Abstract
BACKGROUND AND OBJECTIVE
Hospitals should measure antimicrobial use based on the WHO’s recommended metric, the defined daily dose (DDD). There is no data on antimicrobial usage based on DDD in Saudi Arabia. Thus, this study evaluates the trend in antimicrobial consumption based on this concept.
DESIGN AND SETTING
Observational study in a general hospital in Saudi Arabia from 2006–2008.
METHODS
We analyzed the use of intravenous systemic antibacterial agents (group J01 of the Anatomical Therapeutic Chemical [ATC] classification and the classes of this group) that were administered to hospitalized patients by reviewing the data obtained from the pharmacy records. Antimicrobial consumption was calculated as the number of DDD per 100 bed-days.
RESULTS
Of the total parenteral antibiotics, ciprofloxacin was the most commonly used intravenous antibiotic (67.6%), followed by ceftriaxone (6%), cefazolin (5%), and imipenem-enzyme inhibitor (4.3%). The DDD per 100 patient-days usage of intravenous antimicrobial agents was as follows: J01MA02 ciprofloxacin (parenteral) 82.643, J01DD04 ceftriaxone 7.447, J01DB02 cefazolin 6.166, J01DH51 imipenem-enzyme inhibitor 5.234, J01MA12 levofloxacin 3.188, and J01XA01 vancomycin 2.97. Intravenous ciprofloxacin usage increased from 3.55 to 82.643 DDD/100 patient days.
CONCLUSION
The study showed that the most commonly used intravenous systemic antimicrobial agent was ciprofloxacin. Thus, strategies are needed to specifically target these agents for prescribing improvement.
It is well known that antimicrobial resistance parallels the consumption of antimicrobial therapy. In developing countries, antimicrobial resistance is high and rising.1,2 Unique antibiotic resistance problems such as pan-resistant Acinetobacter baumanii are thought to be of particular concern in the Middle East.3 In addition, the Middle East is the intersection between developed and developing countries and thus the region is exposed to resistant bacteria.2 Many studies have addressed the issue of antimicrobial resistance in Saudi Arabia. Resistance rates are variable depending on the organism and the geographic area of the study. In addition, many factors contribute to the development of resistance throughout the world and specifically in the Middle East, including Saudi Arabia.2 These factors include inappropriate prescriptions, which were found to range between 24% and 80% for patients attending community hospitals.4,5 However, there are no data on antimicrobial usage based on the concept of the defined daily dose (DDD) in Saudi Arabia. Thus, we took this study to evaluate antimicrobial use in a hospital in Saudi Arabia based on this concept.
METHODS
We retrospectively analyzed the data on intravenous systemic antimicrobials dispensed in Saudi Aramco Medical Services Organization (SAMSO), Saudi Arabia, for a 3-year period from 2006 to 2008. SAMSO provides medical care for Saudi Aramco employees and their dependents (spouses, children and parents). Approximately 370 000 individuals are eligible for medical care at SAMSO. The main hospital has 380 beds with five intensive care units (cardiac, medical, surgical, pediatric, and neonatal). On average there are a total of 36 426 admissions annually with an average length of stay of 5.3 days.6
Information on the annual usage of intravenous systemic antimicrobial agents was obtained from the pharmacy register of the annual medication utilization from 2006 to 2008. The pharmacy keeps records and information about all dispensed medications on a monthly and yearly basis. The information and data included the numbers of drugs dispensed by form, type, and route of administration. Data on antibiotics were determined through the number of packages and doses from the hospitals’ pharmacy database for each year. Intravenous systemic antimicrobial agents are equivalent to group J01 of the Anatomical Therapeutic Chemical (ATC) classification system from the WHO Collaborating Centre for Drug Statistics Methodology.7 To estimate antibiotic usage, the total numbers of grams of each antibiotic used were summed for each year, and were divided by the WHO-assigned DDD, thus, estimating the number of days of antibiotic therapy (g/day). DDDs were expressed per 100 patient-days to control the differences of hospital census.8 DDDs/100 patient-days=(annual consumption of antibacterial [g]×100) divided by (DDD [g/d]×total hospitalization days). Other studies report consumption as DDDs per 1000 patient-days.9 The calculation of DDDs/100 patient-days was done using ABC-Calc “Antibiotic Consumption Calculator,” version 3.1 (2006).10 The change in antibiotic usage was compared between 2006 and 2008, and was expressed as a percentage and for this change a P values <.05 were considered statistically significant.
