Abstract
BACKGROUND AND OBJECTIVES
Currently, radiotherapy with concomitant and adjuvant temozolomide has become the standard treatment for glioblastoma. The purpose of this study was to report our experience with radiation plus concomitant temozolomide in 116 patients with glioblastoma multiforme (GBM) and examine the value of different prognostic factors.
DESIGN AND SETTING
Retrospective analysis of 116 patients with newly diagnosed GBM, who were treated at our department between January 1994 and March 2009.
PATIENTS AND METHODS
Age, gender, Karnofsky performance scale (KPS) score, a preoperative history of seizures, extent of surgery, total radiotherapy dose, and use of concomitant and adjuvant temozolomide were evaluated in uni- and multivariate analyses. Survival was determined using the Kaplan-Meier method, and differences were compared using the log rank test. Cox regression analysis was conducted to identify the independent prognostic factors.
RESULTS
The median overall survival time was 9 months, and the 1- and 2-year survival rates were 41.9% and 9.6%, respectively. The univariate analysis revealed that age, KPS score, presence of seizures, radiation doses, and use of concomitant and adjuvant temozolomide were significant prognostic factors. The multivariate analysis confirmed that the age, KPS score, presence of seizures, radiation doses, and use of concomitant and adjuvant temozolomide were independent, significant prognostic factors.
CONCLUSIONS
The results of our analyses demonstrate that radiation with concomitant and adjuvant temozolomide yields encouraging outcomes in patients with GBM, validating the results published in research papers. In addition, age, KPS score, presence of seizures, and radiation doses were identified as prognostic factors.
Malignant gliomas are the most common type of brain tumors. They are the most rapidly growing and destructive of brain tumors.1 In patients who present with glioblastoma multiforme (GBM), in spite of the macroscopic debulking surgery and effective adjuvant radiation therapy (RT), the median survival is generally only 9 to 12 months with <15% of patients surviving 2 years after diagnosis. 2 Temozolomide (TMZ) is an oral alkylating agent with a significant antitumor activity in brain tumors.3 The trial by the European Organization for Research and Treatment of Cancer (EORTC) and the National Cancer Institute of Canada (NCIC) Clinical Trials Group (EORTC 26981/22981-NCIC CE3) was the first study to show that concomitant TMZ and RT yields significant benefits.4 The encouraging results of this study have led to the use of concomitant TMZ with RT followed by 6 cycles of adjuvant TMZ as the standard treatment for GBM.3 It is important, however, to evaluate if the same results can be achieved in clinical practice within other patient populations. The aim of our retrospective analysis was to evaluate the role of the use of RT plus concomitant and adjuvant TMZ and examine the values of different prognostic factors in 116 patients with GBM. We believe that this analysis on the use of concomitant and adjuvant TMZ is the first report from our region.
PATIENTS AND METHODS
We conducted a retrospective analysis of the data on 116 patients with newly diagnosed GBM who were treated at our department between January 1994 and March 2009. The median age was 57.5 years (19–75 years), and the male: female ratio was 1.8. The following prognostic factors were analyzed: age, sex, performance status, history of seizures, extent of surgery, RT dose, and use of concomitant and adjuvant TMZ. Patient distribution regarding the examined factors and patient characteristics are presented in detail in Table 1.
Table 1.
Characteristics of patients treated with radiotherapy.
| Characteristic | n (116) | % |
|---|---|---|
|
| ||
| Age (years) | ||
| ≤50 | 38 | 33 |
| >50 | 78 | 67 |
| Gender | ||
| Male | 75 | 65 |
| Female | 41 | 35 |
| Karnofsky performance status | ||
| <70 | 37 | 32 |
| ≥70 | 79 | 68 |
| Presence of seizures | ||
| Yes | 16 | 14 |
| No | 100 | 86 |
| Extent of surgery | ||
| Complete resection | 66 | 57 |
| Subtotal resection | 50 | 43 |
| Radiotherapy dose | ||
| <60 Gy | 17 | 15 |
| ≥60 Gy | 99 | 85 |
| Concomitant TMZ | ||
| Yes | 21 | 18 |
| No | 95 | 82 |
TMZ: Temozolomide.
