Abstract
BACKGROUND AND OBJECTIVES
Intracranial germ cell tumors (GCTs) are not a common disease. We reviewed the experience of a single institution to determine the variables that affect treatment outcome.
DESIGN AND SETTING
A retrospective review of patients with the diagnosis of intracranial germ cell tumors treated in a single institution (KFSHRC) during the period from March 1985 to December 2007.
PATIENTS AND METHODS
Fifty-seven patients with the diagnosis of intracranial GCT were recorded in the KFSHRC Tumor Registry during the period from 1985 to 2007. Seven patients with a pineal region tumor treated as germinomas in the earlier years without a tissue diagnosis were excluded. This retrospective study was restricted to the remaining 50 patients with a tissue or marker diagnosis: 31 germinomas and 19 non-germinomatous germ cell tumors (NGGCTs).
RESULTS
The 10-year overall survival (OS), event-free survival (EFS) and relapse-free survival (RFS) were 87%, 88% and 96% for patients with germinoma, with a median follow-up of 4.5 (range 2–17) years, compared with 26%, 29% and 46% for patients with NGGCT with a median follow-up of 3 (range 1.5–13) years. For NGGCT, variables favorably influencing OS were younger age (< 16 y vs ≥ 16 y, P=.01), higher radiation dose (>50 Gy vs ≤ 50 Gy; P=.03) and later year of diagnosis (>1990 vs <1990 P=.002).
CONCLUSIONS
Tissue diagnosis of GCTs is mandatory prior to treatment except for patients with elevated markers. In germinoma, localized radiotherapy (RT) for M0 patients may be adequate. Long-term follow-up is needed to define the benefit of adding chemotherapy. For NGGCT, the use of combined modality treatment and RT dose >50 Gy are important factors that influence the outcome. Second-look surgery and resection of residual/refractory tumors is always recommended.
Primary intracranial germ-cell tumors (GCTs) are rare, accounting for 1% to 3% of all brain tumors in children.1 Typically these are midline tumors arising from either the pineal or suprasellar region or occasionally both sites. They are more frequent in boys and have a peak incidence in puberty. These are rare tumors which encompass a multiple unique spectrum of tumor types, the management of which is dependent on the histological diagnosis.2 The optimal management of intracranial GCTS remains to be established. During the last two decades, the principle treatment objective for germinomas has been to determine the minimum effective treatment that will maintain high tumor control rates. For non-germinomatous germ cell tumors (NGGCTs), the objective has been to improve survival rates by an appropriate combination, and as yet an intensification of systemic chemotherapy and radiation treatment.3
PATIENTS AND METHODS
King Faisal Specialist Hospital and Research Center (KFSHRC) provides general tertiary care services and includes the national cancer treatment center for Saudi Arabia. A total of 57 patients with intracranial GCTs were recorded in the Tumor Registry at the KFSHRC Cancer Center between 1985 and 2007. Seven patients treated for germinomas without a tissue diagnosis in the early years of the study were excluded. The medical files of the remaining 50 patients with GCTs were reviewed: 48 had a biopsy-proven diagnosis of intracranial GCTs, and two patients were diagnosed as having NGGCT based on imaging studies and elevated serum and cerebral spinal fluid. All patients underwent imaging of the brain; CT in earlier years of the study and MRI in the recent years. Staging workup included MRI of the spine (35 patients), CSF cytology (34 patients), and serum (50 patients) and CSF (20 patients) human chorionic gonadotrophins (HCG) and alpha-fetoprotein (AFP). There were 31 patients with germinomas, and 19 with NGGCT. Serum markers were elevated in 23 patients: 7/31 patients with germinoma had elevated HCG in the range of 25–100 mIU/mL. Among 19 NGGCT patients, elevated AFP was noted in nine patients, elevated HCG in one patient with choriocarcinoma, and elevated HCG as well as AFP in 4 patients. Overall, CSF cytology was positive in only 3 out of 34 (8%) patients with available data. In addition, there was MRI evidence of gross subarachnoid spinal disease (M3) in six patients.
The median age for the whole group was 12 (range 3–36) years. There were 39 males and 11 females. The tumor was located in the pineal region in 27 patients (54%), in the suprasellar region in 20 patients (40%), and 3 patients had multiple tumors arising in both regions (6%). The common presenting signs and symptoms were increased intracranial pressure (ICP) and hydrocephalus 54% (27 patients), visual impairment (acuity, diplopia, field deficits, blindness) 40% (20 patients), diabetes insipidus 22% (11 patients), endocrinopathies 30% (15 patients), Parinaud syndrome or other ocular palsy 10% (5 patients).
