Abstract
BACKGROUND AND OBJECTIVES
Antibiotics are one of the most overused drugs in the neonatal unit. Our objective was to assess associations between the duration of the initial antibiotic course and subsequent necrotizing enterocolitis (NEC) and/or death in very low birth weight (VLBW) neonates with sterile initial postnatal culture results.
DESIGN AND SETTING
A retrospective cohort analysis of VLBW neonates admitted to a tertiary center during the period from 1 January 2008 to 31 December 2009.
PATIENTS AND METHODS
The study included VLBW neonates who had been inborn and admitted to the neonatal intensive care unit within the first 24 hours after birth. We used descriptive statistics to characterize the study population, and multivariate analyses to evaluate associations between therapy duration, prolonged empirical therapy, and subsequent NEC and/or death.
RESULTS
Of 328 VLBW neonates admitted to our center, 207 (63%) survived >5 days and received initial empirical antibiotic treatment for ≥5 days. The median duration of initial empirical antibiotic therapy was 7 days (range 5–10 days). Those neonates were more likely to be of younger gestational age, lower birth weight, and to have lower Apgar scores (P<.001, .001 and .017, respectively). Each empirical treatment day was associated with increased odds of death (OR 1.45, CI 1.24–1.69), NEC (OR 1.32, CI 1.05–1.6 5), and the composite measure of NEC or death (OR 2.13, CI 1.55–2.93).
CONCLUSION
The use of prolonged initial empirical antibiotic therapy in VLBW neonates with initial sterile culture results may be associated with an increased risk of NEC or death and should be used with caution.
The use of empirical antibiotic therapy for premature infants in the first postnatal days is based on the immaturity of their immune systems, and the high mortality rate among infants who have invasive bacterial infections.1,2 In neonatal intensive care only about 2% to 4% of all neonates receiving empirical antibiotic treatment develop proven serious infections.1,3 In addition, widespread use of broad-spectrum agents carries the potential hazard of increasing antimicrobial resistance and probably even mortality.4–6 Previous reports have described the association between the duration of the initial antibiotic course in very low birth weight (VLBW) neonates, in the absence of positive culture results and the risk of subsequent death or necrotizing enterocolitis (NEC).7,8 Our hypothesis is that multiple factors are associated with the variation in the duration of the initial empirical antibiotic therapy provided for VLBW neonates and that prolonged duration of the initial empirical antibiotic course is associated with an increased risk of death or NEC among VLBW neonates.
PATIENTS AND METHODS
Data were collected retrospectively for VLBW neonates (weighing 700–1500 g at birth) who were born between 1 January 2008 and 31 December 2009 and were admitted to the neonatal intensive care unit of Cairo University Hospital. Neonates included in the current study were inborn and admitted within the first 24 hours after birth. They survived beyond day 5, and had no major birth defects or congenital anomalies, received initial empirical treatment with ≥1 antibacterial agent in the first 3 postnatal days, and did not have early onset sepsis (EOS).
Maternal demographic data included gravidity, multiple gestation, maternal illness, rupture of membranes >24 hours and mode of delivery. Neonates diagnosed with EOS or those who died before 5 days postnatal age were excluded. Late-onset sepsis (LOS) was diagnosed in the presence of culture-positive infections or a positive C-reactive protein result (positive test above 6 mg/L) and bandemia on complete blood count analysis after 3 postnatal days, in the presence of clinical signs of sepsis, if blood culture results were not available. Blood cultures were processed by the clinical microbiology laboratory with the Bactec (Becton Dickinson, Sparks, Maryland, United States) system. The usual practice is to inoculate blood culture results with ≥0.5mL of blood. Qualitative C-reactive protein test (CRP), using a cut point of >6mg/L as a positive result, was done using latex agglutination technique. The total leucocyte counts and platelet counts were measured on an automated counter. The differential leucocytic counts were performed manually on Leishman-stained blood smears by examining 100 cells. Neutrophils were classified as immature (band) forms when the width of the nucleus at any constriction was not less than one third of its widest portion. Bandemia was defined as an immature- to-total neutrophil ratio>0.2.9 Empirical antibiotic therapy was initiated in preterm newborns upon admission to the neonatal intensive care unit. The initial empiric antibiotic combination used was ampicillin and gentamicin.