RT was delivered with cobalt-60 before December 2006. RT was administered using a linear accelerator with 6 to 18 MV photons on the basis of a 3-dimensional treatment planning after December 2006. The planning target volume was determined on the basis of preoperative CT or MRI scans and encompassed the contrast-enhancing area and surrounding edema plus a 2-cm margin.5 After RT at 46 to 50 Gy, the target volume was reduced to a contrast-enhancing area with a 2-cm margin. Thirty-five patients received whole brain irradiation in the initial field. Whole brain irradiation was preferred because of diffuse edema and was not a treatment policy. RT was given at 2 Gy/d, 5 days a week. The total mediaan dose was 60 Gy (range 10–66 Gy). The lower total doses were seen in patients with early disease progression during the course.
Concomitant with RT, 21 patients received daily oral TMZ at 75 mg/m2/d dose (7 days a week) with weekly blood count controls from the first to the last day of RT, up to 49 days. TMZ was administered on an empty stomach, with antiemetics as required, 1 hour before radiation and in the morning on days without radiation.6 During combined RT and TMZ, patients received oral co-trimoxazole on alternate days for 6 weeks as a prophylaxis against Pneumocystis carinii infection. Four weeks after the end of concomitant TMZ and RT, patients received adjuvant TMZ at 150 to 200 mg/m2 on days 1 to 5 with a 28-day interval. The treatment was continued for 6 cycles or until the progression of tumor or toxicity developed. Five patients in the group who were not administered concomitant TMZ received TMZ after RT. During the treatment, all the patients were assessed on a weekly basis by a radiation oncologist. Thereafter, neurologic and performance evaluations were conducted at approximately 3-month intervals. CT/MRI was obtained periodically and/or as indicated by changes in the neurologic or performance status.
Survival was measured from the time of the initial operation until the patient died or until the final analysis. Survival rates were determined by the Kaplan-Meier method. Differences between survival curves were analyzed by the log rank test. Uni- and multivariate analyses were conducted using the Cox proportional hazard model.
RESULTS
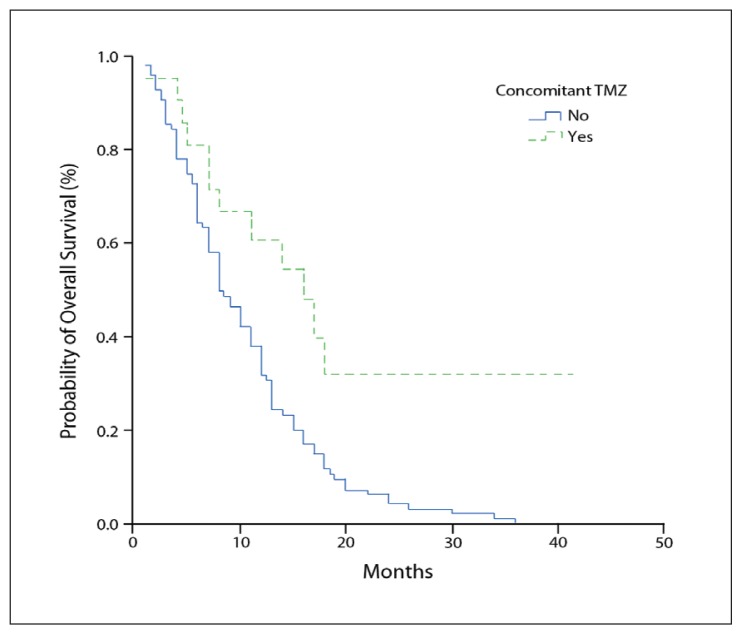
The median actuarial survival for the entire study population was 9 months (standard error 1.01; 95% CI 7.02–10.98). The 1- and 2-year survival rates were 41.9% and 9.6%, respectively. Nine patients were alive at the time of final analyses. The results of the univariate analysis are summarized in Table 2. The univariate analysis showed that age, performance status, history of seizures, RT dose, and use of TMZ were significant prognostic factors. Age was a significant prognostic factor; patients older than 50 years had a poorer prognosis than younger patients (7 vs. 13 months; P=.0008). The median survival for patients with a Karnofsky performance scale (KPS) score of ≥70 was 