Abstract
Recently, several reports demonstrated the efficacy of neoadjuvant chemotherapy (NAC) or chemoradiotherapy (NACRT) for patients with borderline resectable (BRPC) and locally advanced unresectable pancreatic carcinoma (LAPC). The aim of this study was to evaluate the treatment response after NACRT, especially for nerve plexuses, and the optimal resection area for superior mesenteric artery nerve plexuses in BRPC and LAPC patients after NACRT.
A total of 17 patients with BRPC and LAPC received preoperative gemcitabine-based NACRT. The numbers of BRPC and LAPC patients were 13 and 4, respectively. We evaluated nerve plexus invasion by CT before and after NACRT, decided on the resection area of plexus invasion in SMA before NACRT, and compared the preoperative evaluation and clinicopathological findings.
In the plexus of the supra-mesenteric artery (pl-SMA), arterial nerve plexus invasion, in cases <90°, all patients showed the absence of residual cancer in the resected specimen after NACRT. In cases between 90° and 180°, 1 of 2 patients (50%) showed nerve plexus invasion. In cases over 180°, all patients showed nerve plexus invasion. We could perform R0 resection in all 10 cases, and pl-SMA invasion disappeared in 6 of 7 BRPC patients.
We demonstrated the relationship between the angle of nerve plexus tumor invasion and treatment effect after NACRT. We could perform R0 resection in all pl-SMA invasion cases, deciding on the resection area of pl-SMA based on CT before NACRT.
Keywords: NACRT, pancreatic cancer, the plexus of the supra-mesenteric artery
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related death in the United States.[1] Despite recent advances in diagnostic and therapeutic techniques, PDAC remains one of the most lethal malignancies. The 5-year survival rate of patients with primary PDAC, even after complete resection, is lower than 15%,[2] and the overall 5-year survival rate of patients with inoperable PDAC is very low, ranging from 0.4% to 4%.[3,4] Surgery with a curative intent remains the only therapeutic option with the potential for cure.[5,6] However, PDAC often invades major arteries and the portal vein system, and this makes surgical resection complicated and technically demanding. Therefore, a new treatment strategy for these patients is required.
Recently, several reports showed the efficacy of neoadjuvant chemotherapy (NAC) or chemoradiotherapy (NACRT) for patients with borderline resectable (BRPC) and locally advanced unresectable pancreatic carcinoma (LAPC).[7–11] However, it is difficult to decide on the appropriate area for R0 resection, especially when nerve plexuses invasion are near the supra-mesenteric artery (SMA) after NACRT.
The aim of this study was to evaluate the treatment response after NACRT, especially for nerve plexuses invasion in BRPC and LAPC patients, and to validate our surgical strategy on the dissection area of superior mesenteric artery nerve plexuses.
2. Materials and methods
2.1. Study design
This retrospective study reviewed all patients with BRPC or LAPC treated at Hokkaido University Gastroenterological Surgery I with gemcitabine-based NACRT between January 2005 and December 2014. The study was approved by the institutional review board of Hokkaido University.
2.2. Eligibility criteria
All protocols were performed after obtaining informed consent from all patients in accordance with the approved procedure at our hospital. Potential candidates for entry into this study underwent radiographic evaluation before treatment, including thin-slice abdominal computed tomography (CT) and magnetic resonance imaging (MRI). Patients were confirmed to meet the following criteria before the initiation of therapy: no radiographic evidence of metastatic disease, older than 20 years, radiographic evidence of tumor extension beyond the pancreas, resection criteria based on the NCCN guidelines 2016 version 2,[12] confirmed PDAC, based on pathological or cytological examination, before NACRT.
2.3. Neoadjuvant CRT
The NACRT regimen consisted of radiation therapy (50.4 gray in 28 fractions) combined with systemic chemotherapy, involving the intravenous administration of gemcitabine (150 mg/m2), concurrently initiated on days 1, 8, 15, 22, 29, and 36. After NACRT, we performed restaging imaging including CT to explore distant metastasis or the operative indication (Fig. 1).
Figure 1.