The database was analyzed using SPSS statistical software (IBM Corp. Armonk, NY, United States). The Kaplan-Meier method was used to calculate overall survival (OS), event-free survival (EFS) and relapse-free survival (RFS) curves, with 95% confidence intervals (CIs). Overall survival was defined from the date of diagnosis to the date of death or last follow-up. EFS was calculated from the date of diagnosis until the date of the first event, defined as relapse, progression, death from any cause, or last follow-up. RFS was calculated for patients who achieved a complete remission, from the date of diagnosis to the date of first relapse or of last follow-up. Univariate analyses were performed to evaluate variables influencing OS, such as age, gender, histology, radiation tumor dose, radiation volume, extent of surgery, use of chemotherapy, and year of diagnosis using the log rank test.
Overall, 48 patients underwent surgery: craniotomy and biopsy (27), partial resection (18) and total resection (3). For germinomas, craniospinal irradiation (CSRT) was used as a single-modality treatment in the early years of the study. Since 1992, the management guidelines for germinomas in the pediatric age group (<16 years) have changed from CSRT to elective bimodal therapy, use of upfront chemotherapy, with a reduction in the radiation volume and dose in order to reduce radiation toxicity. Three to four cycles of PVB (cisplatin+vinblastine+bleomycin) were followed by whole-brain RT plus boost with a 4 to 5 week gap between chemotherapy and RT. CSRT was only used in the high-risk group, defined as patients with metastatic disease, multiple tumor sites and/or elevated markers (HCG>100). The standard germinoma treatment for adults remained CSRT alone. CSRT was followed by a local boost with a 1–1.5-cm margin around the initial tumor. The RT dose to the whole brain was in the range 18–45 Gy (median, 30.6 Gy). The dose to the spine, when treated, was 18–37 Gy (median 32 Gy) and the dose to the primary tumor area was 30–55 Gy (median 48 Gy). Overall, 43 patients received RT: CSRT (24), whole brain and boost (11) and local RT alone (8). For NGGCT the intent was to use upfront chemotherapy and RT in all patients.
Germinoma patients (n=31)
The median age for the germinoma group was 12 (range, 3–36) years. There were 23 patients <16 years of age (22 were below the age of 14). There were 22 males and nine females. The tumor site was pineal in 16 (51%) and suprasellar in 12 (38%). Multiple midline germinomas were observed in three patients (11%): two patients presented with tumors in two sites (suprasellar and pineal regions), and one patient had diffuse tumor involving the suprasellar, thalamic/hypothalamic regions, the lateral and third ventricles.
The histology was consistent with pure germinoma in 26 patients, and germinoma with trophoblastic elements in 5 patients, in whom the serum HCG was elevated (<100 mIU/mL). The CSF cytology was positive in only 1 of 19 (5%) patients who had lumbar puncture (LP) studies.
A spinal MRI study was available in 23 patients, with gross evidence of subarachnoid spread (M3) in two patients. The extent of surgery was biopsy only in 17 (55%), partial resection in 12 (38%) and total resection in 2 (7%) patients. Adult patients were treated in the adult oncology unit with CSRT only, whereas 20/23 patients younger than the age of 16 years were treated according to the pediatric protocol in the pediatric oncology department. They received primary chemotherapy as part of their treatment. The chemotherapy regimen was PVB (cisplatin+etoposide+bleomyc) in 18 patients and PIV (cisplatin+ ifosfamide+etoposide) in two patients. The number of cycles ranged from one to seven, with most of the patients receiving three to four cycles (15 patients). This was followed by RT in 18 patients. There were two toxic deaths due to sepsis, after one and three cycles of chemotherapy. Overall, 28 patients with germinomas were treated with RT: CSRT plus boost (15), whole brain (WB) plus boost (11) and localized focal RT to tumor bed alone (2). Eight patients treated with CSRT also received chemotherapy.
NGGCT patients (n=19)
The median age was 14 (range, 4–35) years. There were 17 males and 2 females. The tumor site was pineal in 11 (58%), and suprasellar regions in 8 (42%) patients. The histology was mixed GCT in 5 patients (26%), malignant teratoma in 4 (21%), endodermal sinus tumor in 4 (21%), embryonal carcinoma in 2 (10%), choriocarcinoma in 2, and unspecified NGGCT in 2 patients. At the time of initial evaluation 12 patients had MRI spine and 14 had LP for CSF cytology. There was evidence of disease dissemination to the spine in 6 patients: gross positive spinal metastases on MRI (M3) in 4, and positive cytology (M1) in 2 patients. Seventeen of 19 (90%) patients had surgery: biopsy (10), partial resection (6), and total resection (1). Sixteen patients received chemotherapy as part of their initial combined modality therapy (CMT), and 3 patients were treated with CSRT alone in the early years of the study. The chemotherapy regimens included various combinations of cyclophosphamide, ifosfamide, vincristine, vinblastine, cisplatin, carboplatin, etoposide, bleomycin, VP16, methotrexate and prednisone. The use of platinum-based chemotherapy became a standard in our protocols in the year 1990. The chemotherapy regimens used were PVB (11 patients), PIV (4 patients), and MTX/CDDP/VP16 (1 patient). The number of cycles varied between two and six (median four cycles). Radiation treatment was given to 17/19 (90%) patients; CSRT (14 patients), whole brain (WB) plus boost (1 patient), and partial brain plus boost (2 patients). Two patients received no RT: one due to toxic death after three cycles of PVB, while the other died from disease progression after one cycle of PVB. The doses to the primary tumor volume were in the range of 45–55 Gy (median 50.4 Gy). The whole brain when treated, received 28–45 Gy (median 34.2 Gy). The dose to the spine varied between 24–37 Gy (median 34.6 Gy).