12 months, while that for patients with a KPS score of <70 was only 5.5 months. A significant difference was observed in the median survival according to the KPS score (P=.0004). The median survival for 16 patients with a history of seizures prior to diagnosis was 15 months, whereas that for 100 patients with no such history was 8 months. Thus, the presence of seizure activity was associated with better survival (P=.02). The median survival for all patients in the low-dose group was 4 months, whereas that for the high-dose group was 11 months. Thus, the dose of radiation also proved to have a significant impact on survival (P=.0008). The use of concomitant TMZ was another statistically significant prognostic variable in terms of overall survival (median survival 16 vs. 8 months; P=.0019) (Figure 1). The median overall survival of 5 patients in the group who were administered TMZ after, and not concomitantly with, RT was 10 (4.93) months.
Table 2.
Results of univariate analysis for all patients.
| Characteristic | Median survival (months) | 95% CI | P a |
|---|---|---|---|
|
| |||
| Age (years) | .0008 | ||
| ≤50 | 13 | 11.06–14.94 | |
| >50 | 7 | 5.70–8.3 | |
| Gender | NS | ||
| Male | 10 | 7.52–12.48 | |
| Female | 8 | 4.87–11.13 | |
| Karnofsky performance status | 0004 | ||
| <70 | 5.5 | 3.71–7.29 | |
| ≥70 | 12 | 10.13–13.87 | |
| Presence of seizures | .0203 | ||
| Yes | 15 | 9.12–20.88 | |
| No | 8 | 6.37–9.63 | |
| Extent of surgery | NS | ||
| Complete resection | 10 | 6.55–13.45 | |
| Subtotal resection | 8 | 5.28–11.52 | |
| Radiotherapy dose | .0008 | ||
| <60 Gy | 4 | 0.64–7.36 | |
| ≥60 Gy | 11 | 8.94–13.06 | |
| Concomitant TMZ | .0019 | ||
| Yes | 16 | 9.30–22.70 | |
| No | 8 | 6.09–9.91 | |
Versus two characteristics in each category
TMZ: Temozolomide; NS: not significant.
Figure 1.
Kaplan–Meier estimates of overall survival with concomitant temozolomide (TMZ) administration.
The multivariate analysis showed that age (P=.01), KPS score (P=.0093), history of seizures (P=.022), RT dose (P=.0001), and use of TMZ (P=.0071) were significant prognostic factors. The results of the multivariate analysis are summarized in Table 3. During concomitant TMZ and RT, 3 patients (14%) had grade 1 to 2 thrombocytopenia and 2 (9%) had grade 3 thrombocytopenia. For 1 patient (5%), the concomitant TMZ and RT administration was discontinued because of grade 4 hematologic toxicity. No nonhematologic toxicity was reported.
Table 3.
Results of multivariate analysis for all patients.
| Characteristic | P | RR | 95% CI |
|---|---|---|---|
|
| |||
| Age | .01 | 0.0744 | 1.1532–2.8611 |
| Gender | .0559 | 0.0445 | 0.9889–2.4597 |
| Karnofsky performance status | .0093 | −0.0755 | 0.3281–0.8550 |
| Presence of seizures | .0220 | 0.0623 | 1.1026–3.5106 |
| Extent of surgery | .2701 | 0.0000 | 0.8298–1.9480 |
| Radiotherapy dose | .0001 | −0.1287 | 0.1770–0.5547 |
| Concomitant TMZ | .0071 | −0.0793 | 0.2196–0.7873 |
RR: Relative risk/hazard ratio; TMZ: temozolomide.
