Abstract
Leiomyoma is the most common uterine neoplasm. It has several histological variants such as atypical, cellular, myxoid, and epithelioid. Leiomyoma with heterologous elements is a rare variant of leiomyoma, which may contain heterologous elements such as fat, skeletal muscle, and chondroid and osseous tissues. The heterologous sarcomatous differentiation is also rarely seen. We report on a 53-year-old woman who was admitted with abnormal vaginal bleeding and symptoms related to an abdominal mass. She had a huge uterine leiomyoma that contained osteosarcomatous differentiation in several foci. Although malignant progression for leiomyoma is exceedingly rare, when it occurs it may result not only in a leiomyosarcoma but also in a heterologous sarcomatous differention. We have reported histopathological, immunohistochemical, and clinical features of this rare case and reviewed the published studies.
Osteosarcoma is the most common primary malignant tumor of bone, characterized by the osteoid production by the neoplastic cells. Extraskeletal osteosarcoma accounts for 1% to 2% of all soft tissue sarcomas and approximately 2% to 4% of all osteosarcomas. It is usually seen in the thigh, buttock, shoulder, trunk, and retroperitoneum.1 Although osteosarcoma may be seen in the uterus as a component of malignant mixed mesodermal tumors, primery uterine osteosarcoma is a distinctively rare and aggressive tumor of unclear origin. To our knowledge, only 15 cases of primary uterine osteosarcomas have been reported.2,3 Osteosarcomatous differentiation in a leiomyoma has been reported in only one case to date.4 We described the osteosarcomatous differentiation in a uterine leiomyoma of a 53-year-old case. The clinicopathologic and immunohistochemical findings of this exceptional case are presented, and the published studies are reviewed.
CASE
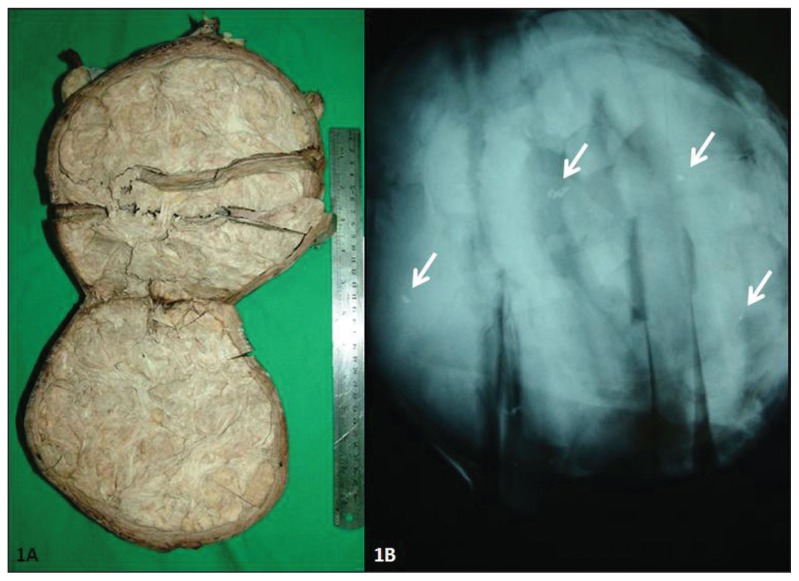
A 53-year-old woman was admitted with abnormal vaginal bleeding and symptoms related to an abdominal mass for 2 months. Ultrasonography was performed, and it showed a 19-cm-diameter solid mass in the uterus. Hysterectomy and bilateral salpingo-oophorectomy were subsequently performed. Macroscopically, the hysterectomy specimen measured 21×21×6 cm. The right and left ovaries were normal in size and appearance. In the posterior wall of the uterine body was a solid mass (19×17×11 cm) consistent with a leiomyoma. The cut surface of this mass was gray-tan colored and solid, and showed the typical whorled pattern of a leiomyoma (Figure 1a). No hemorrhage or necrosis was observed. The endometrial cavity was distorted by the mass. No cervical, endometrial, or ovarian gross pathological lesion was observed. Thirty samples were taken from the mass.
Figure 1.
Macroscopic section (1A) and radiographic appearance (1B) of the mass (Arrows: radiopaque calcification foci).