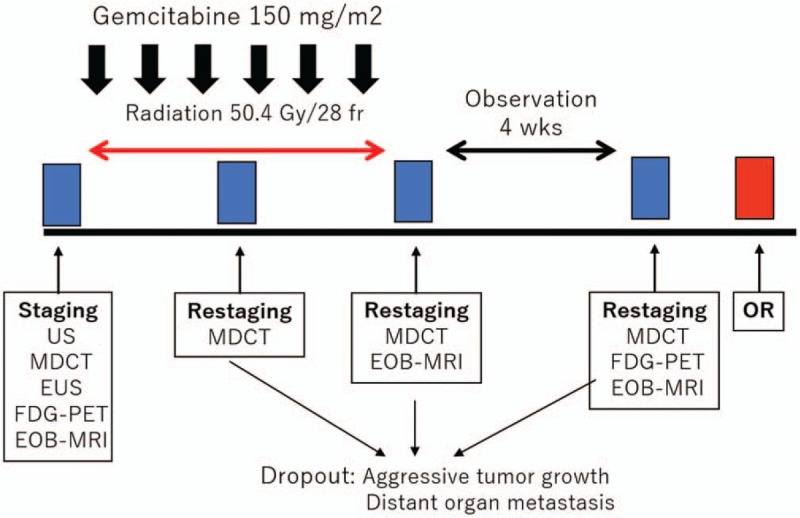
The treatment protocol of NACRT for BRPC and LAPC patients. Radiation was administrated at a total radiation dose of 50.4 Gy, 5 times per week. The intravenous administration of gemcitabine (150 mg/m2) was initiated on days 1, 8, 15, 22, 29, and 36. BRPC = borderline resectable pancreatic carcinoma, LAPC = unresectable pancreatic carcinoma, NACRT = neoadjuvant chemoradiotherapy.
2.4. Response assessment
Restaging was carried out 4 weeks after the end of NACRT by CT. Evaluation of the treatment efficiency for pancreatic cancer was performed according to Response Evaluation Criteria in Solid Tumor (RECIST).[13] Patients with a complete or partial response or stable disease with no distant metastasis underwent surgical exploration to determine whether the tumor was resectable.
2.5. Surgery
Surgical exploration was performed 5 to 6 weeks after the completion of NACRT. The first step of surgery involved total exploration of the abdominal cavity to rule out contraindications to resection, such as hepatic or peritoneal metastasis. After ruling them out, pancreatectomy and systematic lymphadenectomy were performed with a curative intent. Superior mesenteric vein and/or portal vein resection was carried out in cases with possible or definitive tumor invasion. Basically, we performed hemicircumferential dissection of the plexus of the supra-mesenteric artery (pl-SMA). The right side dissection was performed for pancreas head tumor, and the left side dissection for pancreas body and tail tumor. If the tumor showed an abutment exceeding 180° of the circumference of the SMA wall, the resection area was decided on the basis of CT before NACRT (Fig. 2A and B). In principle, combined arterial resection was not performed. All operations were conducted by the same surgical team, and all operative procedures were carried out in the same manner throughout the study period.
Figure 2.