DISCUSSION
The prognosis of GBM is very poor. The 1- and 2-year survival rates for adults diagnosed with GBM were <30% and <15%, respectively.2,7 The median survival is generally only 9–12 months.2 In line with published studies, we obtained a median survival of 9 months, with 1- and 2-year survival probabilities of 41.9% and 9.6%, respectively. Thus, many clinical as well as therapeutic prognostic factors affect the survival of patients.
In agreement with several authors,8,9 we found that age is a significant indicator. We also confirmed the value of age by multivariate analysis (13 vs. 7 months). As reported in previously published articles,10,11 the results of the multivariate analysis in the current study show that the value of clinical status evaluated by the KPS score remains a prognostic factor for survival (12 vs. 5.5 months). Although seizures were the second most common complaint at the time of diagnosis,12 their occurrence has been evaluated in very few studies. The relationship between a history of seizures prior to GBM diagnosis and survival is still controversial. Curran et al13 showed that seizures were not a significant prognostic factor for survival. However, retrospective data support that seizures seem to have a positive prognostic impact.14–18 In our study, seizure occurrence was found to be a significant prognosis factor in uni- and multivariate analyses (15 vs. 8 months). This could be explained by the early diagnosis of the disease because of seizure occurrence.
The controversy about the extent of surgery in patients with malignant gliomas has not yet been completely resolved.19 Retrospective studies strongly suggest that patients with a subtotal resection do not live as long as those with gross total resections.7,20 In a large retrospective study of 416 patients with GBM who were treated at M.D. Anderson Hospital, a volumetric analysis of the extent of resection on postoperative MRI showed that at least 98% tumor resection resulted in a survival advantage compared with less complete resections (13.0 vs. 8.8 months).21 Thereafter, a large, prospectively randomized controlled Phase III trial was conducted. The results of the so-called 5-aminolevulinic acid (ALA) study were published by Stummer et al.22 This study demonstrated that the median overall survival from the time of surgery was 11.8 months in patients with residual contrast-enhancing tumor and was 16.9 months in patients without residual tumor. Subsequently, to assess the benefits of complete resections, Stummer et al19 and Pichlmeier et al23 reanalyzed the data from the patients from the ALA study. These studies demonstrated that patients with complete resection survived significantly longer than patients with incomplete resections. In our study, no correlation was observed between the extent of surgery and survival. This could be because of our lower patient number compared to that in large retrospective studies.
Various studies concerning the radiation dose in GBM have been reported. Walker et al24 reported a dose-response analysis using data from 420 patients treated on Brain Tumor Cooperative Group protocols. 24 A significant improvement in the median survival from 28 to 42 weeks in the groups treated with 50 to 60 Gy radiation doses was found. In addition, a Medical Research Council study of 443 patients also showed a significant survival advantage in patients who received 60 Gy compared to those who received 45 Gy (12 vs. 9 months).25 After confirming the relationship between the dose and response under the dose of 60 Gy, this dose (60 Gy) was accepted as the standard dose. Studies later investigated the effect of doses >60 Gy. The Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group randomized 253 patients to either whole brain irradiation at 60 Gy or at 60 Gy plus a 10 Gy boost to a limited volume.26 No benefit was observed for higher irradiation doses. In contrast, a retrospective study by Tanaka et al27 suggested a survival advantage for patients who were administered a high-dose conformal RT. Sixty-one patients with GBM who were administered conformal RT at 80 to 90 Gy were compared with a group of 60 patients who were administered conventional RT at 60 Gy. The median survivals were calculated as 16.2 versus 12.4 months, respectively. The results of our study suggest that >60 Gy doses achieved better results than <60 Gy doses (11 vs. 4 months). The results of the multivariate analysis showed that the RT dose was found to be the most important independent prognostic factor. In our opinion, the dose-response relationship is not justified at >60 Gy doses because at these doses, there is an increase in the risk of toxicity; thus, well-defined clinical studies are needed to assess this factor. Although conformal RT or intensity modulated RT may enable higher dose administration without an increased risk to surrounding structures, the value of dose escalation using these approaches remains unproven, and they should be used with caution, especially given the additional cost.
