Abstract
Our case of ameloblastoma had a surprisingly long 25 year history, with abnormally large dimensions, a multilocular diffuse-mixed radiographic picture, and was histopathologically diagnosed as granular cell ameloblastoma with desmoplasia. To the best of our knowledge, this is the first ameloblastoma ever reported, that has shown combined features of granular cells, desmoplasia, ameloblastic follicles, plexiform, and acanthomatous patterns. The nature of granular cells in this type of tumor and the significance of their presence have also been reviewed. From the studies on aameloblastomas to date, it seems that the old belief that granular cell ameblastoma is the most aggressive variant of ameloblastoma is a myth, and in all probability, granular cells are just a transitional or matured phase in the life cycle of ameloblastomas, starting with normal stellate reticulum-like cells, leading to a production of granules and finally leading to degeneration and formation of cystic areas.
Ameloblastoma, one of the most enigmatic odontogenic neoplasms of the oral cavity, constitutes 1% to 2% of all cysts and tumors of the jaws. It is the most frequently encountered odontogenic tumor. It was first described by Broca in 1868. Six histopathological variants of ameloblastoma are available: follicular, plexiform, acanthomatous, desmoplastic, basal cell, and granular cell. The granular cell ameloblastoma (GCA) is one of the rarest entities and accounts for only 5% of all ameloblastomas.1 We present a case of ameloblastoma, which had been present for 25 years and had acquired an abnormally large size at the time of presentation. To the best of our knowledge, this is the first ameloblastoma ever reported that showed combined features of granular cells, desmoplasia, ameloblastic follicles, plexiform, and acanthomatous patterns.
CASE
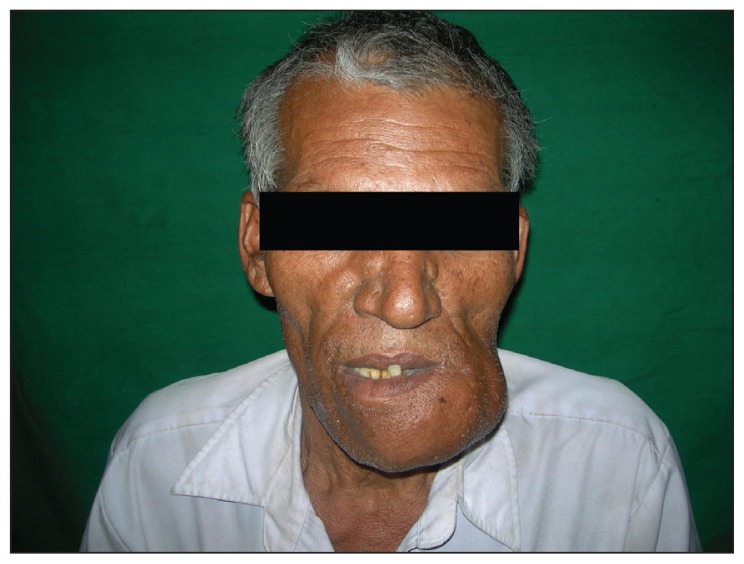
A 50-year-old male patient reported with the chief complaint of a large swelling in the lower jaw for 25 years and a history of trauma 30 years previously. The medical history was noncontributory. The dental history revealed a spontaneous loss of the left mandibular teeth over the last 8 years. Extraoral examination revealed a single, hard, multilobulated swelling of the mandible, extending from the right parasymphysis region to the left angle region, with a small part of the swelling near the left body region tender to palpation. No local rise in temperature or change in color of the overlying skin was noted (Figure 1). No lymph nodes were palpable.
Figure 1.
Extra-oral front view of a single, large, multilobulated swelling of the mandible, extending from the right parasymphysis to the left angle region.