RESULTS
During the study period from 2006 to 2008, the total parenteral antibiotics, ciprofloxacin was the most commonly used intravenous antibiotic (67.6%), followed by ceftriaxone (6%), cefazolin (5%) and imipenem-enzyme inhibitor (4.3%). Table 1 shows the annual usage of intravenous systemic antimicrobial agents as DDD per 100 patient-days was as follows:
Table 1.
Consumption of parenteral antimicrobials as a total and by Anatomical Therapeutic Chemical group in a hospital in Saudi Arabia, 2006–2008.
| DDD per 100 bed-days | % change 2008 vs. 2006 | P | |||
|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | |||
|
| |||||
| J01CA01 ampicillin parenteral | 0.85 | 1.485 | 1.19 | 40.0 | .64 |
| J01CE01 benzylpenicillin parenteral | 0.826 | 0.933 | 0.928 | 12.3 | .36 |
| J01CF04 oxacillin (parenteral) | 0.404 | 1.193 | 0.93 | 130.2 | .54 |
| J01CR02 amoxicillin and enzyme inhibitor (parenteral) | 0.113 | 0.203 | 0.108 | −4.4 | .97 |
| J01CR03 ticarcillin and enzyme inhibitor | 0.604 | 1.424 | 1.329 | 120.0 | .40 |
| J01CR05 pipracillin and enzyme inhibitor | 0.064 | 0.025 | 0.202 | 215.6 | .46 |
| J01DB02 cefazolin | 3.942 | 6.432 | 6.166 | 56.4 | .39 |
| J01DC01 cefoxitin (parenteral) | 0.957 | 1.696 | 1.353 | 41.4 | .64 |
| J01DC02 cefuroxime (parenteral) | 0.67 | 1.319 | 1.194 | 78.2 | .45 |
| J01DD01 cefotaxime | 0.067 | 0.065 | 0.174 | 159.7 | .34 |
| J01DD04 ceftriaxone | 7.274 | 8.363 | 7.447 | 2.4 | .90 |
| J01DD09 ceftazidime | 1.1 | 3.086 | 1.259 | 14.5 | .95 |
| J01DE01 cefepime | 0.149 | 0.151 | 0.827 | 455.0 | .33 |
| J01DF01 aztreonam | 0.012 | 0.062 | 0.055 | 358.3 | .416 |
| J01DH02 meropenem | 0.161 | 0.096 | 0.492 | 205.6 | .43 |
| J01DH51 imipenem and enzyme inhibitor | 3.93 | 4.901 | 5.234 | 33.2 | .17 |
| J01EE01 trimethoprim-sulfamethoxazole (parenteral) | 0.049 | 0.03 | 0.563 | 1049.0 | .35 |
| J01FA01 erythromycin (parenteral) | 0.07 | 0.137 | 0.091 | 30.0 | .80 |
| J01FA10 azithromycin (parenteral) | 0.378 | 0.155 | 0.159 | −57.9 | .34 |
| J01FF01 clindamycin (parenteral) | 0.384 | 0.407 | 0.559 | 45.6 | .25 |
| J01GB03 gentamicin (parenteral) | 0.965 | 1.846 | 2.107 | 118.3 | .19 |
| J01GB06 amikacin | 0.195 | 0.463 | 0.325 | 66.7 | .67 |
| J01MA02 ciprofloxacin (parenteral) | 3.55 | 68.77 | 82.643 | 2228.0 | .23 |
| J01MA12 levofloxacin (parenteral) | 0.588 | 2.491 | 3.188 | 442.2 | .19 |
| J01XA01 vancomycin (parenteral) | 1.422 | 2.536 | 2.97 | 108.9 | .23 |
| J01XB02 polymyxin B (parenteral) | 0.428 | 0.74 | 0.7 | 63.6 | .20 |
| J01XD01 metronidazole (parenteral) | 0.009 | 0.017 | 0.018 | 100.0 | .23 |
J01MA02 ciprofloxacin 82.643
J01DD04 ceftriaxone 7.447
J01DB02 cefazolin 6.166
J01DH51 imipenem-enzyme inhibitor 5.234
J01MA12 levofloxacin 3.188
J01XA01 vancomycin 2.97.