Several randomized clinical trials have examined the role of adjuvant chemotherapy on improving the survival of brain tumor patients.7 In an early meta-analysis, Fine et al28 showed a small survival advantage for chemotherapy following irradiation. Another meta-analysis performed on 12 randomized trials showed that only minimal survival advantage was observed with the addition of adjuvant chemotherapy.29 Despite this small benefit with concomitant administration of chemotherapeutics, new agents and treatment strategies continued to be researched.
TMZ is a lipophilic, second-generation alkylating agent that was developed especially for the treatment of malignant gliomas.20 TMZ induces G2-M arrest in glioma cells, thus synchronizing the cell cycle in a radiosensitive phase.30 In 2002, the presentation of Phase II data of 64 patients with GBM, who were treated with concomitant TMZ and RT, demonstrated an increased 2-year survival rate, and the treatment was well tolerated. 31 Further, a Phase III randomized study (EORTC 26981/22981-NCIC CE3) compared the survival of patients who were administered TMZ concomitantly with (75 mg/m2 daily), and after RT, continued TMZ (150–200 mg/m2, for 5 days, every 4 weeks) versus RT alone; a significant improvement was noted in the median survival from 12.1 to 14.6 months. This study also showed improvement in the 2-year survival rate from 10% to 26%.4 The 5-year analysis of the EORTC-NCIC trial was published in 2009, and the overall survival in the TMZ arm was 9.8%.32 The results of this trial are supported by another randomized study conducted in Greece on 130 patients.6 Patients treated with chemoradiotherapy exhibited better survival than patients treated with RT alone (median survival 13.4 vs. 7.7 months; 1-year survival 56.3% vs. 15.7%). These studies have significantly influenced clinical practice, resulting in the addition of TMZ to RT as the standard treatment for GBM.
A review of nonrandomized studies revealed that the median overall survival in 42 patients who had used concomitant and adjuvant TMZ was 16.4 months, and the 1- and 2-year survival rates were 67.0% and 29.4%, respectively.3 In another study on 41 Asian patients (patients who had received a dose of <50 Gy were excluded), the median survival of patients was 13.4 months, while that of patients treated with TMZ was 19.2 months; in addition, the median survival of the group that received only RT was 11.8 months. The improvement in survival by the addition of TMZ was thus found to be significant.2
In our study, we observed improved survival by using concomitant and adjuvant TMZ in both uni- and multivariate analyses (16 vs. 8 months). The median 1.5-year survival rates of patients administered TMZ and RT alone, were 31.8% and 14.7%, respectively. Both the median and 1.5-year survival rates in the TMZ group in our study were similar to those in the above-mentioned randomized studies. The 1.5-year survival rate of patients treated with RT alone was lower in our study than in the randomized studies. This finding could be explained by the fact that the treatment was discontinued in the patients of this group because of the low KPS score and progress during RT. In the published studies of glioma patients, up to 20% pseudoprogression has been reported to occur in patients who have been treated with concomitant TMZ.33 In our study group, we suspected pseudoprogression in 3 patients during radiological examination, but could not confirm it histopathologically.
In conclusion, the factors influencing survival are age, KPS score, preoperative seizure history, total RT doses, and usage of concomitant and adjuvant TMZ in GBM. With respect to overall survival, the RT dose was found to be the most significant prognostic factor. The median and 1.5-year survival outcomes of the study population in the TMZ group are comparable to the results of large multicentric studies and thus justify the continued use of TMZ in routine clinical practice.
