Abstract
BACKGROUND AND OBJECTIVES
No data are available in Saudi Arabia on the relationship between coronary artery calcification (CAC) and myocardial perfusion scintigraphy (MPS) in asymptomatic women, for determining subclinical coronary artery disease (CAD). The main objective of this study was to investigate the relationship between the presence of CAC and stress-induced myocardial ischemia by MPS in asymptomatic women.
DESIGN AND SETTING
Single-center retrospective study over a 2-year period.
METHODS
One hundred and one women (mean [SD] age, 56 [11] years) without known CAD underwent both MPS and CAC scanning within 3 months. The frequency of ischemia by MPS was compared with the presence or absence of CAC and the number of CAD risk factors.
RESULTS
The prevalence of ischemic MPS was 22% (22/101). Among the 22 patients with ischemic MPS, the CAC score was 0 in 5 patients of 22 (23%), 1 to 200 in 4 patients of 22 (18%), and more than 200 in 13 patients of 22 (59%) (P=.0001). In contrast, among the 79 patients with normal MPS, the CAC score was 0 in 44 of 79 (56%) patients, 1 to 200 in 25 of 79 (32%), and more than 200 in 10 of 79 (13%). The presence or absence of CAC was the single most important predictor of the MPS result (P=.0001).
CONCLUSIONS
Moderate to severe CAC is associated with ischemic MPS in more than 50% of asymptomatic women with 2 or more CAD risk factors. Abnormal MPS is rarely associated with a 0 CAC score. Normal MPS does not exclude subclinical CAD. Therefore, CAC screening is an appropriate initial screening test for CAD in asymptomatic women.
Currently available data continues to indicate an underrecognition and underdiagnosis of coronary artery disease (CAD) as a contributory factor in the high mortality rate in women.1 The substantial under-representation of women in studies of noninvasive cardiac imaging further limits the evidence-based information that is used as a basis in clinical decision making.2 Calcification of the coronary artery is virtually pathognomonic of atherosclerosis.3–5 Clinical and histopathological studies have documented a close correlation between coronary artery calcification (CAC) detected by multi-detector computed tomography (MDCT) and atherosclerotic plaque burden.6,7 In addition, a modest relationship between CAC and the severity of luminal stenosis has been revealed with the use of either conventional angiography8,9 or intravascular ultrasound.10,11 Some studies have suggested that the progression of CAD is more related to atherosclerotic plaque burden12 rather than the extent of diseases detected by angiography.13 CAC is an easily obtained surrogate for plaque burden, and it can detect coronary atherosclerosis in early stages based on the presences and severity of CAC.14,15
Stress myocardial perfusion single photon computed tomography (SPECT) is one of the most commonly performed noninvasive cardiac imaging tests in patients with known or suspected CAD. Gated myocardial perfusion scintigraphy (MPS) provides several parameters that are used to diagnose and risk stratify symptomatic and asymptomatic women. These parameters include stress-induced perfusion defects, global and regional left ventricular function, and left ventricular volumes.16 MPS using contemporary techniques has played an important role in the diagnosis and prognosis of CAD in women. In addition, myocardial perfusion imaging with exercise or pharmacologic stress has been shown to add incremental value to the use of clinical variables or exercise electrocardiogram stress testing alone in the risk stratification of women with an intermediate clinical pretest likelihood of CAD.17 However, MPS imaging has been reported to have technical limitations in women, including false-positive results because of breast attenuation and small left ventricular function.18 Furthermore, MPS is an expensive test and is not recommended for screening of asymptomatic women. Currently, little information is available as to whether CAC can identify asymptomatic women at high risk for myocardial ischemia. Accordingly, the purpose of this study was to determine the relationship between CAC score, number of cardiac risk factors, and the results of MPS in asymptomatic women.
METHODS
A retrospective study was performed on 101 women who underwent rest/stress MPS and CAC scanning within 3 months of each other between January 2009 and November 2010. Patients underwent MPS on a clinical basis for risk stratification, preoperative evaluation, or ongoing research project. CAC scanning was performed either on the basis of physician referral as a screening for subclinical CAD or an ongoing research project. Exclusion criteria included a history of myocardial infarction, prior coronary bypass surgery or percutaneous coronary intervention, prior cardiomyopathy, or known valvular heart disease. The study was approved by our hospital review board.