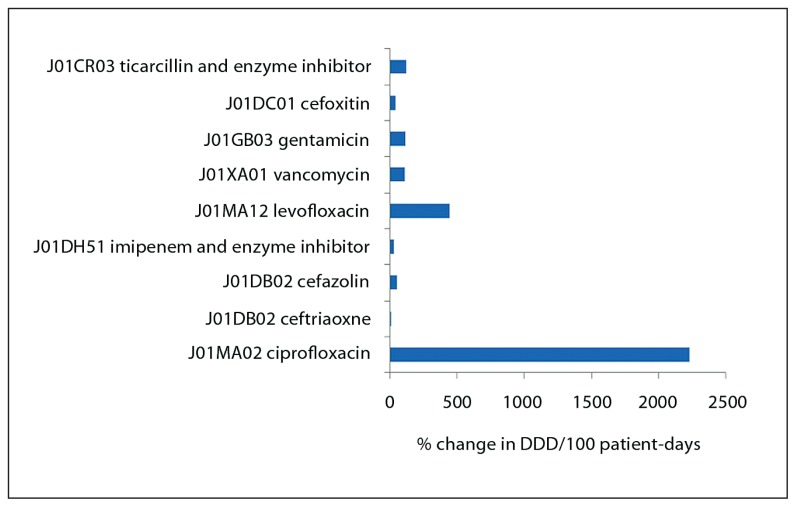
Intravenous ciprofloxacin usage increased from 3.55 to 82.643 DDD/100 patient days (Figure 1). This increase did not reach statistical significance but a linear trend was observed with R2=0.8768. Although vancomycin usage doubled (Figure 1), the actual DDD per 100 patient-days was only 3 in 2008 compared to 1.4 in 2006 (Table 1). Parenteral azithromycin use decreased from 0.378 to 0.159 DDD/100 patient-days in 2006 and 2008, respectively (P=.34). Parenteral levofloxacin use increased from 0.588 to 3.188 DDD/100 patient-days in 2006 and 2008, respectively (P=.19).
Figure 1.
Percent change in defined daily dose/100 patient-days for the top intravenous antimicrobials in 2006 compared to 2008.
DISCUSSION
Fluoroquinolone resistance is an emerging problem in many countries including Saudi Arabia. Hospital-acquired isolates of Klebsiella pneumoniae showed 23% ciprofloxacin resistance11 and Escherichia coli showed a rate of 46% resistance.12 This resistance could be partially explained by increasing the use of fluoroquinolones, not only in the hospital, but also in the community. Thus, the findings in this study of a significant usage of intravenous ciprofloxacin should direct efforts to optimize the usage of this medication. Although the P value was not significant, a linear trend was observed with R2=0.8768. Thus, the finding is indicative of a preferential increase in the use of ciprofloxacin during the study period. In a Danish study, a significant increase in the total consumption of oral ciprofloxacin was linked to the introduction of generic ciprofloxacin, and this in turn correlated with ciprofloxacin resistance in urinary isolates of E coli.13 One reason for increased usage of intravenous antimicrobial therapy may be related to the practice of the use of combination therapy for hospitalized patients.