There were three toxic deaths secondary to chemotherapy. Twenty-six patients had documented endocrinopathies before initiation of treatment (panhypopituitarism, decreased growth hormone, growth retardation, hypothyroidism, hypogonadism, precocious puberty). An additional nine patients required hormone replacement therapy after completion of treatment, which may have been due in part to RT toxicity. Patients were not evaluated formally for neurocognitive deficits; however, difficulty in schooling, and lost grades were common among survivors. To date no second malignant tumors have been observed during follow-up of the survivors.
RESULTS
Germinoma patients (n=31)
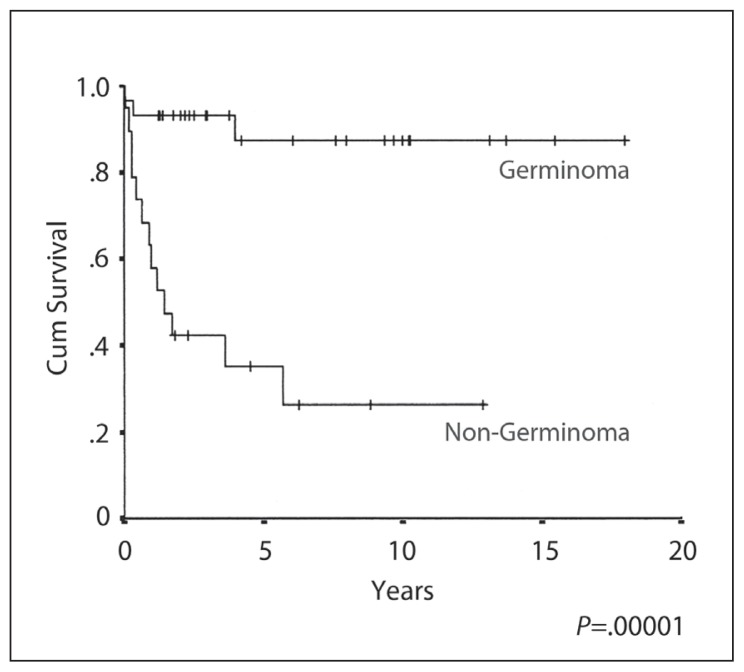
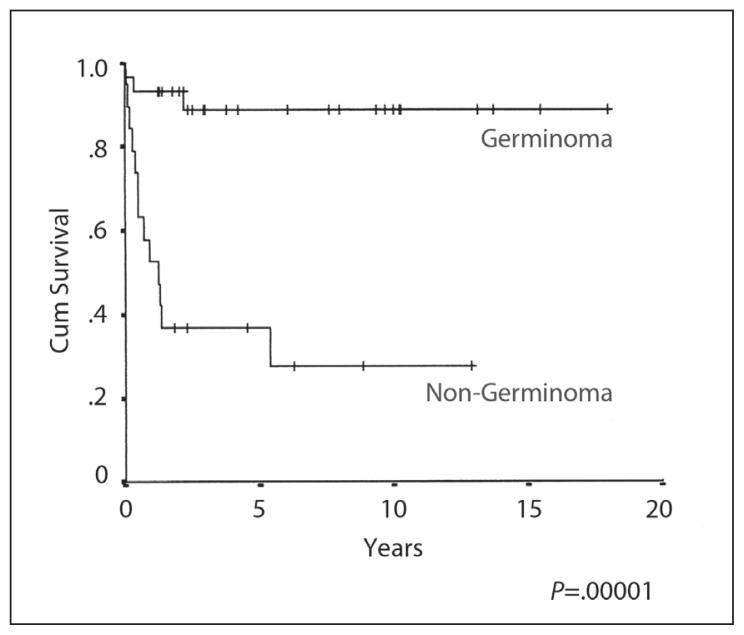
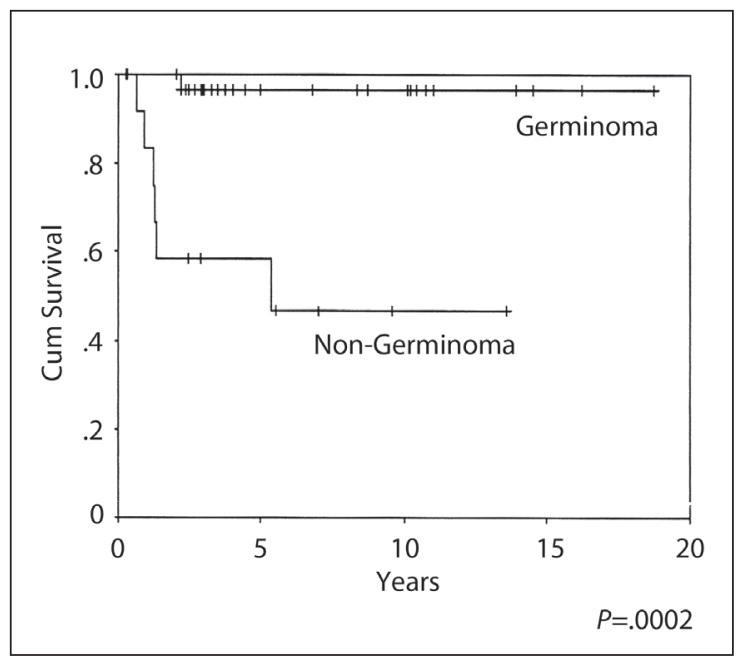
The 10-year OS, EFS and RFS rates were 87%, 88% and 96% respectively (Figures 1–3), with a median follow-up of 4.5 years (range 3–17 years). A complete response (CR) was achieved in 30/31 patients. The response could not be evaluated in one patient who died from sepsis after the first cycle of chemotherapy. The other toxic death occurred in CR after three cycles of PEB, but prior to radiation treatment. There was only one death due to disease relapse. There was no