MPS acquisition and analysis
Patients underwent rest/stress myocardial perfusion studies with either a separate-day protocol or a same-day rest/stress sequence. The choice of tracer and same-day or separate-day protocol was based on logistical requirements. The rest dose in patients who underwent a separate-day rest/stress protocol was 10 to 370 megabecqurel (MBq) of technetium-99 (Tc-99m) sestamibi or tetrofosmin. The stress dose in patients who underwent the rest/stress same-day protocol was 1100 MBq of either (Tc-99m) sestamibi or tetrofosmin. Tc-99m sestamibi or Tc-99m tetrofosmin was injected either during peak treadmill exercise or during peak pharmacological vasodilatation with adenosine (140 μg/kg/min). SPECT imaging was started approximately 15 minutes following injection during exercise or at 30 minutes following pharmacological vasodilatation. Rest SPECT myocardial perfusion imaging was initiated at approximately 60 minutes following injection. SPECT imaging was performed with line source attenuation correction at 90° dual-detector gamma camera (Cardio MD, Philips Medical System, Milpitas, California) equipped with attenuation correction and truncation compensation. The acquisition parameters and post-processing were performed according to the most recent guidelines of the American Society of Nuclear Cardiology for nuclear cardiology procedures.19
All images were reoriented in short, vertical, and horizontal views utilizing AutoSPECT (Cedars-Sinai Medical Center, Los Angeles, California) for visual interpretation by an experienced nuclear medicine physician. The reader was not biased by clinical information and was unaware of the CAC score. Stress and rest perfusion images were scored using 17 tomographic segments, which included 6 segments each for the basal and midventricular slices, and 4 segments for the apical short-axis slices. The final segment was located on the most apical part of the left ventricle.20 Finally, gated short-axis images were processed with the quantitative SPECT software to measure the ejection fraction. In the visual analysis, the 17 segments were scored for perfusion defects on a 4-point system (0=normal; 1=mild; 2=moderate; and 3=severe) for both the stress and rest images. From this analysis, ischemia was defined as a change in the segmental score between stress and rest. Segments with no change between stress and rest were classified as nonreversible. Summed stress and rest scores were calculated by summing the 17 segmental scores in each image set. Utilizing the summed difference score (SDS) in measuring defect reversibility was calculated from the difference between the summed stress and rest scores. An SDS lower than 2 was considered nonischemic, 2 to 7 was considered mild ischemia, and greater than 7 was considered moderate to severe ischemia. The reader made the final determination of an abnormal SPECT study by comparing both the perfusion and functional data.
Calcium scanning and interpretation
The imaging protocol involved acquiring a prospective electrographic-gated single scan on each patient consisting of approximately 30 to 40 slices of 2.5 mm each sufficient to cover the entire heart with triggering at 50% to 80% of the cardiac cycle. Breath-holding instructions were given to minimize misregistration. Scanning required a 3-mm CT slice thickness and a threshold for CAC of ≥130 Hounsfield units (HU) (CT density) involving ≥1 mm2 area/lesion on the high definition 64-slice CT machine (GE Medical System, Waukesha, Wisconsin).
Foci of CAC were identified by an experienced radiographic technologist, confirmed by an experienced cardiovascular radiologist, and scored using semiautomatic commercial software on Advantage Workstation (GE Medical System). The software calculated lesion-specific scores as the product of the area of each calcified focus and peak CT number (scored as 1 if 131 to 199 HU; 2 if 200 to 299 HU; 3 if 300 to 399 HU; and 4 if 400 HU or greater) according to the Agatston method.21 These were summed across all lesions identified within left main, left anterior descending, left circumflex, and right coronary arteries to provide arterial-specific calcium scores, and across arteries to provide the total CAC score.
