CA | 36 | 17 | 47.2 | 0.472 | 0.0178 |
| HA | 38 | 28 | 73.7 | 0.736 | |
| Tetracycline | |||||
| CA | 30 | 15 | 50.0 | 0.500 | 0.092 |
| HA | 34 | 24 | 70.6 | 0.705 | |
CA: community acquired; HA: hospital acquired
Figure 3.
The different infections caused by CA-MRSA and HA-MRSA. MSSA: methicillin susceptible S aureus; MRSA: methicillin resistant S aureus; HA: hospital acquired; CA: community-acquired.
DISCUSSION
In our study, 79 MRSA isolates were identified. The detection of MRSA was performed using the combination of oxacillin and cefoxitin disks. The use these disks in detection of MRSA has been shown in a number of studies to be highly sensitive and specific. Some studies have even proposed the use of these disks as a good substitute for PCR for the mecA gene.14–17
Our results showed that the highest prevalence of MRSA is among old patients aged 56 years old or older. Previous studies demonstrated variable distribution of MRSA in the different age groups. A Saudi study showed MRSA to be most prevalent in the extremes of age.18 An American study reported a pattern of decreasing age during a period of ten years observation while another American study, which was conducted about the same time, showed that the greatest MRSA rate increase was for individuals 17 years and younger. 19,20 These observations may indicate a changing pattern which may be explained by the different causative strains that have different virulence determinants.
The antibiotic susceptibility pattern observed in our study is in accordance with previous studies. All MRSA isolates in the current study were susceptible to vancomycin, linezolid and teicoplanin. Similar results were demonstrated in several other studies.21,22 Resistance to tetracycline, rifampicin, trimethoprim/sulfamethoxazole, levofloxacin, erythromycin, and clindamycin was variable. Resistance to four or more antibiotics was significantly higher in HA-MRSA and so was resistance to levofloxacin, erythromycin and clindamycin. Similar patterns of resistance were shown by Al-Twafiq and Jung et al.8,23 This pattern of resistance may indicate the presence of staphylococcal cassette chromosome mec (SCCmec) –II in our HA-MRSA isolates as indicated by Kilic et al.24 CA-MRSA were fairly susceptible to trimethoprim/sulfamethoxazole and rifampicin which may be good treatment options for these infections. However, rifampicin may be reserved for use in combination therapy to guard against the emergence of resistance to this antibiotic as it is still needed in treatment of infections like TB. The most common infections caused by MRSA were skin, soft tissue, and wound infections; this preponderance is well documented in a number of studies.25,26 Few other MRSA infections such as pneumonia, joint infections, sinusitis, and catheter-associated infections were reported in this study. The outcome of MRSA infections in the current study was not evaluated because there were no complete data. About one-third of MRSA were multidrug-resistant and HA-MRSA were significantly more resistant, especially to levofloxacin, erythromycin, and clindamycin, which may point to excessive use of these antibiotics in hospitals.
REFERENCES
- 1.Elston JW, Barlow GD. Community-associated MRSA in the United Kingdom. J Infect. 2009;59:149–155. doi: 10.1016/j.jinf.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Moore CL, Hingwe A, Donabedian SM, Perri MB, Davis SL, Haque NZ, et al. Comparative evaluation of epidemiology and outcomes of methicillin-resistant Staphylococcus aureus (MRSA) USA300 infections causing community- and healthcare-associated infections. Int J Antimicrob Agents. 2009;34:148–155. doi: 10.1016/j.ijantimicag.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Bassetti M, Nicco E, Mikulska M. Why is community-associated MRSA spreading across the world and how will it change clinical practice? Int J Antimicrob Agents. 2009;34:S15–S19. doi: 10.1016/S0924-8579(09)70544-8. [DOI] [PubMed] [Google Scholar]
- 4.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 5.Fridkin SK, Hageman JC, Morrison M, Sanza LT, Como-Sabetti K, Jernigan JA, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–1444. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 6.Baldan R, Tassan DC, Semeraro G, Costa C, Cichero P, Scarpellini P, et al. Severe community-onset infections in healthy individuals caused by community-acquired MRSA in an Italian teaching hospital, 2006–2008. J Hosp Infect. 2009;72:271–273. doi: 10.1016/j.jhin.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Rozenbaum R, Sampaio MG, Batista GS, Garibaldi AM, Terra GM, Souza MJ, et al. The first report in Brazil of severe infection caused by community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) Braz J Med Biol Res. 2009;42:756–760. doi: 10.1590/s0100-879x2009005000007. [DOI] [PubMed] [Google Scholar]
- 8.Al-Tawfiq JA. Incidence and epidemiology of methicillin-resistant Staphylococcus aureus infection in a Saudi Arabian Hospital, 1999–2003. Infect Control Hosp Epidemiol. 2006;27:1137–1139. doi: 10.1086/507971. [DOI] [PubMed] [Google Scholar]