significant OS and progression-free survival difference on univariate analysis by age, gender, site of disease, elevated HCG, extent of resection, the elective use of chemotherapy or the radiation volume or dose. In-field relapse after RT was not observed.
Figure 1.
Overall survival.
Figure 2.
Event-free survival.
Figure 3.
Relapse-free survival.
NGGCT patients (n=19)
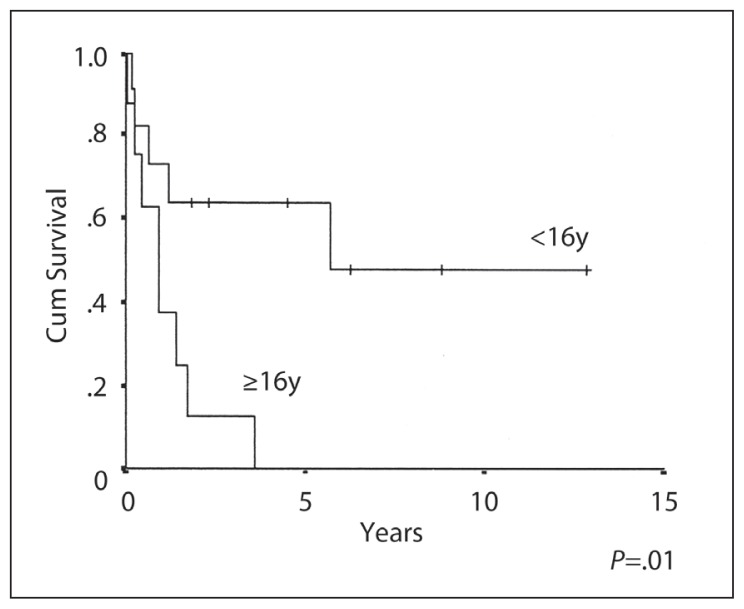
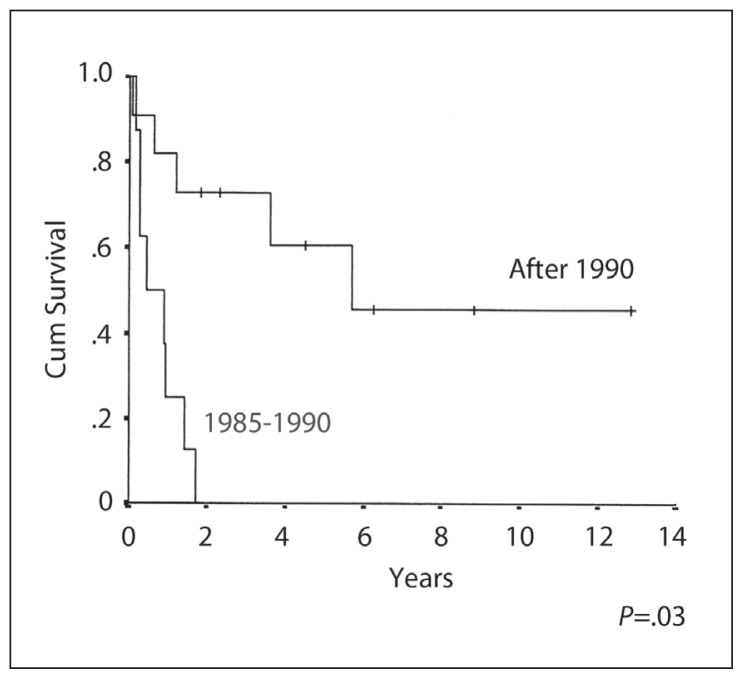
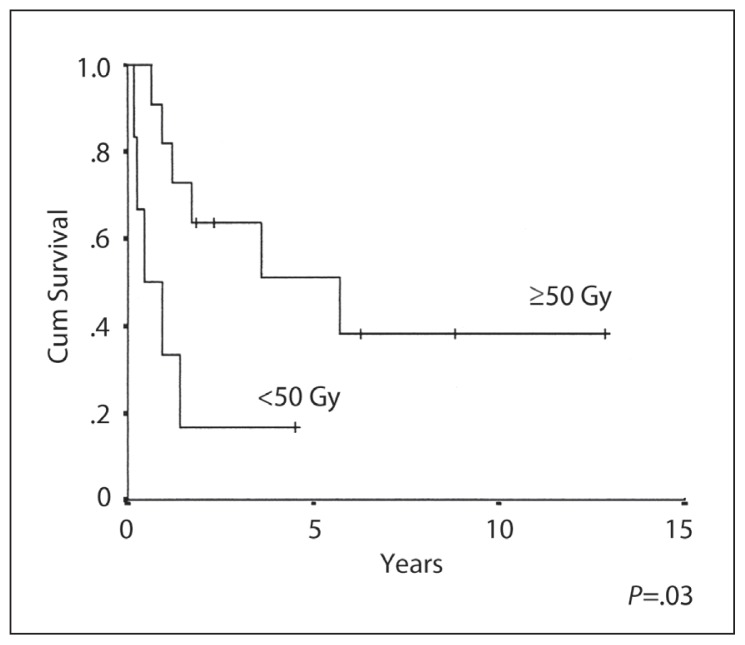
The 10-year OS, EFS and RFS rates were 26%, 29% and 46% respectively (Figures 1–3). The median duration of follow-up was 3 (range 1–13) years. CR was achieved in 13/19 (68%) patients. Six patients (32%) developed progressive disease during initial chemotherapy, and were excluded from the RFS analysis. The significant prognostic variables in univariate analysis were age, (OS 48% at 5 years for patients <16 years old and 0% ≥ 16 years, P=.01) (Figure 4), the time period of diagnosis, (OS at both 5 and 10 years was 45% for patients treated after 1990 and 0% for the period 1985–1990; P=.002) (Figure 5). For the radiation tumor dose, the five-year OS was 52% when the dose was ≥ 50 Gy (11 patients) compared with 17% when the dose was <50 Gy (6 patients) (P=.03) (Figure 6). In patients without spinal metastases at diagnosis, there were no significant differences in either local control or survival rates whether they were treated with CSRT, whole or partial brain RT.
Figure 4.
Overall survival and age in NGGCT.
Figure 5.
Overall survival by the time period of treatment.
Figure 6.
Overall survival and tumor dose in NGGCT.
Eleven patients died of disease, either from progression (PD) (5) or from relapse after an initial CR (6). The sites of relapse were local alone (3 patients), local plus spine (2 patients), local and extra-CNS metastases in one patient (liver and abdominal metastases). Five of the six patients with progressive disease died without achieving a remission.
DISCUSSION
Many important questions regarding the management of intracranial GCTs remain unanswered. The literature consists mainly of retrospective reviews and a small number of prospective single or multiple institution pilot studies.4–6 Prospective randomized studies have not been practical because of the small number of cases. Most single-institution series have included patients without tissue diagnosis, and some have lumped together germinomas and all forms of NGGCT when reporting outcome, making it difficult to determine the optimal management.