The majority of the VLBW neonates included in the current study received preterm formula (Bebelac LBW or S26 Gold LBW) and a few neonates received occasional expressed breast milk feeds in addition to the preterm formula. We defined initial empirical antibiotic treatment as the first antibiotic treatment initiated within the first 3 postnatal days. We defined prolonged initial empirical antibiotic treatment as ≥5 days of initial empirical antibiotic treatment with sterile culture results in reference to the study by Cotten et al.8 The duration of initial empirical antibiotic treatment was defined as the number of days until administration of all initially administered antibiotics was discontinued. For infants with positive blood culture results after the first 3 postnatal days, the duration of initial empirical antibiotic treatment was calculated by using the date of the first positive culture result as the end date; therefore, the duration of initial empirical antibiotic therapy stopped with the first positive culture result as antibiotic administration after that time was not empirical. The outcome variables were NEC, death, and the composite outcome of NEC or death. Necrotizing enterocolitis was classified according to the clinical and radiological modified Bell staging criteria.10 The study design was approved by the Scientific Research Committee of the Paediatrics Department, Faculty of Medicine, Cairo University, Egypt. Data confidentiality was preserved according to the Revised Helsinki Declaration of Bioethics.11
Data were coded and entered using the statistical package SPSS version 15 (IBM Corp, Armonk, New York, United States). Data were summarized using mean, standard deviation (SD) and (range) for quantitative variables and number and percent for qualitative variables. Comparison between groups was done using the chi-square test for qualitative variables, independent sample t test for normally distributed quantitative variables, while the Mann Whitney test was used for qualitative variables which are not normally distributed. Logistic regression analysis was done for predictors for neonatal outcome (NEC, death, NEC or death) and LOS. The logistic regression model included maternal, perinatal, and neonatal variables shown previously to be associated with NEC and death.12–14 These variables included: gestational age, small-for-gestational age status, gender, early enteral feeding (<5 d) rupture of membranes for >24 hours, maternal illness (hypertension, hemorrhage, lupus), multiple births and Apgar score at 5 minutes of <5. All variables were entered as categorical variables except for gestational age and duration of initial empirical antibiotic treatment, which were entered as continuous variables. P<.05 was considered significant.
RESULTS
Of 328 VLBW neonates admitted to our center throughout a 2-year period from January 2008 to December 2009, 207 [102 (49.3%) males and 105 (50.7%) females] survived >5 days, received initial empirical antibiotic treatment, and had sterile initial culture results through the first 3 postnatal days and were therefore included in the current study. One hundred and forty-one neonates (68%) were delivered by cesarean delivery. The mean (SD) gestational age of the study cohort was 31.2 (2.3) weeks and their birth weights ranged from 700 to 1499 grams [mean (SD); 1260.7 (194.3) g]. Fifty-eight neonates (28%) were small for gestational age, while 24 neonates (11.6%) had a 5 minute Apgar score <5. Maternal hypertension was present in 40% of the cases, while prolonged rupture of membranes (>24 hours) complicated 27.1% of the pregnancies. One-hundred and seventeen mothers (56.5%) were mutigravida, and 61 mothers (29.5%) had multiple gestation pregnancies.