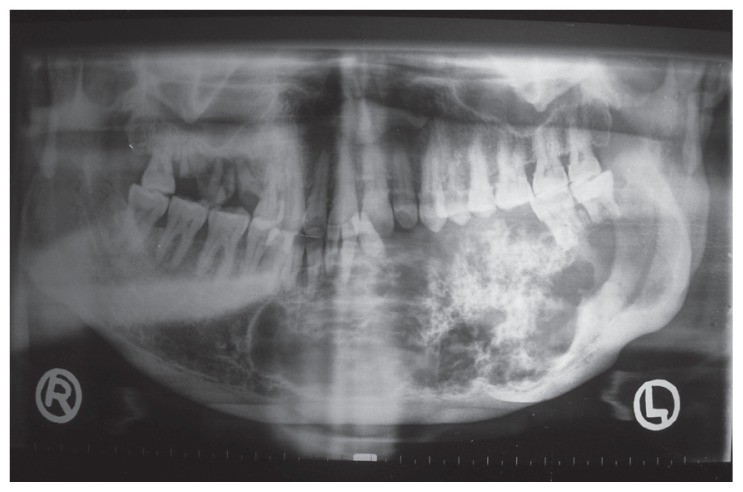
On intraoral examination, a bony hard swelling extended from the 38 to 44 region, involving the left buccal vestibule, labial vestibule, and floor of the mouth, resulting in the displacement of the tongue. Teeth 33, 34, 35, and 36 were missing, with mobility present in the remaining teeth associated with the lesion, and mucosal ulceration in region 33 was evident. Radiographically, the orthopantomograph showed a mixed radiolucent-radiopaque multilocular lesion, extending from the right parasymphysis to the left angle region and from the superior alveolar crest to the inferior border of the mandible. A well-defined, sclerotic, scalloped border with diffuse flecks of calcification, along with a displacement of 31 and 32 and root resorption of 41, 32, 37, and 38 were also evident (Figure 2).
Figure 2.
Orthopantomograph showing a mixed radiolucentradiopaque multilocular lesion, extending from the right parasymphysis to the left angle region of the mandible, with welldefined, sclerotic, scalloped borders. Also notice displacement of teeth 31 and 32 and root resorption of 41, 32, 37 and 38.
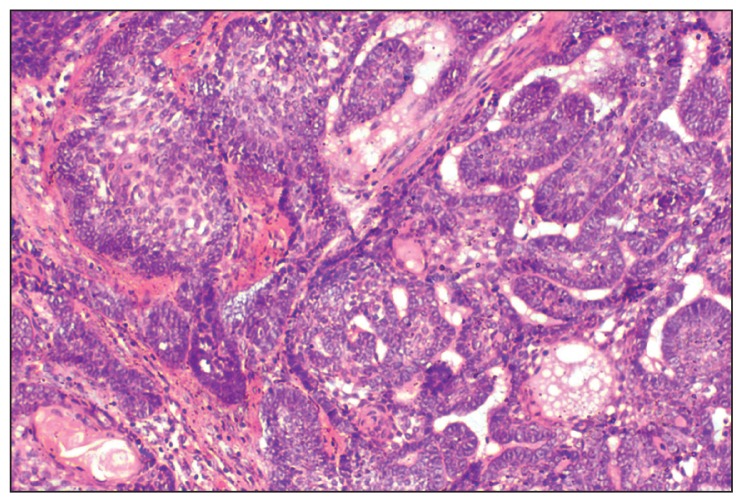
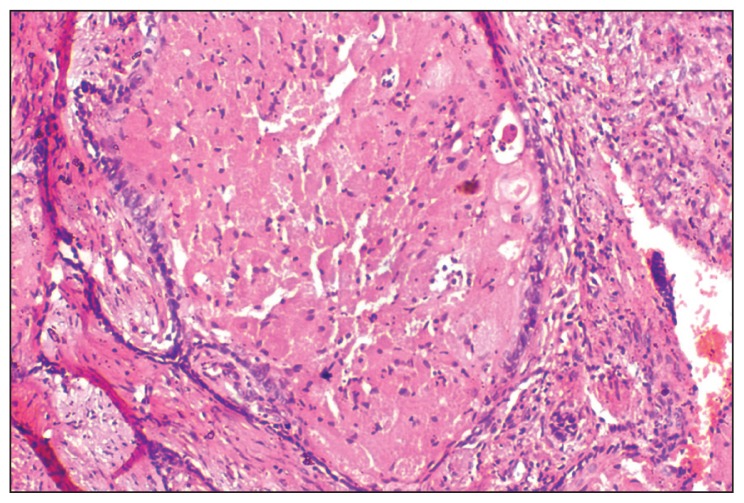
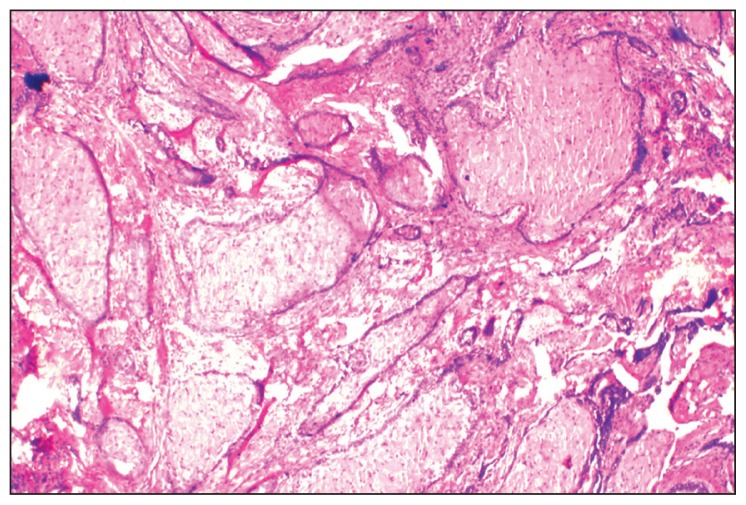
A provisional diagnosis of ameloblastoma, calcifying epithelial odontogenic tumor, or a fibro-osseous lesion was made and a biopsy was performed. On microscopic examination, HE stained sections revealed ameloblastic epithelium arranged in follicles, cords, and strands. Cystic degeneration, squamous metaplasia, and keratin formation of stellate reticulum-like cells were also found (Figure 3). Transformation of the central stellate reticulum-like cells into large polyhedral cells with course granular eosinophilic cytoplasm, having an eccentric nucleus and poorly demarcated cell membranes was evident in a majority of ameloblastic follicles (Figure 4). A few areas also depicted thin strands and cords of odontogenic epithelium in a dense fibrous stroma (Figure 5). Based on all these histopathological findings, a diagnosis of GCA with desmoplasia was made.
Figure 3.
Photomicrograph showing ameloblastic epithelium arranged in interconnecting strands or follicles, some of them showing cystic degeneration, squamous metaplasia and keratin formation (HE stain, 20×).
Figure 4.
Photomicrograph showing granular cells filled with coarse eosinophilic granules, eccentric nucleus and an inconspicuous cytoplasmic membrane, so that the adjacent cells seem to be merging with each other (HE stain, 20×).
Figure 5.
Photomicrograph showing granular cell changes in the stellate reticulum-like cells of the ameloblastic follicles and compressed odontogenic strands and cords in a dense fibrous stroma (HE stain, 4×)