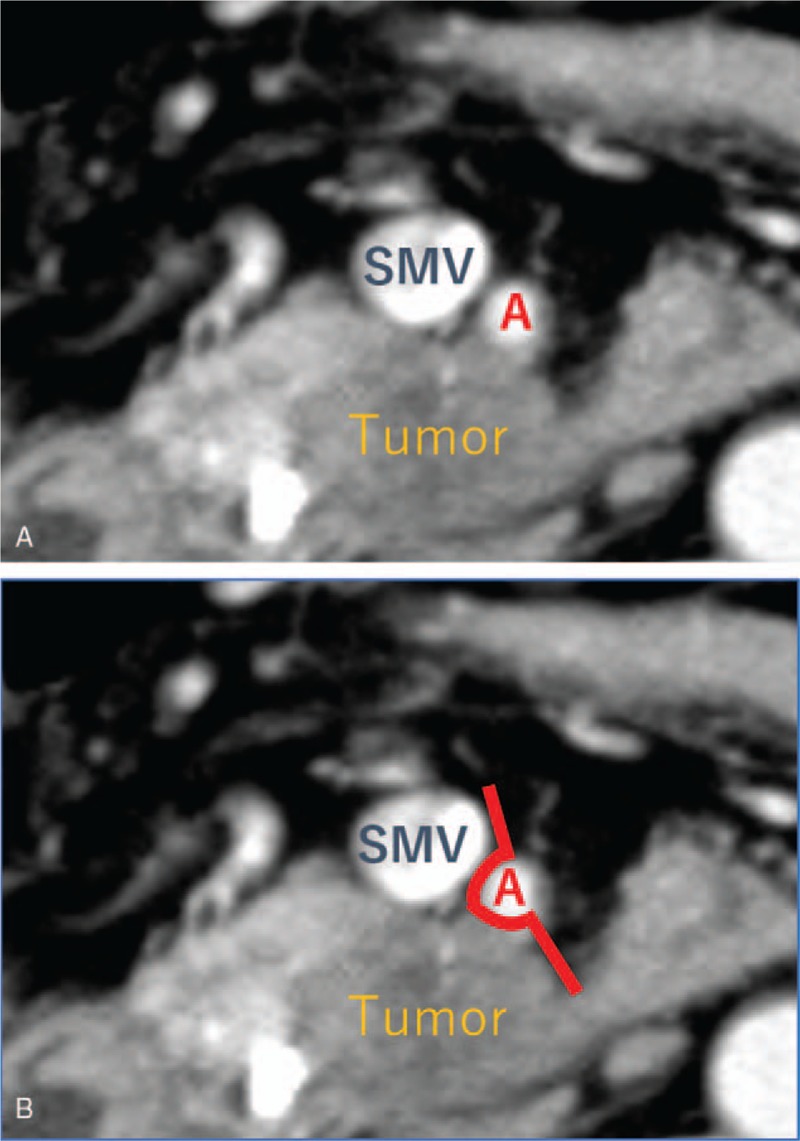
Representative example of how to decide on the resection area of pl-SMA. A BRPC patient. (A) A pancreatic head tumor showed an abutment not exceeding 180° of the circumference of the SMA wall, from 4 to 9 o’clock. (B) We decided on a resection area from 4 to 11 o’clock (bold line). BRPC = borderline resectable pancreatic carcinoma, pl-SMA = the plexus of the supra-mesenteric artery.
Adjuvant chemotherapy was basically applied unless contraindicated by the patient's condition. In short, the patients received treatments according to the protocols available at the time of treatment. Thus, the patients were given gemcitabine[14] or S-1.[15] Chemotherapy was initiated at < 2 months after the operation in all patients who were considered eligible for the treatment.
2.6. Pathologic examination
Macroscopic and microscopic examinations were performed of every pancreatic specimen according to the same procedure. Each specimen was oriented based on anatomic structures and surgical marks. Before cutting the specimen, sections of the margins were submitted. We produced shave sections of the bile duct margin, pancreatic margin, and retroperitoneal margin. Then, the specimen was cut into 5-mm slices, parallel to this first section. All specimens were fixed with 10% buffered formalin, and embedded in paraffin. Deparaffinized sections were stained with hematoxylin-eosin and examined by light microscopy.
2.7. Statistical analysis
Patient records were prospectively entered into our database and completed by information obtained from a retrospective review of hospital and physician's records. Analysis was performed using EZR.
3. Results
3.1. Patient demographics and tumor characteristics before NACRT
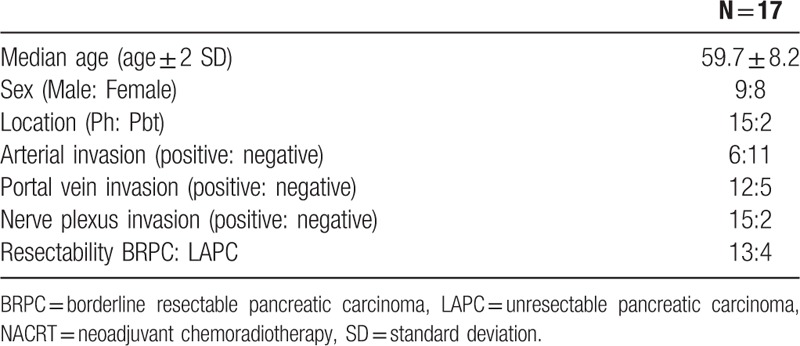
The mean patient age was 59.7 years old (SD, ±8.2 years). Nine patients were men and 8 were women. The location of the tumor was the pancreatic head in 15 patients and the body and/or tail in 2. Twelve cases were portal vein and/or supra-mesenteric vein invasion-positive and 5 were negative. Fifteen patients were nerve plexus invasion-positive and 2 were negative. Thirteen patients had BRPC, and 4 patients had LAPC (Table 1).