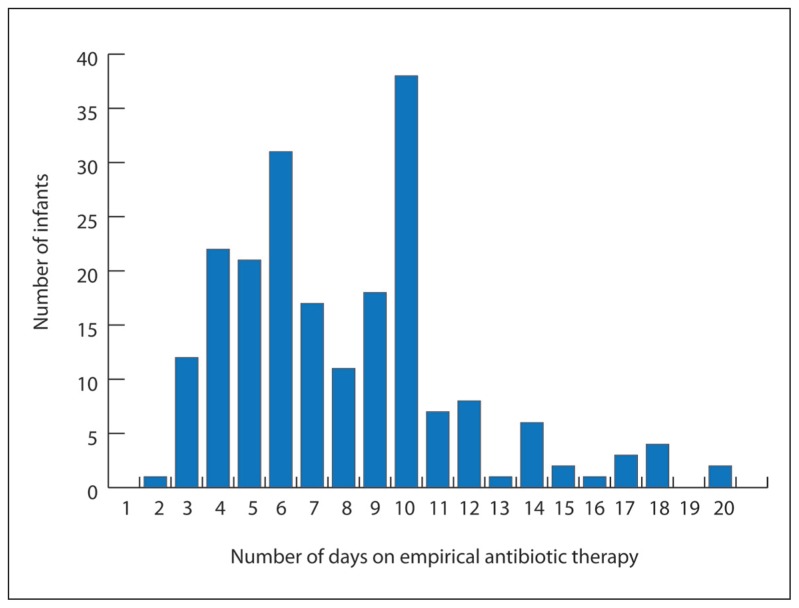
All neonates were treated with a combination of two antibiotics; ampicillin and gentamicin. One-hundred and seventy-three neonates (83.6%) in the study cohort received initial empirical antibiotic treatment for ≥5days. The mean (SD) duration of initial empirical antibiotic treatment was 8 (3.6) days (Figure 1). Neonates who received initial empirical antibiotic treatment for ≥5 days in the absence of positive culture results were more likely to be of younger gestational age, lower birth weight, and to have lower Apgar scores (P<.001, .001, and .017, respectively). Their mothers were more likely to have had maternal hypertension (P=.017) (Table 1).
Figure 1.
Numbers of study infants according to duration of initial empirical antibiotic treatment.
Table 1.
Characteristics associated with prolonged initial empirical antibiotic treatment.
| Variable | Prolonged initial empirical antibiotic therapy | P | |
|---|---|---|---|
|
| |||
| No (n= 34) | Yes (n=173) | ||
| Maternal demographic features | |||
| Multigravida, n (%) | 16 (47.1) | 101 ( 58.4) | .223 |
| Cesearean section, n (%) | 22 (64.7) | 119 (68.8) | .641 |
| Multiple gestations, n (%) | 9 (26.5) | 52 (30.1) | .675 |
| Hypertension, n (%) | 6 (17.6) | 74 (42.8) | .013 |
| Rupture of membranes >24hrs, n (%) | 7 (20.6) | 49 (28.3) | .353 |
| Neonatal demographic features | |||
| Gestational age, mean (SD), wk | 32.56 (1.67) | 30.97 (2.32 | <.001 |
| Birthweight, mean (SD), g | 1365.32 (117.32) | 1240.15 (199.93) | <.001 |
| Female, n (%) | 14 (41.2) | 91 (52.6) | .223 |
| Small for gestational age, n (%) | 11 (32.4) | 47 (27.2) | .538 |
| 5 minute Apgar score <5, n (%) | 0 (0) | 24 (13.9) | .017 |
Data are mean (SD) or number (%)
The mean (SD) duration of admission of the study cohort was 21.1 (11.6) days. Thirty-four neonates (16.4%) were diagnosed as having NEC (Bell stage 2 or more), with a mean (SD) age at diagnosis of 13.4 (4.2) days. Only one of the neonates included in the current study had surgical NEC. Eighty (38.6%) neonates died, and 92 (44.4%) patients developed the composite outcome of NEC or death.