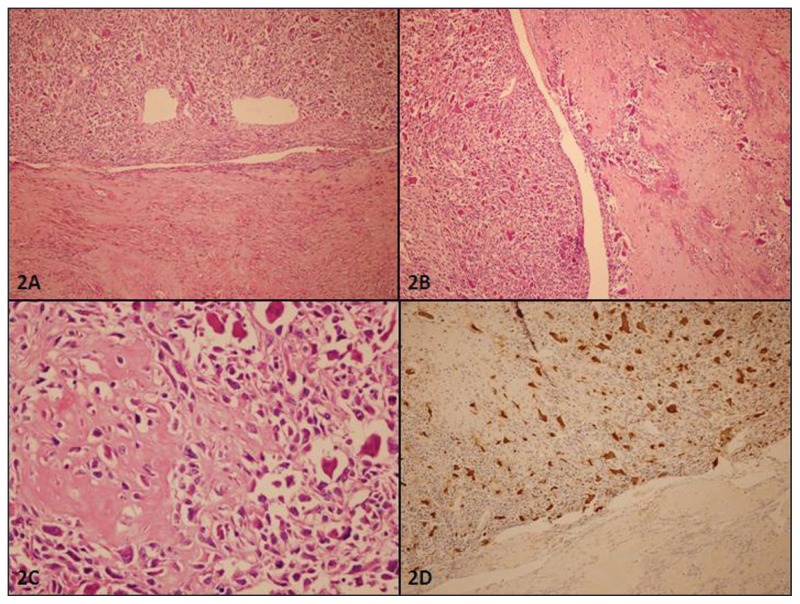
The microscopic examination showed that the mass consisted of interlacing bundles of the smooth muscle cells typical of leiomyoma. Some hyaline changes were seen. A sharp demarcation was observed from the surrounding myometrium. The cervix, endometrium, neither ovary, and tuba uterina contained no pathologic lesion. Since atypical smooth muscle cells were seen in a focus and hyaline necrosis-degenerative changes were observed in some areas of the leiomyoma, a concern arose for a uterine smooth muscle tumor of uncertain malignant potential or a leiomyosarcoma, and the case was referred to our department for consultation. The mass appeared as a typical leiomyoma with some hyaline changes and dense collagenous bundles on microscopical examination. Microscopic foci of bizarre cells were observed in two sections. The mitotic count ranged from 2 to 3 per 10 high-power fields (HPF) in several foci of the mass. Furthermore, a 15-mm focus separated from the leiomyoma with dense collagenous bundles that appeared in a block, some of which showing osseous metaplasia. In this focus, atypical cells were present with round to spindle, pleomorphic, and hyperchromatic nuclei and eosinophilic cytoplasms. In several areas, some of these cells were also producing osteoid. Many osteoclasttype giant cells were seen between these atypical cells. The atypical mitotic count in this focus ranged from 40 to 50 per 10 HPF. Microscopic findings were interpreted as an osteosarcomatous differentiation in a uterine leiomyoma (Figure 2).
Figure 2.
Histopathological features of the mass. A: Junction of the leiomyoma (lower part) and osteosarcomatous (upper part) areas are seen (HE stain, ×100). B: Atypical mesenchymal cells and numerous osteoclast type giant cells are near the osseous metaplasia area (HE stain, ×100). C: Atypical mesenchymal cells have high mitotic count and were producing osteoid (HE stain, ×400). D: Osteoclast type giant cells are positive with CD 68 (Diamino benzidine, ×100).
Immunohistochemistry was performed for this paraffin block. These atypical cells and leiomyoma cells were positive for vimentin (Clone V9, Neomarkers, Fremont, CA, United States) and smooth muscle actin (SMA) (Clone 1A4, Neomarkers). Desmin (Clone D33, DBS, Pleasanton, CA, United States) was positive for leiomyoma but negative for the osteosarcomatous focus. Estrogen receptor (ER) (Clone SP1, Neomarkers) and progesterone receptor (PR) (Clone PR1, Neomarkers) were positive (average 40%–50% of the cells) for leiomyoma but negative for the osteosarcomatous focus. CD-68 (Clone KP1, Neomarkers) showed positivity limited to the osteoclast-type giant cells. Cytokeratin (AE1/AE3, Neomarkers) and EMA (Clone GP1.4, Novocastra, Newcastle Upon Tyne, UK) were negative for both components. The Ki-67 (Clone MM1, Novocastra) index was 2% for leiomyoma and 30% for osteosarcomatous focus (Figure 2).