Statistical analysis
Total calcium scores were classified into 3 categories: 0=calcium absent; 1 to 200=minimal to mild; and more than 200=moderate to severe; the prevalence of an ischemic MPS was compared across calcium score categories using the chi-square test for trend. A multiple logistic regression was performed to examine whether log (CAC +1) was independently associated with the likelihood of abnormal MPS result after adjustment for other CAD risk factors. All continuous variables are expressed as mean (SD). A value of P<.05 was considered to indicate a statistical significance.
RESULTS
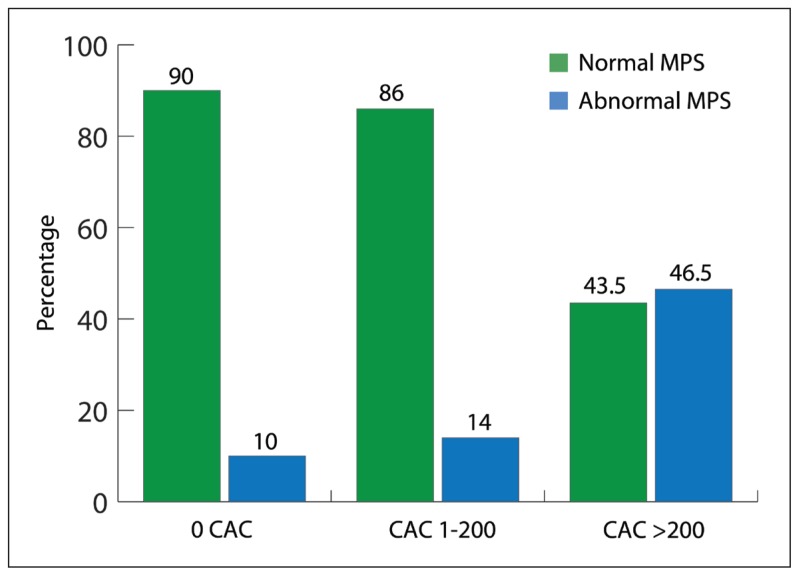
The characteristics of the study population stratified by MPS are shown in Table 1. Of the 101 patients, 22 had abnormal MPS (22%) compared with normal MPS, and patients with abnormal MPS were significantly older and had significantly higher CAC. Interestingly, no significant correlation was observed between ischemic MPS and other traditional risk factors of CAD such as diabetes, hypertension, smoking, high cholesterol, or a positive family history for premature CAD. As shown in Figure 1, a very strong correlation was observed between the presence and the severity of CAC and MPS result. Only 5 patients (10%) out of 45 patients with zero CAC had abnormal MPS. However, patients with a CAC score of more than 200 had more than a 50% chance of having abnormal MPS (P≤.0001).
Table 1.
Characteristics of the study population.
| Parameter | Overall n= 101 women |
Normal MPS n=79 |
Abnormal MPS n=22 |
P |
|---|---|---|---|---|
|
| ||||
| Mean age (SD) (years) | 56 (11) | 56 (10) | 56 (12) | .0021 |
|
| ||||
| Risk factors | ||||
| High Cholesterola | 24 (24) | 19 (24) | 5 (23) | .897 |
| Diabetesb | 45 (44.6) | 33 (24) | 12 (45) | .285 |
| Hypertensionc | 52 (51.5) | 36 (47) | 16 ( 73) | .024 |
| Smoking | 23 (23) | 19 (24) | 4 (18) | .562 |
| Positive family history | 22 (22) | 19 (24) | 3 (14) | .295 |
|
| ||||
| CAC score | ||||
| 0 | 49 (48.5) | 44 (56) | 5 (23) | <.0001 |
| 1–200 | 29 (32) | 25 (32) | 4 (18) | <.0001 |
| More than 200 | 23 (23) | 10 (13) | 13 ( 59) | <.0001 |
Values are nymber and percent of column total except for age.
MPS=myocardial perfusion scintigraphy; CAC=coronary artery calcification.
Defined as serum total cholesterol ≥230 mg/dL (6.2 mmol/L) or treatment with lipid lowering drugs.
Defined as fasting blood sugar more than 126 dL (7 mmol/L) or treatment with insulin and or oral hypoglycemic drugs.
Defined as systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≤90 mm Hg and/or the use of antihypertensive medication.
Figure 1.