All neonates diagnosed with NEC received prolonged initial antibiotic treatment compared to 80.3 % of the patients who were not diagnosed with NEC (P=.005). Moreover, among the study cohort, all of the patients who died had received prolonged initial empirical antibiotic treatment compared to 73.2% of the patients who were discharged, a difference that was statistically highly significant (P<.001). Similarly, 100% of the patients who were classified as having the composite outcome of NEC or death had received prolonged initial empirical antibiotic treatment compared to 70% of patients not classified as having this composite outcome, a difference which was also statistically highly significant (P<.001) (Table 2).
Table 2.
Duration of initial empirical antibiotic treatment and proportion of neonates who received prolonged initial empirical antibiotic treatment according to the outcome of necrotizing enterocolitis (NEC) and/or death.
| Outcome | P | ||
|---|---|---|---|
| No | Yes | ||
|
| |||
| NEC or death, n | 115 | 92 | |
| Duration of initial treatment, mean (SD),d | 5.8 (2.3) | 10.8 (3.0) | <.001 |
| Prolonged initial treatment, N (%) | 81 (70) | 92 (100) | <.001 |
| NEC, n (%) | 173 | 34 | |
| Duration of initial treatment, mean (SD),d | 7.3 (3.3) | 11.7 (3.1) | <.001 |
| Prolonged initial treatment, n (%) | 139 (80.3) | 34 (100) | .005 |
| Death, n (%) | 127 | 80 | |
| Duration of initial treatment, mean (SD),d | 6.4 (3.1) | 10.6 (2.8) | <.001 |
| Prolonged initial treatment, n (%) | 93 (73) | 80 (100) | <.001 |
Data are mean (SD) or number (%).
By logistic regression analysis a low gestational age, multiple pregnancy, maternal hemorrhage, maternal hypertension, Apgar at 5 min <5, and duration of initial empirical antibiotic therapy were found to significantly predict NEC (P=.011, .007, .037, .04, .001 and .018, respectively), with each additional day of empirical antibiotic therapy increasing the odds of NEC (OR 1.31, CI 1.04–1.65) (Table 3).
Table 3.
Logistic regression analysis testing for significant predictors of necrotizing enterocolitis (NEC) and/or death.
| Variable | NEC | Death | NEC or Death | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | |||||||
|
| ||||||||||||
| Gestational Age | 0.63 | 0.44 | 0.90 | .011 | 0.64 | 0.51 | 0.81 | <.001 | 0.35 | 0.22 | 0.55 | <.001 |
| Gender | 1.54 | 0.33 | 7.12 | .575 | 1.19 | 0.52 | 2.70 | .679 | 0.95 | 0.29 | 3.13 | .944 |
| Multiple Pregnancy | 9.45 | 1.86 | 47.92 | .007 | 1.19 | 0.46 | 3.07 | .710 | 4.22 | 0.97 | 18.27 | .054 |
| Maternal hypertension | 8.85 | 1.10 | 71.09 | .040 | 2.74 | 1.12 | 6.68 | .027 | 2.55 | 0.73 | 8.91 | .142 |
| Maternal hemorrhage | 33.38 | 1.22 | 908.32 | .037 | 3.63 | 0.82 | 15.96 | .087 | 14.05 | 1.74 | 113.52 | .013 |
| PROM | 2.66 | 0.64 | 11.00 | 0175 | 1.08 | 0.44 | 2.67 | .854 | 1.31 | 0.32 | 5.31 | .701 |
| SGA | 1.17 | 0.17 | 7.66 | .870 | 2.42 | 0.88 | 6.67 | .086 | 4.35 | 0.91 | 20.71 | .064 |
| Apgar at 5 min <5 | 30.87 | 4.03 | 236.09 | .001 | 0.88 | 0.24 | 3.27 | .860 | 35.75 | 1.17 | 1084.22 | .040 |
| Duration of empirical antibiotic treatment | 1.31 | 1.04 | 1.65 | .018 | 1.44 | 1.23 | 1.68 | <.001 | 2.13 | 1.54 | 33.40 | <.001 |
| Early enteral feeding < 5 days | 0.09 | 0.01 | 0.58 | .011 | 6.15 | 1.48 | 25.45 | .012 | 3.64 | 0.39 | 2.93 | .252 |
OR: Odds ratio, PROM: premature rupture of membranes; SGA: small for gestational age.