The main carbapenem being used was an imipenem-enzyme inhibitor and the rate of utilization was higher than the reported rate from other studies.14 The finding in our study is consistent with those from other countries.15 Vancomycin utilization remains relatively low at about 1.4–2.9 DDD/100 patient-days. This rate falls within the 10% to 50% of the National Healthcare Safety Network (NHSN) data16 and is close to the mean utilization of vancomycin of 1.9/100 bed-days in a study from 29 public hospitals and private clinics in Chile.17 This finding may explain the low prevalence of vancomycin-resistant enterococci (VRE) in Saudi Arabia. In a study of 4276 patients from a tertiary care referral hospital, VRE was identified in only six (0.14%) patients.18
Our study has a few limitations. First, we included only intravenous antimicrobial therapy in the results, which was related to the fact that the data could not be separated for outpatient and inpatient for oral antimicrobial agents. Second, the data represented one hospital in the Saudi Arabia and thus may not be a representative of the whole country. Third, similar to what was noted previously,19 the data included children and adults; however, DDD are only defined for adults by the WHO Collaborating Centre for Drug Statistics Methodology. Fourth, the actual usage of all (intravenous and oral) systemic antimicrobials could not be calculated in this study. To allow for comparison with benchmarking data such as the NHSN16 it is important to have specific unit antimicrobial consumption data.20 Such data would be more informative if it had been correlated to the resistance pattern in each unit.9,21 Despite these limitations, the study is the first from Saudi Arabia to shed light on antimicrobial utilization using the DDD concept. The discrepancy in antimicrobial utilization between different hospitals is a reflection of different policies at diverse institutions. In future studies, it is important to include education and an antimicrobial stewardship program to particularly control the use of quinolones. In addition, we need to assess antibiotic indication, duration, and to include oral antimicrobial therapy in the inpatient as well as the outpatient setting.
Acknowledgments
The author acknowledges the use of Saudi Aramco Medical Services Organization (SAMSO) facilities for the data and study. Opinions expressed in this article are those of the authors and not necessarily of SAMSO. SAMSO did not fund the study. The study was approved according to our institute requirement (Number 11-1904).
REFERENCES
- 1.Okeke IN, Laxminarayan R, Bhutta ZA, Duse AG, Jenkins P, O’Brien TF, Pablos-Mendez A, Klugman KP. Antimicrobial in developing countries. Part I: recent trends and current status. Lancet Infect Dis. 2005;5:481–93. doi: 10.1016/S1473-3099(05)70189-4. [DOI] [PubMed] [Google Scholar]
- 2.Al-Tawfiq JA, Stephens G, Memish ZA. Inappropriate antimicrobial use and potential solutions: a Middle Eastern perspective. Expert Rev Anti Infect Ther. 2010 Jul;8(7):765–74. doi: 10.1586/eri.10.56. [DOI] [PubMed] [Google Scholar]
- 3.Scott P, Deye G, Srinivasan A, Murray C, Moran K, Hulten E, Fishbain J, Craft D, Riddell S, Lindler L, Mancuso J, Milstrey E, Bautista CT, Patel J, Ewell A, Hamilton T, Gaddy C, Tenney M, Christopher G, Petersen K, Endy T, Petruccelli B. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. 2007;44(12):1577–84. doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 4.Bilal NE, Gedebou M, Al-Ghamdi S. Endemic nosocomial infections and misuse of antibiotics in a maternity hospital in Saudi Arabia. APMIS. 2002;110(2):140–7. doi: 10.1034/j.1600-0463.2002.110204.x. [DOI] [PubMed] [Google Scholar]
- 5.Al-Ghamdi S, Gedebou M, Bilal NE. Nosocomial infections and misuse of antibiotics in a provincial community hospital, Saudi Arabia. J Hosp Infect. 2002;50(2):115–21. doi: 10.1053/jhin.2001.1149. [DOI] [PubMed] [Google Scholar]