REFERENCES
- 1.Combs SE, Gutwein S, Schulz-Ertner D, Kampen MV, Thilmann C, Edler L, et al. Temozolomide combined with irradiation as postoperative treatment of primary glioblastoma multiforme. Strahlenther Onkol. 2005;181:372–7. doi: 10.1007/s00066-005-1359-x. [DOI] [PubMed] [Google Scholar]
- 2.Back MF, Ang EL, Ng WH, See SJ, Lim CC, Chan SP, et al. Improved median survival for glioblastoma multiforme following introduction of adjuvant temozolomide chemotherapy. Ann Acad Med Singapore. 2007;36:338–42. [PubMed] [Google Scholar]
- 3.Jalali R, Basu A, Gupta T, Munshi A, Menon H, Sarin R, et al. Encouraging experience of concomitant temozolomide with radiotherapy followed by adjuvant temozolomide in newly diagnosed glioblastoma multiforme: Single institution experieance. Br J Neurosurg. 2007;21:583–7. doi: 10.1080/02688690701604574. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, Van Den Brent MJ. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Narayana A, Recht L, Gutin PH. Central nervous system tumors. In: Hoppe RT, Phillips TL, Roach M, editors. Textbook of Radiation Oncology. 3rd ed. Philadelphia: Elsevier Saunders; 2010. pp. 421–45. [Google Scholar]
- 6.Athanassiou H, Synodinou M, Maragoudakis E, Paraskevaidis M, Verigos C, Misailidou D, et al. Randomized phase II study of temozolamide and radiotherapy compared with radiotherapy alone in newly diagnosed glioblastoma multiforme. J Clin Oncol. 2005;23:2372–7. doi: 10.1200/JCO.2005.00.331. [DOI] [PubMed] [Google Scholar]
- 7.Brandes AA, Tosoni A, Franceschi E, Reni M, Gatta G, Vecht C. Glioblastoma in adults. Crit Rev Oncol Hematol. 2008;67:139–52. doi: 10.1016/j.critrevonc.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Ulutin C, Fayda M, Aksu G, Cetinayak O, Kuzhan O, Ors F, et al. Primary glioblastoma multiforme in younger patients: A single-institution experience. Tumori. 2006;92:407–11. doi: 10.1177/030089160609200507. [DOI] [PubMed] [Google Scholar]
- 9.Donato V, Papaleo A, Castrichino A, Banelli E, Giangaspero F, Salvati M, et al. Prognostic implication of clinical and pathologic features in patients with glioblastoma multiforme treated with concomitant radiation plus temozolomide. Tumori. 2007;93:248–56. doi: 10.1177/030089160709300304. [DOI] [PubMed] [Google Scholar]
- 10.Shinoda J, Sakai N, Murase S, Yano H, Matsuhisa T, Funakoshi T. Selection of eligible patients with supratentorial glioblastoma multiforme for gross total resection. J Neurooncol. 2001;52:161–71. doi: 10.1023/a:1010624504311. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa K, Aoki Y, Fujimaki T, Tago M, Terahara A, Karasawa K, et al. High-dose conformal radiotherapy influenced the pattern of failure but did not improve survival in glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1998;40:1141–9. doi: 10.1016/s0360-3016(97)00911-5. [DOI] [PubMed] [Google Scholar]
- 12.Salcman M. Supratentorial gliomas: Clinical features and surgical therapy. In: Wilkins RH, Rengachary SS, editors. Neurosurgery. New York: McGraw-Hill; 1985. pp. 579–90. [Google Scholar]
- 13.Curran WJ, Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–10. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 14.McKeran RO, Thomas DG. The clinical study of gliomas. In: Thomas DG, Graham DI, editors. Brain Tumors: Scientific Basis, Clinical Investigation, and Current Therapy. London: Butterworth; 1980. pp. 194–230. [Google Scholar]
- 15.Winger MJ, Macdonald DR, Cairncross JG. Supratentorial anaplastic gliomas in adults. The prognostic importance of extent of resection and prior low-grade glioma. J Neurosurg. 1989;71:487–93. doi: 10.3171/jns.1989.71.4.0487. [DOI] [PubMed] [Google Scholar]