A lower gestational age, maternal hypertension and duration of initial empirical antibiotic therapy were found to be significant predictors for death (P=.001, .004 and .001, respectively), with each additional day of empirical antibiotic therapy significantly increasing the odds for death (OR 1.44, CI 1.23–1.68) (Table 3). For the composite outcome of NEC or death, by logistic regression analysis using the same multivariate model, a lower gestational age, maternal hemorrhage, Apgar at 5 min <5 and duration of initial empirical antibiotic therapy, were found significant (P=.001, .013, .04, and .001, respectively), with each additional day of empirical antibiotic therapy doubling the odds for this outcome (OR 2.13, CI 1.54–2.93) (Table 3).
Among 88 neonates who had been mechanically ventilated (mean [SD] duration of 9.3 [5.4] days), 86 (97.7%) had received prolonged initial empirical antibiotic therapy (>5 days), while only 2 (2.3%) had not received prolonged empirical therapy. Moreover, the mean (SD) duration of initial empirical antibiotic therapy among the ventilated newborns was significantly higher than the non-ventilated newborns (10.2 [2.9] days versus 6.4 [3.2] days, respectively, P<.001). Of those who had been ventilated and had received prolonged empirical antibiotic therapy, 31.4% (27/86) developed NEC, 82.6% (71/86) died, and 90.7% (78/86) developed the composite outcome of NEC or death.
LOS was diagnosed in 95 neonates (45.9%); 92 (96.8%) received prolonged initial empirical antibiotic therapy. Logistic regression analysis to test for significant predictors of LOS using the same multivariate model showed that an Apgar score of <5 at 5 minutes and duration of initial empirical antibiotic therapy were both significant predictors of LOS (P=.037 and <.001, respectively), with each additional day of initial empirical antibiotic therapy significantly increasing the odds of the neonate developing LOS (OR 1.27, CI 1.12–1.44).
The most commonly isolated organisms were Klebsiella (19.2%), Acinetobacter (6.7%), coagulase-negative Staphylococci (CONS) (6.7%), Pseudomonas (2.9%), Streptococcus viridians (2.9%), Enterobacter (2.9%) and Escherishia coli (1.9%).
Early initiation of enteral feeds (before 5 days post-natal age) was present in 88.4% of the study population, with a mean (SD) age of onset feeding of 3 (1.1) days. Comparing the VLBW neonates who received early enteral feeding with those who did not on the occurrence of NEC revealed that of 183 VLBW neonates who received early enteral feeding 166 (90.7%) did not suffer from NEC while 17 (9.3%) suffered from NEC, while of 24 neonates who did not receive early enteral feeding, 7 (29.2%) neonates did not suffer from NEC while 17 (70.8%) suffered from NEC; the difference between the two groups was statistically significant (P=.001).
Early initiation of enteral feeding was associated with significantly reduced odds of developing NEC (OR 0.09, CI 0.01–0.58, P=.011) (Table 3). Among the neonates who received early enteral feeding (<5 days postnatal age), 110 (60.1%) did not suffer from the composite outcome of NEC or death, against 73 (39.9%) who suffered from the composite outcome of NEC or death (P=.001). However, no statistically significant difference was found between neonates who received early enteral feeding and those who did not regarding the outcome of death (P=.097).
DISCUSSION
More than half of the neonates (83.6%) in the study cohort received initial empirical antibiotic treatment for ≥5days. A significantly larger proportion of infants with the composite outcome of NEC or death had received prolonged initial antibiotic treatment, compared with infants without this outcome (100% vs 70%). Results were similar for infants with NEC alone or death alone. Multivariate analyses revealed that each additional day of empirical antibiotic therapy was associated with increased odds of NEC, death, and the composite outcome of NEC or death.