The gross specimen of the mass was requested from the first laboratory and specimen radiography was performed, which revealed minute radiopaque foci (Figure 1b). Thirty new tissue blocks were taken from these radiopaque foci. We found six additional osteosarcomatous foci that measured from 5 to 10 mm. No epithelial or other sarcomatous component was found. All osteosarcomatous foci had similar histopathological features. The foci were independent and discontinuous, both macroscopically and microscopically. The histopathological features and immunohistochemical profile were in keeping with an osteosarcoma differentiating in a uterine leiomyoma. Thorax and abdominal CT performed after the diagnosis showed no metastasis. The patient was stable after a follow-up period of 7 months.
DISCUSSION
Uterine smooth muscle tumors are seen commonly, and most are leiomyomas. Its malignant counterpart is leiomyosarcoma, which is also the most common uterine nonepithelial malignant tumor. Leiomyosarcoma represents about 1.3% of uterine malignancies. Although most leiomyosarcomas arise de novo, malignancy may develop in pre-exisiting leiomyomas as well.5 Leiomyomas may have unusual growth patterns, and many histopathological variants, such as atypical, cellular, mitotically active, myxoid, and epithelioid have been described.5,6 Leiomyoma with heterologous elements is a rare variant of leiomyoma and may contain heterologous elements such as fat and skeletal muscle, and chondroid and osseous tissues. Adipose tissue is the most common heterologous component in leiomyoma, which is then called a lipoleiomyoma.5,6
The heterologuos sarcomatous differentiation is rarely seen in a uterine leiomyoma. To our knowledge, only nine previous cases of heterologuous sarcomatous differentiation within a uterine leiomyoma have been documented.4,7–14 Three of the nine cases had multipletype sarcomas (two cases had leiomyosarcoma, liposarcoma, and osteosarcoma; one case had leiomyosarcoma, chondrosarcoma, and osteosarcoma) and were named malignant mesenchymomas.11–13 The term “malignant mesenchymoma” as proposed by Stout defines at least two distinct types of malignant mesenchymal component in the same mesenchymal tumor.15,16 In our cases no other sarcomatous component besides osteosarcoma was present. The other six cases had single-type sarcoma (two epithelioid angiosarcomas, one rhabdomyosarcoma, one fibrosarcoma, one myxoid liposarcoma, and one osteosarcoma).4,7–10 One of the nine cases, described by Wang et al,4 had osteosarcomatous differentiation similar to our case. Their cases had a centrally located osteosarcomatous component in an 8-cm leiomyoma. The leiomyoma component had a purely benign appearance and was in the peripheral region of the mass. The osteosarcomatous component composed almost 80% percent of all leiomyoma. The immunohistochemical profile of this case was also almost the same as our case: SMA, desmin, ER, PR positive in the leiomyomatous component and only SMA and focally desmin positive; ER, PR negative in the osteosarcomatous component.
The heterologous sarcomatous differentiation may occur as a component of uterine malignant mixed Müllerian tumors or Müllerian adenosarcoma, which by definition should also contain an epithelial component. 5,6 In our case, there was no epithelial differentiation, and epithelial markers, cytokeratin, and EMA were negative. Since we did not find other sarcomatous components, we ruled out malignant mesenchymoma. Leiomyosarcomas may have heterologous comoponents and osteoclastic-type giant cells.5,6 In our case the smooth muscle tumor had no malignant features except the osteosarcomatous foci.
In the leiomyomatous areas, the mitotic count was low and showed no necrosis. The pathogenesis of heterologous sarcoma arising from leiomyoma is unclear. Multiple reasons may be suggested for the malignant heterologous sarcomatous differentiation.5,13 The divergent differentiation of stem cells toward osteoprogenitor cells may be the reason of this osteosarcomatous differentiation without any benign precursor. In our opinion, the most plausible theory is a malignant change arising in a focus of osseous metaplasia. In our case, there was multiple sarcomatous foci (six sections contain osteosarcoma areas), all in close proximity of focal ossification areas. These findings are also more supportive of the later theory that the osteosarcomatous diferentiation arises from an ossification focus in leiomyoma.
In conclusion, we describe the second case of osteosarcomatous differentiation in a uterine leiomyoma. Although malignant progression for leiomyoma is exceedingly rare, when it occurs it may not result only in a leiomyosarcoma but also in heterologous sarcomatous differention, even in several foci.