Table 1.
Patient demographics before NACRT.
3.2. Treatment response after NACRT
Of the 17 patients planned to receive neoadjuvant chemoradiotherapy, a median of 5 cycles (range: 3–6) of neoadjuvant gemcitabine were administered. No serious adverse effects associated with neoadjuvant chemotherapy and no treatment-related deaths occurred.
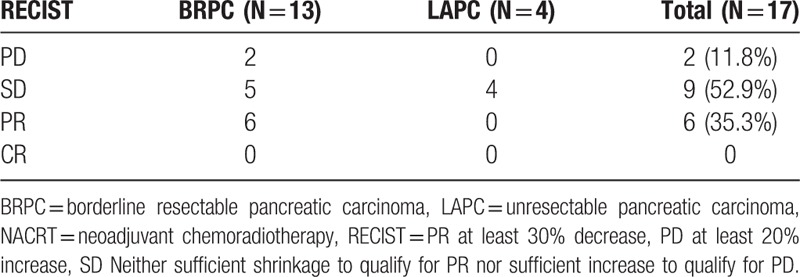
Radiological responses included partial remission in 6 patients, stable disease in 5 patients, and progressive disease in 2 patients among BRPC patients, and stable disease in 4 LAPC patients. No patient achieved complete remission (Table 2).
Table 2.
Treatment response after NACRT.
3.3. Comparison with CT evaluation of pre-NACRT and pathological findings
Comparisons with CT evaluation of pre-NACRT and clinicopathological factors with PDAC patients who received NACRT are shown in Table 3. Among the 17 patients, 5 of the 13 BRPC patients showed downstaging and 2 of the 4 LAPC patients showed downstaging. Only 4 patients had positive lymph nodes. BR patients showed lower rates of nerve plexus invasion. Seven (53.8%) of the BRPC patients were Evans classification IIb, and 3 (75%) of the LAPC patients were Evans classification IIb or III.
Table 3.
Comparison with CT evaluation of pre-NACRT and pathological findings.
3.4. CT evaluation and pathological response in pl-SMA patients
Ten patients, 7 BRPC and 3 LAPC patients, showed pl-SMA invasion before NACRT. Based on our resection strategy for pl-SMA, we performed R0 resection in all cases, and pl-SMA invasion disappeared in 6 of the 7 BR patients. Three of the 10 patients survived with no recurrence (Table 4).
Table 4.
CT Evaluation and pathological response in pl-SMA patients.
3.5. Arterial pl invasion between CT before NACRT and pathological findings in pl-SMA invasion-positive patients after NACRT
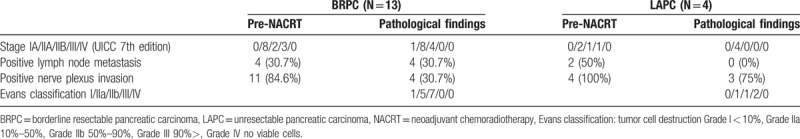
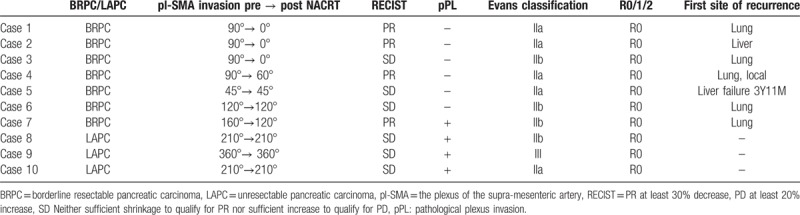
Fifteen of the 10 patients showed SMA contact before NACRT: 5 patients <90°, 2 patients between 90° and 180°, and 3 patients over 180°. In cases of <90°, all patients showed the absence of residual cancer in the resected specimens after NACRT. In the cases between 90° and 180°, 1 of the 2 patients (50%) showed pl-SMA invasion. In the cases over 180°, all patients showed pl invasion (Table 5). As shown in Figure 3, a 63-year-old female suffered from pancreatic head carcinoma that had contact with SMA of 120° before NACRT (Fig. 3A). After NACRT, the radiological tumor response was stable disease, and the tumor contact with SMA did not show any change (Fig. 3B). The macroscopic appearance of PDAC following NACRT can be seen in only small clusters within 2.1 cm. There is no tumor in pl-SMA. The initial size of the tumor is unidentifiable (Fig. 3C). Microscopically, extensive fibrosis is present surrounding the neural band. In addition, there are monocytes that invade the fibrosis (Fig. 3D).