The frequency of abnormal (ischemic) stress single photon computed tomography (MPS) according to coronary artery calcification score (CAC).
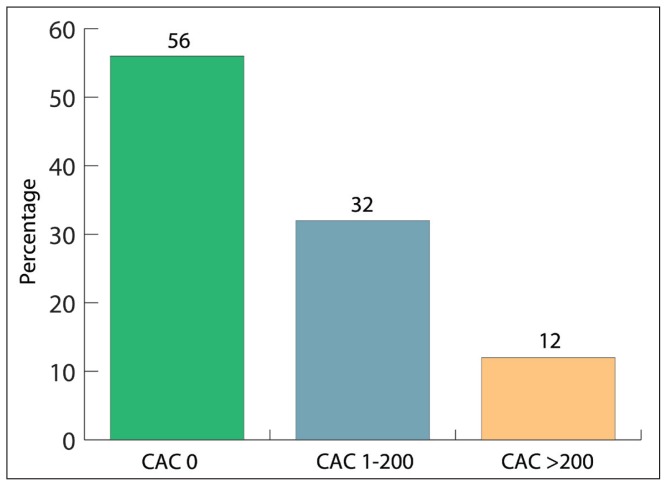
A strong correlation was observed between the presence and the severity of CAC with age, diabetes, and hypertension, but no correlation was observed between CAC and high cholesterol, smoking, or family history. A total of 35 of 79 patients (45%) with normal MPS had CAC of more than zero. About 12.7% had severe CAC (CAC score more than 200) (Figure 2).
Figure 2.
The distribution of coronary artery calcification score (CAC) in patients with normal stress single photon computed tomography (MPS) (P<.001 across all groups).
Diabetes was present in 45 patients of the total population study. A total of 17 of 45 patients (37.8%) had no detectable CAC, but 12 (26.7%) and 16 (35.6%) patients had a CAC score of 1 to 200 and more than 200, respectively, with statistically significant P value (.020). However, no correlation was observed between diabetes and abnormal MPS (P=.268). Multivariable logistic regression analysis showed that the log CAC score was the most and the only predictor for abnormal MPS, odds ratio 2, and P value of less than .001. Other traditional CAD risk factors, e.g., diabetes, hypertension. age, family history, and high cholesterol, were not predictors for abnormal MPS in a multivariate regression analysis.
DISCUSSION
The main finding of our study is a strong relationship between the CAC score obtained by MDCT and the presence of ischemia as measured by stress MPS. The CAC score can accurately classify patients into low-risk or high-risk groups in asymptomatic women. The absence of CAC or CAC score in less than 200 asymptomatic women is associated with a low probability of abnormal MPS; in contrast women with a CAC score of more than 200 and 2 or more CAD risk factors have more than a 50% chance of having an abnormal (e.g., ischemic) MPS. This result may be exploited to improve the selection and use of a more expensive test, such as MPS, for identifying asymptomatic women with high risk for cardiovascular events.
Predictors of Ischemic MPS
In our study, the best predictors of ischemic MPS were CAC score and age. For example, asymptomatic 58-year old or older females with a CAC score of more than 200 have a 50% or more chance of having abnormal MPS, but asymptomatic relatively younger females (e.g., 56-year old or younger) with a zero CAC score have less than a 10% chance of having an abnormal MPS. This result is consistent with previous studies that demonstrate that the angiographic extent and severity of CAD are closely related to the CAC score,22,23 and the angiographic stenosis severity best predicts an abnormal MPS.24 Overall, the data suggests that CAC is more sensitive than MPS in asymptomatic women in detecting subclinical atherosclerosis and subsequent risk factor modification.