REFERENCES
- 1.Rosenberg AE, Heim S. Extraskeletal osteosarcoma. In: Fletcher CD, Unni KK, Mertens F, editors. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon: IARC Press; 2002. pp. 182–3. [Google Scholar]
- 2.Hardisson D, Simón RS, Burgos E. Primary Osteosarcoma of the Primary osteosarcoma of the uterine corpus: Report of a case with immunohistochemical and ultrastructural study. Uterine Corpus: Report of a Case. Gynecol Oncol. 2001;82:181–6. doi: 10.1006/gyno.2001.6262. [DOI] [PubMed] [Google Scholar]
- 3.Su M, Tokairin T, Nishikawa Y, Yoshioka T, Takahashi O, Watanabe H, et al. Primary osteosarcoma of the uterine corpus: case report and review of the literature. Pathol Int. 2002;52:158–63. doi: 10.1046/j.1440-1827.2002.01325.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang RC, Wen MC, Wang J, Ho SC, Jan YJ. Osteosarcoma Arising in a Long-Standing Uterine Leiomyoma: A Case Report and Literature Review. Int J Surg Pathol. 2011;19:99–103. doi: 10.1177/1066896908327037. [DOI] [PubMed] [Google Scholar]
- 5.Zaloudek CJ, Hendrickson MR. Mesenchymal tumors of the uterus. In: Kurman RJ, editor. Blaustein’s Pathology of the Female Genital Tract. New York: Springer Verlag; 2002. pp. 561–615. [Google Scholar]
- 6.Oliva E. Pure Mesenchymal and Mixed Müllerian Tumors of the Uterus. In: Nucci MR, Oliva E, editors. Gynecologic Pathology. China: Elsevier Churchill Livingstone; 2009. pp. 261–330. [Google Scholar]
- 7.Tallini G, Price FV, Carcangiu ML. Epithelioid angiosarcoma arising in uterine leiomyomas. Am J Clin Pathol. 1993;100:514–8. doi: 10.1093/ajcp/100.5.514. [DOI] [PubMed] [Google Scholar]
- 8.Drachenberg CB, Faust FJ, Borkowski A, Papadimitriou JC. Epithelioid angiosarcoma of the uterus arising in a leiomyoma with associated ovarian and tubal angiomatosis. Am J Clin Pathol. 1994;102:388–9. doi: 10.1093/ajcp/102.3.388. [DOI] [PubMed] [Google Scholar]
- 9.Takano M, Kikuchi Y, Aida S, Sato K, Nagata I. Embryonal rhabdomyosarcoma of the uterine corpus in a 76-year-old patient. Gynecol Oncol. 1999;75:490–4. doi: 10.1006/gyno.1999.5593. [DOI] [PubMed] [Google Scholar]
- 10.Bodner-Adler B, Bodner K, Czerwenka K, Leodolter S, Mayerhofer K. Fibrosarcoma of the uterus: A case report. Anticancer Res. 2001;21:3651–2. [PubMed] [Google Scholar]
- 11.den Bakker MA, Hegt VN, Sleddens HB, Nuijten AS, Dinjens WN. Malignant mesenchymoma of the uterus, arising in a leiomyoma. Histopathology. 2002;40:65–70. doi: 10.1046/j.1365-2559.2002.01290.x. [DOI] [PubMed] [Google Scholar]
- 12.Kew CC, Putti TC, Razvi K. Malignant mesenchymoma arising from a uterine leiomyoma in the menopause. Gynecol Oncol. 2004;95:712–5. doi: 10.1016/j.ygyno.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Sheldon EC, Howe R, Selman T, Mann C, Ganesan R. Uterine malignant mesenchymoma, arising in a leiomyoma, with pulmonary metastases. Histopathology. 2007;50:397–400. doi: 10.1111/j.1365-2559.2007.02589.x. [DOI] [PubMed] [Google Scholar]
- 14.Hong R, Lim SC, Jung H. A myxoid liposarcoma arising in a leiomyoma of the uterus: A case report. Arch Gynecol Obstet. 2008;277:445–8. doi: 10.1007/s00404-007-0486-2. [DOI] [PubMed] [Google Scholar]
- 15.Stout AP. Mesenchymoma, the mixed tumor of mesenchymal derivatives. Ann Surg. 1948;127:278–90. doi: 10.1097/00000658-194802000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss SW, Goldblum JR. Malignant soft tissue tumors of uncertain type. In: Weiss SW, Goldblum JR, editors. Soft Tissue Tumors. China: Elsevier; 2008. pp. 1161–220. [Google Scholar]