Table 5.
Nerve plexus invasion between CT before NACRT and pathological findings in pl-SMA invasion-positive patients after NACRT.
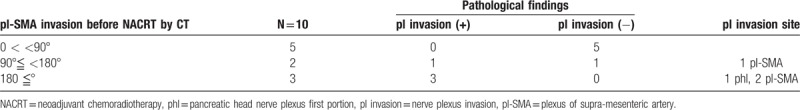
Figure 3.
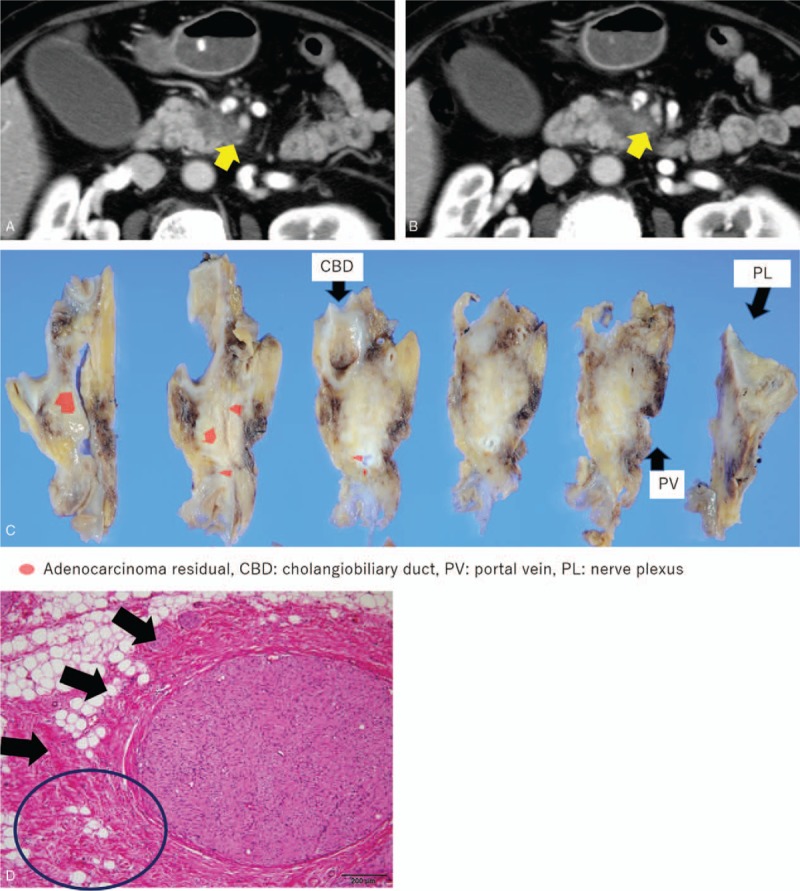
Representative case of BRPC, case 6, a 63-year-old female, pancreatic head carcinoma that had contact with SMA of 120° before NACRT (A). After NACRT, the tumor size was stable, and contact with the SMA remained unchanged (B). The macroscopic appearance of PDAC following NACRT can be seen only in small clusters within 2.1 cm. There is no tumor in pl-SMA. The initial size of the tumor is unidentifiable (C). Microscopically, extensive fibrosis is present surrounding the neural band. In addition, there are monocytes that invade the fibrosis (circled area in [D]). BRPC = borderline resectable pancreatic carcinoma, NACRT = neoadjuvant chemoradiotherapy, PDAC = pancreatic ductal adenocarcinoma, pl-SMA = the plexus of the supra-mesenteric artery, SMA = the plexus of the supra-mesenteric artery.