- 9.Al-Mendalawi MD. Severe community-acquired infection caused by methicillin-resistant Staphylococcus aureus in Saudi Arabian children. Saudi Med J. 31:461. author reply 461–462. [PubMed] [Google Scholar]
- 10.Bukhari EE, Al-Otaibi FE. Severe community-acquired infection caused by methicillin-resistant Staphylococcus aureus in Saudi Arabian children. Saudi Med J. 2009;30:1595–1600. [PubMed] [Google Scholar]
- 11.Ahmad S, Alenzi FQ, Al-Juaid NF, Ahmed S. Prevalence and antibiotic susceptibility pattern of methicillin resistant Staphylococcus aureus at Armed Forces Hospital in Saudi Arabia. Bangladesh Med Res Counc Bull. 2009;35:28–30. doi: 10.3329/bmrcb.v35i1.1983. [DOI] [PubMed] [Google Scholar]
- 12.Bukharie HA. Increasing threat of community-acquired methicillin-resistant Staphylococcus aureus. Am J Med Sci. 340:378–381. doi: 10.1097/MAJ.0b013e3181e95fdc. [DOI] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute. Fifteenth informational supplement. Wayen PA: CLSI; 2005. Performance standards for antimicrobial susceptibility testing. Document M100–S15. [Google Scholar]
- 14.Adaleti R, Nakipoglu Y, Karahan ZC, Tasdemir C, Kaya F. Comparison of polymerase chain reaction and conventional methods in detecting methicillin-resistant Staphylococcus aureus. J Infect Dev Ctries. 2008;2:46–50. doi: 10.3855/jidc.321. [DOI] [PubMed] [Google Scholar]
- 15.Baddour MM, AbuElKheir MM, Fatani AJ. Comparison of mecA polymerase chain reaction with phenotypic methods for the detection of methicillin-resistant Staphylococcus aureus. Curr Microbiol. 2007;55:473–479. doi: 10.1007/s00284-007-9015-6. [DOI] [PubMed] [Google Scholar]
- 16.Anand KB, Agrawal P, Kumar S, Kapila K. Comparison of cefoxitin disc diffusion test, oxacillin screen agar, and PCR for mecA gene for detection of MRSA. Indian JMedMicrobiol. 2009;27:27–29. [PubMed] [Google Scholar]
- 17.Kerttula AM, Mero S, Pasanen T, Vuopio-Varkila J, Virolainen A. Evaluation of phenotypic and molecular methods for screening and detection of methicillin-resistant Staphylococcus aureus. Scand J Infect Dis. 2008;40:663–666. doi: 10.1080/00365540801955216. [DOI] [PubMed] [Google Scholar]
- 18.McMullen KM, Warren DK, Woeltje KF. The changing susceptibilities of methicillin-resistant Staphylococcus aureus at a midwestern hospital: the emergence of “community-associated” MRSA. Am J Infect Control. 2009;37:454–457. doi: 10.1016/j.ajic.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Baddour MM, Abuelkheir MM, Fatani AJ. Trends in antibiotic susceptibility patterns and epidemiology of MRSA isolates from several hospitals in Riyadh, Saudi Arabia. Ann Clin Microbiol Antimicrob. 2006;5:30. doi: 10.1186/1476-0711-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bordon J, Master RN, Clark RB, Duvvuri P, Karlowsky JA, Ayesu K, et al. Methicillin-resistant Staphylococcus aureus resistance to non-beta-lactam antimicrobials in the United States from 1996 to 2008. Diagn Microbiol Infect Dis. 67:395–398. doi: 10.1016/j.diagmicrobio.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Draghi DC, Sheehan DF, Hogan P, Sahm DF. Current antimicrobial resistance profiles among methicillin-resistant Staphylococcus aureus encountered in the outpatient setting. Diagn Microbiol Infect Dis. 2006;55:129–133. doi: 10.1016/j.diagmicrobio.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Neela V, Sasikumar M, Ghaznavi GR, Zamberi S, Mariana S. In vitro activities of 28 antimicrobial agents against methicillin-resistant Staphylococcus aureus (MRSA) from a clinical setting in Malaysia. Southeast Asian J Trop Med Public Health. 2008;39:885–892. [PubMed] [Google Scholar]
- 23.Jung SI, Shin DH, Park KH, Shin JH. Antimicrobial susceptibility and clonal relatedness between community- and hospital-acquired methicillin-resistant Staphylococcus aureus from blood cultures. J Microbiol. 2006;44:336–343. [PubMed] [Google Scholar]
- 24.Kilic A, Li H, Stratton CW, Tang YW. Antimicrobial susceptibility patterns and staphylococcal cassette chromosome mec types of, as well as Panton-Valentine leukocidin occurrence among, methicillin-resistant Staphylococcus aureus isolates from children and adults in middle Tennessee. J Clin Microbiol. 2006;44:4436–4440. doi: 10.1128/JCM.01546-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magilner D, Byerly MM, Cline DM. The prevalence of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) in skin abscesses presenting to the pediatric emergency department. NCMedJ. 2008;69:351–354. [PubMed] [Google Scholar]
- 26.Pardo L, Machado V, Mollerach M, Mota MI, Tuchscherr LP, Gadea P, et al. Characteristics of Community-Associated Methicillin-Resistant Staphylococcus aureus (CA-MRSA) Strains Isolated from Skin and Soft-Tissue Infections in Uruguay. Int J Microbiol. 2009:472126. doi: 10.1155/2009/472126. [DOI] [PMC free article] [PubMed] [Google Scholar]