The impact of CAD risk factors on CAC score
Using univariate analysis, our data suggests a clear association between CAC and age, diabetes, and hypertension; in contrast no relationship was observed between CAC and other CAD risk factors such as family history of premature CAD, smoking history, and high cholesterol. However, in a multivariate analysis the presence of CAC was the only predictor for abnormal MPS. It is well known that conventional risk factors do correlate with CAC25 even though CAC is superior to conventional risk factors in predicting outcomes. Postmenopausal women present a striking example of the inability of conventional risk factor analysis to predict the presence or absence of subclinical atherosclerosis. A study by Hecht and Superko showed no difference in any lipid parameters or in the Framingham risk score between postmenopausal women with and without calcified plaques, rendering a therapeutic decision that is plaque-imaging based extremely problematic.26
Normal MPS and presence of CAC
In our study approximately 45% of asymptomatic women with normal MPS have detectable CAC. In fact 13% have a CAC score of more than 200. This result indicates a limited role for all stress testing (e.g., stress MPS, stress echocardiography, or treadmill stress test) in asymptomatic women. Stress testing does not effectively screen for subclinical atherosclerosis. The presence of CAC implies atherosclerosis, but not necessarily the presence of coronary stenosis; in contrast MPS requires the presence of a hemodynamically significant lesion, either fixed or dynamic, before any abnormality becomes evident.27
Relationship between Diabetes, CAC, and MPS
Diabetic patients should be given special consideration because diabetes is considered a CAD risk equivalent. Our data showed a strong relationship between diabetes and CAC score (P=.002), but no statistical significance between diabetes and ischemic MPS was demonstrated. Diabetic patients with a zero CAC score have the same excellent prognosis as patients without diabetes, and it is reasonable to treat patients with a zero CAC scores less aggressively than would be dictated as a CAD risk equivalent. On comparison, diabetic patients with an elevated CAC score have a poor prognosis than patients without diabetes and a similar CAC score and subsequently should be treated more aggressively.28
Prior studies
This is the first study in Saudi Arabia to investigate the relationship between the CAC score and the MPS result. However, a few studies are available that investigate the interrelationship between CAC and MPS in the general population (men and women). He et al noted a threshold phenomenon with a very low abnormal MPS and a CAC score of less than 100 and 46% with abnormal MPS in patients with CAC score more than 400.29 The observed difference in frequency of abnormal MPS (our patients with moderate to severe CAC have 56% abnormal MPS) in our study is due to many factors, such as gender and ethnic difference, and most importantly our patients have more risk factors for CAD, such as being older than 55 years, with a high prevalence of diabetes and other CAD risk factors. Another possible explanation for this difference is the criteria being utilized in the interpretation of MPS. Berman et al showed the same threshold phenomenon between CAC and MPS. He concluded that in most patients a low CAC score appeared to obviate noninvasive testing, but a normal MPS was frequently associated with coronary calcification that indicates subclinical atherosclerosis.30 In our recently published data, a visual estimation of CAC on the CT component of positron emmission tomography (PET)-CT in cancer patients was highly consistent with the current study. We found that the presence of CAC by visual detection is associated with 53% abnormal MPS, but the absence of CAC was rarely associated with abnormal MPS.31
Study Limitations
The total study population was small (only 101 asymptomatic women) and patients with a CAC score of more than 200 was the smallest group (23 patients out of 101 of total study population). Another important issue was that our patients were asymptomatic and had multiple CAD risk factors, such as being older than 55 years and having diabetes. Further studies utilizing a larger patient population with fewer CAD risk factors are greatly needed.
In conclusion, CAC screening by MDCT in asymptomatic women with multiple risk factors predicts silent myocardial ischemia diagnosed by stress MPS. Therefore, CAC screening is recommended as an appropriate initial imaging test in this subset of patients for risk stratification. Further testing with more definitive testing, such as stress MPS, is recommended for patients with a CAC score of more than 200 with 2 or more CAD risk factors. Aggressive risk factor modification and therapy are highly recommended in low-risk patients with a CAC score of less than 200.