4. Discussion
This is the first report to describe the clinicopathological treatment effect of NACRT regarding pl-SMA invasion. We showed the relationship between the angle of nerve plexus tumor invasion before NACRT and the treatment effect after NACRT. And, we could perform R0 resection for all pl-SMA invasion cases, if the pl-SMA resection area was decided on the basis of CT before NACRT.
Several reports showed that patients who underwent complete pancreatic cancer resection had a favorable survival rate in comparison with surgical margin-positive cases.[16–18] However, surgery for BRPC and LAPC in contact with a major artery including the hepatic artery and SMA is highly controversial. The survival of patients who underwent combined resection and reconstruction of major arteries was reported to be significantly correlated with higher mortality rates.[19–21] More patients would be indicated for surgical treatment if tumor removal without co-resection of the arteries could be deemed oncologically feasible. Recently, neoadjuvant chemotherapy or chemoradiotherapy has been advocated for patients with BRPC and LAPC by several investigators.[7,22–25] Higher R0 resection rates after NACRT for patients with BRPC and LAPC have been reported.[7–10,26,27] A higher R0 resection rate might have contributed to the better overall survival of patients with arterial contact who received NACRT. However, little information is available on treatment strategies including artery-preserving surgery for BRPC and LAPC in contact with major arteries. The typical findings associated with this factor are the presence of reticular opacities abutting the arteries, and the grainy appearance of dense periarterial tissue on tri-phasic contrast-enhanced MDCT. According to our results, we can perform R0 resection of pl-SMA in all cases if we decide on the resection area based on CT before NACRT
Nerve plexus dissection has been routinely advocated in Japan for achieving margin-negative resection, because pancreatic cancer tends to extend along the nerve plexus. The 2 important issues are achieving the resection margin, especially to the SMA, and preserving pl-SMA at least to the hemicircle to avoid uncontrollable postoperative diarrhea. Inoue et al[28] reported that following right hemicircum-ferential pl-SMA dissection, 29% of patients suffered from diarrhea, which could be controlled successfully using opioids. Nimura et al[29] reported the results of a randomized controlled trial, which found that postoperative weight loss associated with severe diarrhea for more than 6 months was more frequently encountered in patients undergoing an extended surgical procedure than in those undergoing a standard procedure. Furthermore, postoperative adjuvant chemotherapy improves the survival of patients with resected pancreatic cancer.[15,30] It is important for patients to undergo R0 resection and receive adjuvant chemotherapy to achieve improved survival. So, balanced resection may be required to allow patients to successfully complete the planned dose of adjuvant chemotherapy. To achieve R0 and balanced resection, our findings indicate that we can reduce the resection area of pl-SMA based on intraoperative frozen-section analysis of the resection margin after NACRT.
The tumor size and stage after NACRT are key indicators of the therapeutic effect. However, RECIST criteria may not accurately reflect the tumor response.[7,31] In this study, radiographic downstaging of disease in patients with BRPC and LAPC after NACRT was rare. The replacement of the tumor with fibrosis, which is observed on the histologic examination of resected patients, and radioinduced edema, may explain why the radio response to NACRT is modest and does not correlate well with the histologic response.[32–35] Therefore, we recommend the aggressive use of surgery in patients who have BRPC and LAPC with a suitable performance status, fully controlled comorbidities, and an absence of metastatic disease after NACRT.
In conclusion, we have demonstrated the relationship between the angle of nerve plexus tumor invasion and treatment effect after NACRT. We could perform R0 resection in all patients with pl-SMA invasion.
Author contributions
Conceptualization: Takahiro Einama, Hirofumi Kamachi, Yuzuru Sakamoto, Kenji Wakayama, Tatsuya Orimo, Hideki Yokoo, Toshiya Kamiyama, Norio Katoh, Yusuke Uchinami, Tomoko Mitsuhashi, Akinobu Taketomi.