The tumor was surgically approached under general anesthesia. A submandibular incision was made from the left angle to the right angle region and a layerwise dissection was carried out. An intraoral incision was made distal to the right mandibular second premolar to the left angle region. An angled reconstruction plate was adapted. A partial mandibulectomy was carried out by sectioning of bone from the right mandibular second premolar to the left angle region. The reconstruction plate was secured using three screws on either side and a suction drain was placed. A layerwise closure was done extraorally, and a single-layer closure was carried out intraorally. The patient was kept on antibiotics postoperatively, and the healing was uneventful.
DISCUSSION
Numerous theories have been proposed on the origin and nature of these granular cells in ameloblastomas. These granular cells are epithelial in origin and several ultrastructural and histochemical studies have described them as lysosomes. Many authors have reported the immunohistochemical (IHC) findings of GCA, but the actual contents within the granular cells are largely undefined. Nasu et al speculate that with age, the unnecessary/aged components progressively increase in the cytoplasm of some of the tumor cells, but the ability of lysosomes to dispose of these materials decreases, hence their cytoplasm becomes packed with lysosomal granules. 2 Tandler and Rossi (1977) thought that these lysosomes might have been a result of some genetic alteration in the granular cells.3 A recent IHC and ultrastructural study by Kumamoto et al suggests that the cytoplasmic granularity might be attributed to the increased apoptotic cell death of the neoplastic granular cells and their subsequent phagocytosis by the adjacent granular cells.4
The most recent studies on GCA by Ara et al suggest that the synthesis of signaling molecules, such as beta-catenin and Wnt-5a is upregulated in the granular cells of GCA, but their transportation or secretion is impaired, resulting in their accumulation within granular cells, as autophagosomes. Also, the heparanase enzyme, which is associated with invasiveness, fails to be activated, resulting in accumulation within these cells, in its latent form.5 Nevertheless, the true nature of these granules still remains a mystery.
Recent IHC studies also suggest that the survival potential as well as the proliferative index of the neoplastic granular cells in GCA is the least among all the variants of ameloblastomas.6,7 Taking all these into consideration, probably the old belief that GCA is the most aggressive variant of ameloblastoma is just a myth, and in all probability, granular cells are just a transitional or matured phase in the lifecycle of ameloblastomas, starting with normal stellate reticulum-like cells leading to a production of granules and finally leading to degeneration and formation of cystic areas.8 This hypothesis has been supported by the case report of Tsukada et al in which the microscopic picture of the tissue excised during a span of 33 years showed a gradual change from normal ameloblastic cells to granular cells and finally to a degenerated tissue.9 However, no proof is available as yet and further investigation is required in this matter.