REFERENCES
- 1.Mieres JH, Shaw LJ, Arai A, Budoff MJ, Flamm SD, Hundley WG, et al. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: Consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation. 2005;111:682–96. doi: 10.1161/01.CIR.0000155233.67287.60. [DOI] [PubMed] [Google Scholar]
- 2.Shaw LJ, Peterson ED, Johnson LL. Non-invasive stress testing. In: Charney P, editor. Coronary Artery Disease in Women: What All Physicians Need to Know. Philadelphia, Pa: American College of Physicians; 1999. pp. 327–50. [Google Scholar]
- 3.Frink RJ, Achor RW, Brown AL, Jr, Kincaid OW, Brandenburg RO. Significance of calcification of the coronary arteries. Am J Cardiol. 1970;26:241–7. doi: 10.1016/0002-9149(70)90790-3. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy JH, Palmer FJ. Incidence and significance of coronary artery calcification. Br Heart J. 1974;36:499–506. doi: 10.1136/hrt.36.5.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rifkin RD, Parisi AF, Folland E. Coronary calcification in the diagnosis of coronary artery disease. Am J Cardiol. 1979;44:141–7. doi: 10.1016/0002-9149(79)90263-7. [DOI] [PubMed] [Google Scholar]
- 6.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–62. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 7.Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;1:126–33. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 8.Bielak LF, Rumberger JA, Sheedy PF, 2nd, Schwartz RS, Peyser PA. Probabilistic model for prediction of angiographically defined obstructive coronary artery disease using electron beam computed tomography calcium score strata. Circulation. 2000;102:380–5. doi: 10.1161/01.cir.102.4.380. [DOI] [PubMed] [Google Scholar]
- 9.Leber AW, Knez A, Mukherjee R, White C, Huber A, Becker A, et al. Usefulness of calcium scoring using electron beam computed tomography and noninvasive coronary angiography in patients with suspected coronary artery disease. Am J Cardiol. 2001;88:219–23. doi: 10.1016/s0002-9149(01)01629-0. [DOI] [PubMed] [Google Scholar]
- 10.Schmermund A, Baumgart D, Gorge G, Seibel R, Gronemeyer D, Ge J, et al. Coronary artery calcium in acute coronary syndromes: A comparative study of electron-beam computed tomography, coronary angiography, and intracoronary ultrasound in survivors of acute myocardial infarction and unstable angina. Circulation. 1997;96:1461–9. doi: 10.1161/01.cir.96.5.1461. [DOI] [PubMed] [Google Scholar]
- 11.Mintz GS, Pichard AD, Popma JJ, Kent KM, Satler LF, Bucher TA, et al. Determinants and correlates of target lesion calcium in coronary artery disease: A clinical, angiographic and intravascular ultrasound study. J Am Coll Cardiol. 1997;29:268–74. doi: 10.1016/s0735-1097(96)00479-2. [DOI] [PubMed] [Google Scholar]
- 12.Wayhs R, Zelinger A, Raggi P. High coronary artery calcium scores pose an extremely elevated risk for hard events. J Am Coll Cardiol. 2002;39:225–30. doi: 10.1016/s0735-1097(01)01737-5. [DOI] [PubMed] [Google Scholar]
- 13.Mock MB, Ringqvist I, Fisher LD, Davis KB, Chaitman BR, Kouchoukos NT, et al. Survival of medically treated patients in the coronary artery surgery study(CASS) registry. Circulation. 1982;66:562–8. doi: 10.1161/01.cir.66.3.562. [DOI] [PubMed] [Google Scholar]
- 14.Rumberger JA. Cost effectiveness of coronary calcification scanning using electron beam tomography in intermediate and high risk asymptomatic individuals. J Cardiovasc Risk. 2000;7:113–9. doi: 10.1177/204748730000700205. [DOI] [PubMed] [Google Scholar]
- 15.Greenland P, Smith SC, Jr, Grundy SM. Improving coronary heart disease risk assessment in asymptomatic people: Role of traditional risk factors and noninvasive cardiovascular tests. Circulation. 2001;104:1863–7. doi: 10.1161/hc4201.097189. [DOI] [PubMed] [Google Scholar]
- 16.Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer JV, Berman DS, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging--executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging) Circulation. 2003;108:1404–18. doi: 10.1161/01.CIR.0000080946.42225.4D. [DOI] [PubMed] [Google Scholar]