These results were comparable to those of Cotten et al8 who found that longer durations of initial empirical antibiotic treatment were more likely to be associated with NEC or death and NEC alone, compared with shorter durations of initial empirical antibiotic treatment. They observed about a 4% increase in the odds of an infant in their study cohort having NEC or dying with each additional day of initial empirical antibiotic treatment. The increase with each additional day was almost doubled for NEC alone, with about a 7% increase in the odds for each additional day of initial empirical antibiotic treatment. Duration of initial empirical antibiotic use was strongly associated with increased mortality rates in their study, with about a 16% increase in the adjusted odds for each additional day of initial empirical antibiotic treatment.
Antibiotic administration is known to perturb the composition of the intestinal microbiota, resulting in suppression of anaerobic bacteria (with the exception of clostridia, which remain at detectable levels) and increased numbers of potentially pathogenic bacteria such as Klebsiella, Enterobacter, Citrobacter, and Pseudomonas.15,16
The choice of perinatal antimicrobial agents may facilitate the appearance of organisms that cause late-onset neonatal sepsis.17 A previous study suggested that selection of cefotaxime instead of gentamicin for the first 3 postnatal days was associated with higher mortality risk, even for most preterm infants.4 In the current study, logistic regression analysis showed that each additional day of initial empirical antibiotic therapy significantly increased the odds of the neonate developing late-onset sepsis. Similarly, Cotten et al8 found a significant association between initial antibiotic course of ≥5 days and the combined outcome of LOS or death (OR: 1.21 [95% CI: 1.03–1.42]).
Theoretically, gut stimulation could prevent fastinginduced mucosal atrophy, thus preventing bacterial translocation and episodes of endotoxemia, mucosal inflammation, or sepsis.18 In the current study by multivariate analysis, early initiation of trophic feeding was associated with reduced odds of developing NEC, reinforcing previous conclusions that gut stimulation protocols are beneficial to VLBW infants.19
This study provides evidence of an association between longer duration of initial empirical antibiotic courses started in the first postnatal days and death and NEC for VLBW infants whose initial blood culture results are sterile. A limitation of our study is that we could not compare the outcomes of different regimens of initial empirical antibiotic therapy as all neonates in our center receive ampicillin and an aminoglycoside for initial therapy. The limitations of the retrospective design of this study include lack of information on antenatal steroids, maternal intrapartum antibiotics, and use of umbilical catheter as well as inability to retrieve information and on the effects of drugs and on the exact amount of expressed breast milk received by the patients. Multivariate logistic regression analyses were used in attempts to correct for the significance of factors, such as gestational age, birthweight and Apgar score that contribute to the overall risk of a specific outcome. Further prospective studies are needed to determine causation and to determine whether limiting the duration of initial antibiotic treatment with sterile culture results might reduce the risk of NEC and death for VLBW infants.
To our knowledge, this is the first study done to search for an association between the duration of the initial antibiotic course in VLBW neonates, in the absence of positive culture results and the risk of subsequent death or NEC in Egyptian neonates.
Antibiotics are one of the most overused drugs in the neonatal unit. While appropriate usage is definitely helpful, indiscriminate use of antibiotics could lead to emergence of multidrug resistance in previously susceptible isolates.20 Therefore, we propose that antibiotic therapy can be stopped after 48 to 72 hours in those infants who are started on antibiotics for the presence of perinatal risk factors if the clinical course is not compatible with sepsis and the cultures are sterile.