The involvement of the anterior mandibular region, the mixed radiolucent-radiopaque picture, and the presence of thin strands and cords in a dense fibrous stroma point out that the present case had a prominent desmoplastic component as well. Sivapathasundaram et al argued that the desmoplasia of stroma in desmoplastic ameloblastoma is also a maturative change in a solid ameloblastoma.10 Hence, the presence of both granular as well as desmoplastic variants, along with a long history of 25 years, support this hypothesis.
In conclusion, the present case was an extremely rare lesion because first, the average duration of symptoms in mandibular ameloblastomas until the first diagnosis is 13 months,11 but in this case it was 25 years. Second, such extensive lesions, as in this case, were found in only 4% of the mandibular ameloblastomas.11 Last and most important, this is the first time that an ameloblastoma showing combined features of granular cells, desmoplasia, ameloblastic follicles, plexiform, and acanthomatous patterns has been reported to the best of our knowledge.
REFERENCES
- 1.Cranin AN, Benett J, Solomon M, Quarcoo S. Massive Granular cell ameloblastoma with metastasis: Report of a case. J Oral Maxillofac Surg. 1987;45:800–4. doi: 10.1016/0278-2391(87)90207-2. [DOI] [PubMed] [Google Scholar]
- 2.Nasu M, Tagaki M, Yamamoto H. Ultrastructural and histochemical studies of granular cell ameloblastoma. J Oral Pathol. 1984;13:448–56. doi: 10.1111/j.1600-0714.1984.tb01445.x. [DOI] [PubMed] [Google Scholar]
- 3.Tandler B, Rossi EP. Granular cell ameloblastoma: Electron microscopic observations. J Oral Pathol. 1977;6:401–12. doi: 10.1111/j.1600-0714.1977.tb01807.x. [DOI] [PubMed] [Google Scholar]
- 4.Kumamoto H, Kimi K, Ooya K. Immunohistochemical and ultrastructural investigation of apoptotic cell death in Granular cell ameloblastoma. J Oral Pathol Med. 2001;30:245–50. doi: 10.1034/j.1600-0714.2001.300409.x. [DOI] [PubMed] [Google Scholar]
- 5.Ara SG, Han PP, Tamamura R, Nagatsuka H, Hu H, Katase N, et al. Immunolocalization of cell signaling molecules in the granular cell ameloblastoma. J Oral Pathol Med. 2007;36:609–14. doi: 10.1111/j.1600-0714.2007.00580.x. [DOI] [PubMed] [Google Scholar]
- 6.Kumamoto H, Kimi K, Ooya K. Immunohistochemical analysis of apoptosis related factors (Fas, fas Ligand, caspase-3 and single stranded DNA) in ameloblastomas. J Oral Pathol Med. 2001;30:596–602. doi: 10.1034/j.1600-0714.2001.301004.x. [DOI] [PubMed] [Google Scholar]
- 7.Kumamoto H, Ohki K, Ooya K. Expression of p63 and p73 in ameloblastomas. J Oral Pathol Med. 2005;34:220–6. doi: 10.1111/j.1600-0714.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 8.Smith J, Sollee N, Drake J, Blankenship J. Granular cell ameloblastoma with remarkable mucin production. Oral Surg Oral Med Oral Pathol. 1966;21:499–505. doi: 10.1016/0030-4220(66)90408-7. [DOI] [PubMed] [Google Scholar]
- 9.Tsukada Y, Pava S, Pickren J. Granular cell ameloblastoma with metastasis to the lungs. Cancer. 1965;18:916–25. doi: 10.1002/1097-0142(196507)18:7<916::aid-cncr2820180722>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.Sivapathasundaram B, Einstein A, Syed RI. Desmoplastic ameloblastoma in Indians: Report of 5 cases and review of literature. Indian J Dent Res. 2007;18:218–21. doi: 10.4103/0970-9290.35836. [DOI] [PubMed] [Google Scholar]
- 11.Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: Biological profile of 3677 cases. Eur J Cancer B Oral Oncol. 1995;31B:86–99. doi: 10.1016/0964-1955(94)00037-5. [DOI] [PubMed] [Google Scholar]