Data curation: Hirofumi Kamachi, Yosuke Tsuruga, Toshihiro Sakata, Kazuaki Shibuya, Yuzuru Sakamoto, Shingo Shimada, Kenji Wakayama, Tatsuya Orimo, Hideki Yokoo, Toshiya Kamiyama, Norio Katoh, Yusuke Uchinami, Tomoko Mitsuhashi, Akinobu Taketomi.
Formal analysis: Takahiro Einama, Hirofumi Kamachi, Shingo Shimada, Norio Katoh, Akinobu Taketomi.
Investigation: Hirofumi Kamachi, Akinobu Taketomi.
Methodology: Takahiro Einama, Hirofumi Kamachi, Kazuaki Shibuya, Norio Katoh, Akinobu Taketomi.
Project administration: Takahiro Einama, Hirofumi Kamachi, Akinobu Taketomi.
Supervision: Toshiya Kamiyama, Yusuke Uchinami, Tomoko Mitsuhashi, Akinobu Taketomi.
Writing – original draft: Takahiro Einama.
Footnotes
Abbreviations: BRPC = borderline resectable, CT = computed tomography, LAPC = locally advanced unresectable pancreatic carcinoma, MRI = magnetic resonance imaging, NAC = neoadjuvant chemotherapy, NACRT = neoadjuvant chemoradiotherapy, PDAC = pancreatic ductal adenocarcinoma, pl-SMA = the plexus of the supra-mesenteric artery, RECIST = Response Evaluation Criteria in Solid Tumor.
The authors have no funding and no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Matsuno S, Egawa S, Fukuyama S, et al. Pancreatic Cancer Registry in Japan: 20 years of experience. Pancreas 2004;28:219–30. [DOI] [PubMed] [Google Scholar]
- [3].Bramhall SR, Allum WH, Jones AG, et al. Treatment and survival in 13,560 patients with pancreatic cancer, and incidence of the disease, in the West Midlands: an epidemiological study. Br J Surg 1995;82:111–5. [DOI] [PubMed] [Google Scholar]
- [4].Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer 2001;37(suppl 8):S4–66. [DOI] [PubMed] [Google Scholar]
- [5].Nakao A, Takeda S, Inoue S, et al. Indications and techniques of extended resection for pancreatic cancer. World J Surg 2006;30:976–82. discussion 983-974. [DOI] [PubMed] [Google Scholar]
- [6].Cameron JL, Riall TS, Coleman J, et al. One thousand consecutive pancreaticoduodenectomies. Ann Surg 2006;244:10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012;118:5749–56. [DOI] [PubMed] [Google Scholar]
- [8].Sho M, Akahori T, Tanaka T, et al. Importance of resectability status in neoadjuvant treatment for pancreatic cancer. J Hepatobiliary Pancreat Sci 2015;22:563–70. [DOI] [PubMed] [Google Scholar]
- [9].Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015;261:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fujii T, Yamada S, Murotani K, et al. Inverse probability of treatment weighting analysis of upfront surgery versus neoadjuvant chemoradiotherapy followed by surgery for pancreatic adenocarcinoma with arterial abutment. Medicine (Baltimore) 2015;94:e1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yanagimoto H, Ishii H, Nakai Y, et al. Improved survival with combined gemcitabine and S-1 for locally advanced pancreatic cancer: pooled analysis of three randomized studies. J Hepatobiliary Pancreat Sci 2014;21:761–6. [DOI] [PubMed] [Google Scholar]
- [12].Al-Hawary M. Role of imaging in diagnosing and staging pancreatic cancer. J Natl Compr Canc Netw 2016;14(5 suppl):678–80. [DOI] [PubMed] [Google Scholar]
- [13].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- [14].Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473–81. [DOI] [PubMed] [Google Scholar]
- [15].Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016;388:248–57. [DOI] [PubMed] [Google Scholar]
- [16].Murakami Y, Satoi S, Sho M, et al. National comprehensive cancer network resectability status for pancreatic carcinoma predicts overall survival. World J Surg 2015;39:2306–14. [DOI] [PubMed] [Google Scholar]
- [17].Murakami Y, Uemura K, Sudo T, et al. Survival impact of neoadjuvant gemcitabine plus S-1 chemotherapy for patients with borderline resectable pancreatic carcinoma with arterial contact. Cancer Chemother Pharmacol 2016;79:37–47. [DOI] [PubMed] [Google Scholar]