REFERENCES
- 1.Stoll BJ, Hansen NI, Higgins RD, Fanaroff AA, Duara S, Goldberg R, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002–2003. Pediatr Infect Dis J. 2005;24:635–9. doi: 10.1097/01.inf.0000168749.82105.64. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman D, Fairchild KD. Clinical microbiology of bacterial and fungal sepsis in very-lo w-birthweight infants. Clin Microbiol Rev. 2004;17:638–80. doi: 10.1128/CMR.17.3.638-680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez Sastre JB, Fernandez Colomer B, Coto Cotallo GD, Ramos Aparicio A. Trends in the epidemiology of neonatal sepsis of vertical transmission in the era of group B streptococcal prevention. Acta Paediatr. 2005;94:451–7. doi: 10.1111/j.1651-2227.2005.tb01917.x. [DOI] [PubMed] [Google Scholar]
- 4.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics. 2006;117:67–74. doi: 10.1542/peds.2005-0179. [DOI] [PubMed] [Google Scholar]
- 5.de Man P, Verhoeven BA, Verbrugh HA, Vos MC, van den Anker JN. An antibiotic policy to prevent emergence of resistant bacilli. Lancet. 2000;355:973–8. doi: 10.1016/s0140-6736(00)90015-1. [DOI] [PubMed] [Google Scholar]
- 6.Mtitimila EI, Cooke RW. Antibiotic regimens for suspected early neonatal sepsis. Cochrane Database Syst Rev. 2004;4:CD004495. doi: 10.1002/14651858.CD004495.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159(3):392–7. doi: 10.1016/j.jpeds.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotten M, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sánchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123:58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manroe BL, Weinberg AG, Rosenfeld CR. Neonatal blood count in health and disease. Reference values for neutrophilic cells. Pediatrics. 1979;95:89. doi: 10.1016/s0022-3476(79)80096-7. [DOI] [PubMed] [Google Scholar]
- 10.Walsh M, Kleigman R. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Medical Association. Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects, the 59th WMA General Assembly; 2008; Seoul, South Korea. [Google Scholar]
- 12.Uauy RD, Fanaroff AA, Korones SB, Phillips EA, Phillips JB, Wright LL. Necrotizing enterocolitis in very low birth weight infants: biodemographic and clinical correlates. J Pediatr. 1991;119:630–38. doi: 10.1016/s0022-3476(05)82418-7. [DOI] [PubMed] [Google Scholar]
- 13.Ambalavanan N, Carlo WA, Bobashev G, Mathias E, Liu B, Poole K, et al. Prediction of death for extremely low birth weight neonates. Pediatrics. 2005;116:1367–73. doi: 10.1542/peds.2004-2099. [DOI] [PubMed] [Google Scholar]
- 14.Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolits. Pediatrics. 2005;115:696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- 15.Adlerberth I. Establishment of a normal intestinal microflora in the newborn infant. In: Hanson LA, Yolken RH, editors. Probiotics, Other Nutritional Factors and Intestinal Microflora. Lippincott-Raven; Philadelphia, Pa, USA: 1999. pp. 63–78. (Nestlè Nutrition Workshop Series). [Google Scholar]
- 16.Bennet R, Eriksson M, Nord CE. The fecal microflora of 1–3-month-old infants during treatment with eight oral antibiotics. Infection. 2002;30(3):158–60. doi: 10.1007/s15010-002-2140-z. [DOI] [PubMed] [Google Scholar]
- 17.Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996. Pediatrics. 2001;107(1) doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 18.Kliegman RM. The Relationship of neonatal feeding practices and the pathogenesis and prevention of necrotizing enterocolitis. Pediatrics. 2003;111:671–2. doi: 10.1542/peds.111.3.671. [DOI] [PubMed] [Google Scholar]
- 19.Berseth CL, Bisquera JA, Paje VU. Prolonging small feeding volumes early in life decreases the incidence of NEC in very low birth weight infants. Pediatrics. 2003;111:529–34. doi: 10.1542/peds.111.3.529. [DOI] [PubMed] [Google Scholar]
- 20.Sankar MJ, Sankar J, Chawla D, Nangia S. Antibiotic usage in neonates - Guidelines and current practices. Neonatology. 2009;23:68–77. [Google Scholar]