- 16.Miller PJ, Hassanein RS, Giri S, Kimler GF, O’Boynick P, Evans RG. Univariate and multivariate statistical analysis of high-grade gliomas: The relationship of radiation dose and other prognostic factors. Int J Radiat Oncol Biol Phys. 1990;19:275–80. doi: 10.1016/0360-3016(90)90534-q. [DOI] [PubMed] [Google Scholar]
- 17.Mineo JF, Bordron A, Baroncini M, Ramirez C, Maurage CA, Blond S, et al. Prognosis factors of survival time in patients with glioblastoma multiforme: A multivariate analysis of 340 patients. Acta Neurochir (Wien) 2007;149:245–53. doi: 10.1007/s00701-006-1092-y. [DOI] [PubMed] [Google Scholar]
- 18.Ozbek N, Cakir S, Gursel B, Meydan D. Prognostic significance of seizure in patients with glioblastoma multiforme. Neurol India. 2004;52:76–8. [PubMed] [Google Scholar]
- 19.Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W, Tonn JC, et al. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery. 2008;62:564–76. doi: 10.1227/01.neu.0000317304.31579.17. [DOI] [PubMed] [Google Scholar]
- 20.Fiveash JB, Nordal RA, Markert JM, Ahmed RS, Nabors LB. High-grade gliomas. In: Gunderson LL, Tepper JE, editors. Clinical Radiation Oncology. 2nd ed. Philadelphia: Churcill-Livingstone; 2007. pp. 515–37. [Google Scholar]
- 21.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, DeMonte F, Lang FF, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–8. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 22.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 23.Pichlmeier U, Bink A, Schackert G, Stummer W ALA Glioma Study Group. Resection and survival in glioblastoma multiforme: An RTOG recursive partitioning analysis of ALA study patients. Neuro Oncol. 2008;10:1025–34. doi: 10.1215/15228517-2008-052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725–31. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- 25.Bleehen NM, Stenning SP. A Medical Research Council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. The Medical Research Council Brain Tumour Working Party. Br J Cancer. 1991;64:769–74. doi: 10.1038/bjc.1991.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson DF, Diener-West M, Horton J, Chang CH, Schoenfeld D, Nelson JS. Combined modality approach to treatment of milignant gliomas-re-evaluation of RTOG 7401/ECOG 1374 with long-term follow-up: A joint study of the Radiation Therapy Oncology Group and the Eastern Cooperative Oncology Group. NCI Monogr. 1988;6:279–84. [PubMed] [Google Scholar]
- 27.Tanaka M, Ino Y, Nakagawa K, Tago M, Todo T. High-dose conformal radiotherapy for supratentorial malignant glioma: A historical comparison. Lancet Oncol. 2005;6:953–60. doi: 10.1016/S1470-2045(05)70395-8. [DOI] [PubMed] [Google Scholar]
- 28.Fine HA, Dear KB, Loeffler JS, Black PM, Canellos GP. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71:2585–97. doi: 10.1002/1097-0142(19930415)71:8<2585::aid-cncr2820710825>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 29.Stewart LA. Chemotherapy in adult high-grade glioma: A systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–8. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 30.Hirose Y, Berger MS, Pieper RO. p53 Effects both the duration of G2/M arrest and the fate of temozolomide-treated human glioblastoma cells. Cancer Res. 2001;61:1957–63. [PubMed] [Google Scholar]
- 31.Stupp R, Dietrich PY, Kraljevic SO, Pica A, Maillard I, Maeder P, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002;20:1375–82. doi: 10.1200/JCO.2002.20.5.1375. [DOI] [PubMed] [Google Scholar]
- 32.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 33.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–61. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]