For germinoma, CSRT is the standard treatment with an OS approaching 100%. The minimum effective dose and volume of radiation alone, in non-disseminated cases, remains undetermined. The influence of adding effective chemotherapy on these parameters is under investigation. A controversial issue is whether the spine needs to be irradiated when there is no evidence of dissemination. For localized germinomas, the standard of care has shifted from CSRT to localized RT with4,7–9 or without chemotherapy.10,11 The overall EFS rates appear to be the same for patients treated with RT or RT plus chemotherapy. CMT with neo-adjuvant chemotherapy has been explored with the objective of reducing radiation volumes and doses in young patients with limited disease (M0). Whereas there is an agreement upon the use of lower RT doses for subclinical disease with bimodal treatment (24–30 Gy CSRT, 40–45 Gy to the primary) in patients with disseminated disease,12 controversies still exist regarding the definition of focal RT field volume for localized disease. For patients with limited disease, the initial RT volume varies from whole brain to partial brain or ventricular radiotherapy.4,5 Conclusions are hard to draw, given the variations in patient selection and dose and volume for radiation given. In the SIOP, SFOP and MAKEI experiences, using chemotherapy with focal RT to tumor bed plus margin was associated with an excess relapse in the ventricular area of about 10% compared to the standard CSRT.4–6 The spinal failure rates reported in the literature vary from 6%–36%.4,5,13 On the other hand, a limited review that assessed five retrospective studies published after 1997, estimated an isolated spinal relapse rate of 9% in patients who received no spinal RT.14 Two US studies that combined multicenter experiences in the treatment of intracranial GCT, concluded that CSRT is not indicated in the treatment of localized germinoma.15
The benefit of chemotherapy in germinomas is not yet proven; it is not clear whether with longer follow-up, CMT would substantially reduce late effects in comparison to CSRT, or whether CMT could be inferior because of the reduction in RT doses and volumes. Also, chemotherapy-related toxicity is not adequately reported in all studies. In a study from the Joint Center for Radiation Therapy, and Dana Farber Cancer Institute, Boston, that included 40 patients with the diagnosis of intracranial germinoma, the authors concluded that lower doses of CS-RT without chemotherapy appear to produce equally effective DFS and OS as do higher doses of RT or combination chemotherapy and RT.10
In a recent study from Japan, 165 patients with localized intracranial germinoma were treated with cranial RT without spinal irradiation. Fifteen patients (9.1%) developed spinal recurrences. In this study, large intracranial germinomas (>4 cm) and multifocal disease were independent risk factors for spinal relapse. Radiation field, RT dose and the use of chemotherapy did not affect the occurrence of spinal recurrences. Salvage treatment with chemotherapy and RT was effective in controlling the recurrent disease.16
Among the 31 patients with germinomas in our series, there was only one incidence of failure in a patient who was initially treated with chemotherapy followed by whole-brain RT. He developed isolated relapse in the spine, and died of disease progression despite salvage therapy.
In NGGCT, older reports on RT alone gave poor results with an OS of about 25%. Chemotherapy alone has been abandoned due to CNS progression and a relapse rate of 50%. Multimodality treatment integrating surgery, chemotherapy plus RT has become the standard over the last 25 years and has resulted in an OS increase to about 70%.17,18 This survival improvement has been attributed to advances in diagnostic imaging and surgical technique, the use of platinum-based multi-agent chemotherapy, and CSRT. The Japanese group undertook a Phase II study to establish post-surgical combined chemotherapy and radiation therapy for primary intracranial GCTs, using different chemotherapy regimens for different prognostic groups based on histology.19 Also, a collaborative effort led to the development of the International Germ Cell Cancer Collaborative Group (IGCCCG) risk criteria.20
Outcomes of NGGCTs depend on tumor type, hence the importance of establishing an accurate histological diagnosis.21 This is often complicated by difficulties in obtaining adequate biopsy, and sampling errors in such heterogeneous tumors. Biopsy is mandatory except in patients where tumor markers are elevated at the time of presentation. While the desired extent of resection in germinomas is unclear,22 total or subtotal resection, when practical with acceptable morbidity, was a common practice for NGGCT. The extent of initial surgical resection showed a prognostic significance in some series,23 but not in others.8 The introduction of highly effective cisplatin-based neoadjuvant chemotherapy obviated the need for upfront extensive resection in NGGCTs, since delayed resections have proven to be more complete and technically less difficult. In the SIOP 96 study, 40 patients with NGGCTs underwent surgery: 26 stereotactic procedures, 10 open biopsies and 33 attempted resections. There were 14 significant surgical complications, including three deaths. It was concluded that primary surgery was not beneficial for NGGCT patients.18
Recently, minimally invasive interventions such as endoscopic tumor biopsy (ETB) were introduced for establishing tissue diagnosis in pineal and third ventricle tumors.24 These interventions have gained preference over open surgical techniques in many centers, since they seem to have lower complications and mortality than open craniotomy and sterotactic biopsy. It is not quite clear whether ETB is as accurate as stereotactic or open surgical biopsy. In a neurosurgical series from the UK, failure to provide an adequate endoscopic biopsy for accurate histological characterization has been reported in only 24% of the cases.24
There is scarce data addressing the appropriate RT field in NGGCT. Bimodal therapy with localized RT has been used for localized tumors. The question remains whether there are indications for CSRT other than tumor dissemination. In-field relapse or progression during RT is not uncommon, and RT doses >50 Gy are used. Salvage by second-line therapy is possible, but with a lower success rate than for germinoma.
In our series, except for one patient, local relapse in the primary site was a component of all failures. After chemotherapy for NGGCT of the brain or gonads, patients may continue to have residual radiologic abnormalities that may represent teratoma, necrosis, or residual GCT.25 Teratoma is unresponsive to chemotherapy, and second surgical resection is the only effective treatment. This also applies to patients whose tumor markers have not normalized after initial chemotherapy, where second complete surgical resection may be more critical than recourse to more chemotherapy. This indicates the need for more aggressive local control primarily, whether in surgical approaches, and possibly higher RT doses in patients with residual disease. It also highlights the need for more intensive chemotherapy.