- 6.Al-Tawfiq JA. Distribution and epidemiology of Candida species causing fungemia at a Saudi Arabian hospital, 1996–2004. Int J Infect Dis. 2007 May;11(3):239–44. doi: 10.1016/j.ijid.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 7.WHO Collaborating Centre for Drug Statistics Methodology. ACT index with DDDs 2001. WHO Collaborating Centre for Drug Statistics Methodolog y; Oslo, Norway: [Google Scholar]
- 8.World Health Organization. ATC Index with DDDs. Oslo, Norway: WHO; 2004. [Accessed 24 Oct 2009]. Collaborating Centre for Drug Statistics Methodology. Available at: http://www.whocc.no/atcddd/ [Google Scholar]
- 9.Fridkin SK, Steward CD, Edwards JR, Pryor ER, McGowan JE, Jr, Archibald LK, Gaynes RP, Tenover FC. Surveillance of antimicrobial use and antimicrobial resistance in United States hospitals: project ICARE phase 2. Clin Infect Dis. 1999;29:245–52. doi: 10.1086/520193. [DOI] [PubMed] [Google Scholar]
- 10.ABC Calc - Antibiotic consumption calculator. Version 3.1. 2006. [Last accessed Jan 2009]. available at: http://www.escmid.org/research_projects/study_groups/esgap/abc_calc/
- 11.Al-Tawfiq JA, Antony A. Antimicrobial resistance of Klebsiella pneumoniae in a Saudi Arabian hospital: results of a 6-year surveillance study, 1998–2003. J Infect Chemother. 2007 Aug;13(4):230–4. doi: 10.1007/s10156-007-0532-9. [DOI] [PubMed] [Google Scholar]
- 12.Al-Tawfiq JA. Increasing antibiotic resistance among isolates of Escherichia coli recovered from inpatients and outpatients in a Saudi Arabian hospital. Infect Control Hosp Epidemiol. 2006 Jul;27(7):748–53. doi: 10.1086/505336. [DOI] [PubMed] [Google Scholar]
- 13.Jensen US, Muller A, Brandt CT, Frimodt-Møller N, Hammerum AM, Monnet DL DANRES study group. Effect of generics on price and consumption of ciprofloxacin in primary healthcare: the relationship to increasing resistance. J Antimicrob Chemother. 2010 Jun;65(6):1286–91. doi: 10.1093/jac/dkq093. [DOI] [PubMed] [Google Scholar]
- 14.Pešić G, Jović Z, Vasić K. Application of the ATC/DDD Methodology to Compare Antibiotic Utilization in two university Hospital Surgical Departments. Med Biol. 2005;3(12):174–178. [Google Scholar]
- 15.Zarb P, Ansari F, Muller A, Vankerckhoven V, Davey PG, Goossens H. Drug Utilization 75% (DU75%) in 17 European Hospitals (2000–2005): Results from the ESAC-2 Hospital Care Sub Project. Curr Clin Pharmacol. 2011 Feb 1;6(1):62–70. doi: 10.2174/157488411794941322. [DOI] [PubMed] [Google Scholar]
- 16.National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004 Dec;32(8):470–85. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 17.Fica CA, Cabello MA, Juliet LC, Prado DP, Bavestrello FL. [Intravenous antimicrobial use among different hospital in Chile during 2005]. Rev Chilena Infectol. 2008 Dec;25(6):419–27. [PubMed] [Google Scholar]
- 18.Qadri SM, Postle AG. Vancomycin-resistant enterococci (VRE) as normal flora of the intestine in patients at a tertiary care hospital. Ann Saudi Med. 1996 Nov;16(6):625–8. doi: 10.5144/0256-4947.1996.625. [DOI] [PubMed] [Google Scholar]
- 19.Müller-Pebody B, Muscat M, Pelle B, Klein BM, Brandt CT, Monnet DL. Increase and change in pattern of hospital antimicrobial use, Denmark, 1997–2001. J Antimicrob Chemother. 2004 Dec;54(6):1122–6. doi: 10.1093/jac/dkh494. [DOI] [PubMed] [Google Scholar]
- 20.Edwards JR, Peterson KD, Mu Y, Banerjee S, Allen-Bridson K, Morrell G, Dudeck MA, Pollock DA, Horan TC. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009 Dec;37(10):783–805. doi: 10.1016/j.ajic.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 21.White RL, Friedrich LV, Mihm LB, Bosso JA. Assessment of the relationship between antimicrobial usage and susceptibility: differences between the hospital and specific patient-care areas. Clin Infect Dis. 2000;31(1):16–23. doi: 10.1086/313916. [DOI] [PubMed] [Google Scholar]