- [18].Kato H, Usui M, Isaji S, et al. Clinical features and treatment outcome of borderline resectable pancreatic head/body cancer: a multi-institutional survey by the Japanese Society of Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2013;20:601–10. [DOI] [PubMed] [Google Scholar]
- [19].Bachellier P, Rosso E, Lucescu I, et al. Is the need for an arterial resection a contraindication to pancreatic resection for locally advanced pancreatic adenocarcinoma? A case-matched controlled study. J Surg Oncol 2011;103:75–84. [DOI] [PubMed] [Google Scholar]
- [20].Bockhorn M, Burdelski C, Bogoevski D, et al. Arterial en bloc resection for pancreatic carcinoma. Br J Surg 2011;98:86–92. [DOI] [PubMed] [Google Scholar]
- [21].Christians KK, Pilgrim CH, Tsai S, et al. Arterial resection at the time of pancreatectomy for cancer. Surgery 2014;155:919–26. [DOI] [PubMed] [Google Scholar]
- [22].Lee JL, Kim SC, Kim JH, et al. Prospective efficacy and safety study of neoadjuvant gemcitabine with capecitabine combination chemotherapy for borderline-resectable or unresectable locally advanced pancreatic adenocarcinoma. Surgery 2012;152:851–62. [DOI] [PubMed] [Google Scholar]
- [23].Sahora K, Kuehrer I, Eisenhut A, et al. NeoGemOx: gemcitabine and oxaliplatin as neoadjuvant treatment for locally advanced, nonmetastasized pancreatic cancer. Surgery 2011;149:311–20. [DOI] [PubMed] [Google Scholar]
- [24].Kim EJ, Ben-Josef E, Herman JM, et al. A multi-institutional phase 2 study of neoadjuvant gemcitabine and oxaliplatin with radiation therapy in patients with pancreatic cancer. Cancer 2013;119:2692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang J, Estrella JS, Peng L, et al. Histologic tumor involvement of superior mesenteric vein/portal vein predicts poor prognosis in patients with stage II pancreatic adenocarcinoma treated with neoadjuvant chemoradiation. Cancer 2012;118:3801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tzeng CW, Balachandran A, Ahmad M, et al. Serum carbohydrate antigen 19-9 represents a marker of response to neoadjuvant therapy in patients with borderline resectable pancreatic cancer. HPB (Oxford) 2014;16:430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol 2015;22:1153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Inoue Y, Saiura A, Yoshioka R, et al. Pancreatoduodenectomy with systematic mesopancreas dissection using a supracolic anterior artery—first approach. Ann Surg 2015;262:1092–101. [DOI] [PubMed] [Google Scholar]
- [29].Nimura Y, Nagino M, Takao S, et al. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: long-term results of a Japanese multicenter randomized controlled trial. J Hepatobiliary Pancreat Sci 2012;19:230–41. [DOI] [PubMed] [Google Scholar]
- [30].Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267–77. [DOI] [PubMed] [Google Scholar]
- [31].Gillen S, Schuster T, Meyer Zum Buschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].White R, Lee C, Anscher M, et al. Preoperative chemoradiation for patients with locally advanced adenocarcinoma of the pancreas. Ann Surg Oncol 1999;6:38–45. [DOI] [PubMed] [Google Scholar]
- [33].Spitz FR, Abbruzzese JL, Lee JE, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol 1997;15:928–37. [DOI] [PubMed] [Google Scholar]
- [34].Hoffman JP, Lipsitz S, Pisansky T, et al. Phase II trial of preoperative radiation therapy and chemotherapy for patients with localized, resectable adenocarcinoma of the pancreas: an Eastern Cooperative Oncology Group Study. J Clin Oncol 1998;16:317–23. [DOI] [PubMed] [Google Scholar]
- [35].Verbeke C, Lohr M, Karlsson JS, et al. Pathology reporting of pancreatic cancer following neoadjuvant therapy: challenges and uncertainties. Cancer Treat Rev 2015;41:17–26. [DOI] [PubMed] [Google Scholar]