In the current series, the study time period showed to be a significant variable; there were no survivors among the eight patients with NGGCT treated between 1985–1989 as compared to a 45% five-year survival rate for patients treated after 1990 (11 patients). This could be attributed to improved anesthesia, neurosurgical and radiation therapy techniques, and better staging with the introduction of MRI at KFSHRC since 1990. Among the eight patients who were treated before 1990, none had MRI study of the spine. Some of these patients may have had disseminated disease that could have contributed to the poor outcome. The most important variable seems, however, to be the use of more aggressive platinum-based pediatric protocols, and better aggressive supportive treatment at our institution since 1990.
The superior OS in patients younger than 16 years of age (5-year OS 48% for <16 years old and 0% ≥ 16 years, P=0.01), is probably attributed to the use of more aggressive chemotherapy and supportive treatment pediatric protocols for this age group, as compared to the adult protocols used at our institution. In the early years of the study, three adult patients with NGGCT were treated with RT only without chemotherapy, which could be another contributing factor.
Tissue diagnosis of GCTs is mandatory prior to treatment except for patients with elevated markers. In germinoma, localized RT for M0 patients may be adequate. The determination of the RT volume and minimum effective dose remains to be determined. Long-term follow-up is needed to define the benefit of adding chemotherapy. For NGGCT, the use of CMT, and RT dose >50 Gy are important factors that influence outcome. The determination of which patients may be treated with local RT is the main question. Second-look surgery and resection of residual/refractory tumors is always recommended.
REFERENCES
- 1.Jennings M, Gelman R, Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg. 1985;63:155–67. doi: 10.3171/jns.1985.63.2.0155. [DOI] [PubMed] [Google Scholar]
- 2.Schild S, Scheithauer B, Haddock M. Histologically confirmed pineal tumors and other germ cell tumors of the brain. Cancer. 1996;78:2564–71. doi: 10.1002/(sici)1097-0142(19961215)78:12<2564::aid-cncr16>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson JC, Punt J, Hale J, Saran F, Calaminus G Germ Cell Tumour Working Groups of the United Kingdom Children’s Cancer Study Group (UKCCSG) and International Society of Paediatric Oncology (SIOP) Neurosurgical management of pediatric germ cell tumors of the central nervous system- a multi-disciplinary team approach for the new millennium. Br J Neurosurg. 2002;16:93–5. doi: 10.1080/02688690220131688. [DOI] [PubMed] [Google Scholar]
- 4.Bouffet E, Baranzelli MC, Patte C, Portas M, Edan C, Chastagner P, et al. Combined treatment modality for intracranial germinomas: Results of a multicentre SFOP experience. Societe Francaise d’Oncologie Pediatrique. Br J Cancer. 1999;79:1199–204. doi: 10.1038/sj.bjc.6690192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alapetite C, Ricardi U, Saran F. Whole ventricular irradiation in combination with chemotherapy in intracranial germinomas: The consensus of the SIOP CNS GCT study group. Med Pediatr Oncol. 2002;39:984–9. [Google Scholar]
- 6.Cabminus G. Update SIOP CNS GCT 96. Abstracts 2nd International Symposium CNS GCT; 2005. [Google Scholar]
- 7.Bamberg M, Kortmann RD, Calaminus G, Becker G, Meisner C, Harms D, et al. Radiation therapy for intracranial germinoma: Results of the German cooperative prospective trials MAKEI 83/86/89. J Clin Oncol. 1999;17:2585–92. doi: 10.1200/JCO.1999.17.8.2585. [DOI] [PubMed] [Google Scholar]
- 8.Cefalo G, Giami M, Lombardi F. Intracranial germinoma: Does a cisplatinum based chemotherapeutic regimen permit to avoid central nervous system irradiation. Med Pediatr Oncol. 1995;25:303. [Google Scholar]
- 9.Kellie SJ, Boyce H, Dunkel IJ, Diez B, Rosenblum M, Brualdi L, et al. Primary chemotherapy for intracranial non-germinomatous germ cell tumors: Results of the Second International Central Nervous System Germ Cell Tumor Study Group Protocol. J Clin Oncol. 2004;22:846–53. doi: 10.1200/JCO.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Aoyama H, Shirato H, Ikeda J, Fujieda K, Miyasaka K, Sawamura Y. Induction chemotherapy followed by low-Dose Involved-field radiotherapy for intracranial germ cell tumors. J Clin Oncol. 2002;20:857–65. doi: 10.1200/JCO.2002.20.3.857. [DOI] [PubMed] [Google Scholar]