- 17.Mieres JH, Shaw LJ. Stress myocardial perfusion imaging in the diagnosis and prognosis of women with suspected coronary artery disease. Cardiol Rev. 2003;11:330–6. doi: 10.1097/01.crd.0000088275.80332.28. [DOI] [PubMed] [Google Scholar]
- 18.Mieres JH, Shaw LJ, Hendel RC, Miller DD, Bonow RO, Berman DS, et al. American Society of Nuclear Cardiology consensus statement: Task Force on Women and Coronary Artery Disease--the role of myocardial perfusion imaging in the clinical evaluation of coronary artery disease in women [correction] J Nucl Cardiol. 2003;10:95–101. doi: 10.1067/mnc.2003.130362. [DOI] [PubMed] [Google Scholar]
- 19.Holly TA, Abbott BG, Al-Mallah M, Calnon DA, Cohen MC, DiFilippo FP, et al. Single photonemission computed tomography. J Nucl Cardiol. 2010;17:941–73. doi: 10.1007/s12350-010-9246-y. [DOI] [PubMed] [Google Scholar]
- 20.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 21.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 22.Rumberger JA, Sheedy PF, Breen JF, Schwartz RS. Electron beam computed tomographic coronary calcium score cutpoints and severity of associated angiographic lumen stenosis. J Am Coll Cardiol. 1997;29:1542–8. doi: 10.1016/s0735-1097(97)00093-4. [DOI] [PubMed] [Google Scholar]
- 23.Guerci AD, Spadaro LA, Goodman KJ, Lledo-Perez A, Newstein D, Lerner G, et al. Comparison of electron beam computed tomography scanning and conventional risk factor assessment for the prediction of angiographic coronary artery disease. J Am Coll Cardiol. 1998;32:673–9. doi: 10.1016/s0735-1097(98)00299-x. [DOI] [PubMed] [Google Scholar]
- 24.Mahmarian JJ, Steingart RM, Forman S, Sharaf BL, Coglianese ME, Miller DD, et al. Relation between ambulatory electrocardiographic monitoring and myocardial perfusion imaging to detect coronary artery disease and myocardial ischemia: an ACIP ancillary study. The Asymptomatic Cardiac Ischemia Pilot (ACIP ) Investigators. J Am Coll Cardiol. 1997;29:764–9. doi: 10.1016/s0735-1097(96)00572-4. [DOI] [PubMed] [Google Scholar]
- 25.Kuller LH, Matthews KA, Sutton-Tyrrell K, Edmundowicz D, Bunker CH. Coronary and aortic calcification among women 8 years after menopause and their premenopausal risk factors: the healthy women study. Arterioscler Thromb Vasc Biol. 1999;19:2189–98. doi: 10.1161/01.atv.19.9.2189. [DOI] [PubMed] [Google Scholar]
- 26.Hecht HS, Superko HR. Electron beam tomography and National Cholesterol Education Program guidelines in asymptomatic women. J Am Coll Cardiol. 2001;37:1506–11. doi: 10.1016/s0735-1097(01)01211-6. [DOI] [PubMed] [Google Scholar]
- 27.Schmermund A, Denktas AE, Rumberger JA, Christian TF, Sheedy PF, 2nd, et al. Independent and incremental value of coronary artery calcium for predicting the extent of angiographic coronary artery disease: Comparison with cardiac risk factors and radionuclide perfusion imaging. J Am Coll Cardiol. 1999;34:777–86. doi: 10.1016/s0735-1097(99)00265-x. [DOI] [PubMed] [Google Scholar]
- 28.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43:1663–9. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 29.He ZX, Hedrick TD, Pratt CM, Verani MS, Aquino V, Roberts R, et al. Severity of coronary artery calcification by electron beam computed tomography predicts silent myocardial ischemia. Circulation. 2000;101:244–51. doi: 10.1161/01.cir.101.3.244. [DOI] [PubMed] [Google Scholar]
- 30.Berman DS, Wong ND, Gransar H, Miranda-Peats R, Dahlbeck J, Hayes SW, et al. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol. 2004;44:923–30. doi: 10.1016/j.jacc.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 31.Fathala A, Aljefri A, Alsugair A, Abouzied M. Coronary artery calcification detected by PET/CT scan as a marker of myocardial ischemia/coronary artery disease. Nucl Med Commun. 2011;32:273–8. doi: 10.1097/MNM.0b013e328341a741. [DOI] [PubMed] [Google Scholar]