- 11.Hardenbergh PH, Golden J, Billet A, Scott RM, Shrieve DC, Silver B, et al. Intracranial germinoma: The case for lower dose radiotherapy. Int J Radiat Oncol Biol Phys. 1997;39:419–26. doi: 10.1016/s0360-3016(97)00330-1. [DOI] [PubMed] [Google Scholar]
- 12.Shibamoto Y, Takahashi M, Abe M. Reduction of the radiation dose for intracranial germinoma: a prospective study. Brit J Cancer. 1994;70:984–9. doi: 10.1038/bjc.1994.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibamoto Y, Abe M, Yamashita J, Takahashi M, Hiraoka M, Ono K, et al. Treatment results of intracranial germinoma as a function of the irradiated volume. Int J Radiat Oncol Biol Phys. 1988;15:285–90. doi: 10.1016/s0360-3016(98)90006-2. [DOI] [PubMed] [Google Scholar]
- 14.Rogers SJ, Mosleh-Shirazi MA, Saran FH. Radiotherapy for localised intracranial germinoma: time to sever historical ties? Review. Lancet Oncol. 2005;6:509–19. doi: 10.1016/S1470-2045(05)70245-X. [DOI] [PubMed] [Google Scholar]
- 15.Paulino AC, Wen BC, Mohhideen MN. Controversies in the management of intracranial germinomas. Oncology (Huntingt) 1999;13:513–21. [PubMed] [Google Scholar]
- 16.Haas-Kogan DA, Missett BT, Wara WM, Donaldson SS, Lamborn KR, Prados MD, et al. Radiation Therapy for intracraniial germ cell tumors. Int J Radiat Oncol Biol Phys. 2003;56:511–8. doi: 10.1016/s0360-3016(02)04611-4. [DOI] [PubMed] [Google Scholar]
- 17.Ogawa K, Yoshi Y, Shikama N, Nakamura K, Uno T, Onishi H, et al. Spinal recurrence recurrence from intracranial germinoma: Risk factors and treatment outcome for spinal recurrence. Int J Radiat Oncol Biol Phys. 2008;172:1347–54. doi: 10.1016/j.ijrobp.2008.03.055. [DOI] [PubMed] [Google Scholar]
- 18.Calaminus G, Bamberg M, Baranzelli MC, Benoit Y, di Montezemolo LC, Fossati-Bellani F, et al. Intracranial germ cell tumors: A comprenensive update of the European data. Neuropediatrics. 1994;25:26–32. doi: 10.1055/s-2008-1071577. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson JC, Punt J, Hale J, Saran F, Calaminus G Germ Cell Tumour Working Groups of the United Kingdom Children’s Cancer Study Group (UKCCSG) and International Society of Paediatric Oncology (SIOP) Neurosurgical management of pediatric germ cell tumors of the central nervous system- a multi-disciplinary team approach for the new millennium. Br J Neurosurg. 2002;16:93–5. doi: 10.1080/02688690220131688. [DOI] [PubMed] [Google Scholar]
- 20.Matsutani M Japanese Pediatric Brain Tumor study Group. Combined chemotherapy and radiation therapy for CNS germ cell tumors – the Japanese experience. J Neuro Oncol. 2001;54:311–6. doi: 10.1023/a:1012743707883. [DOI] [PubMed] [Google Scholar]
- 21.IGCCCG. International germ cell consensus classification: A prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- 22.Sawamura Y, Ikeda J, Shirato H, Tada M, Abe H. Germ cell tumors of the central nervous system: treatment consideration based on 111 cases and their long term clinical outcomes. Euro J Cancer. 1998;34:104–10. doi: 10.1016/s0959-8049(97)10045-4. [DOI] [PubMed] [Google Scholar]
- 23.Krieger M, Diez B, Kellie J, et al. The impact of surgical resection on the outcome of CNS germinomas: The results of the International CNS GCT Study Group. NS3-Abstracts for The Second Int Symposium on CNS Germ Cell Tumors; Nov; LA, California. 2005. [Google Scholar]
- 24.Fizzy K, Tjulandin S, Salvioni, Germa-Liuch JR. Viable malignant cells after primary chemotherapy for disseminated nonseminomatous germ cell tumors: Prognostic factors and role of postsurgery chemotherapy- Results from an International Study Group. J Clin Oncol. 2001;19:2647–57. doi: 10.1200/JCO.2001.19.10.2647. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien DF, Hayhurst C, Pizer B, Mallucci CL. Outcomes in patients undergoing single-trajectory third ventriculostomy and endoscopic biopsy for midline tumors presenting with hydrocephalus. J Neurosurg. 2006;105(Suppl3):219–26. doi: 10.3171/ped.2006.105.3.219. [DOI] [PubMed] [Google Scholar]
- 26.Aide N. Enlarging residual mass of a nonseminomatous germ cell tumor: Growing teratoma syndrome or cancer recurrence. Diagnosis in Oncology. J Clin Oncol. 2007;25:4494–6. doi: 10.1200/JCO.2007.12.7530. [DOI] [PubMed] [Google Scholar]