Supplemental Digital Content is available in the text
Keywords: diagnostic error, hemophagocytic lymphohistiocytosis, quality improvement
Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a highly fatal, hyperinflammatory syndrome in adults triggered by an underlying illness in most cases. As such, suspicion of HLH dictates further investigation to identify the HLH trigger and determine treatment. HLH is clinically challenging due to diverse presentations and underlying triggers, provider unfamiliarity, and bleeding complications. Clinically, we observed diagnostic error from incorrect testing and cognitive biases (interleukin-2 confused with soluble interleukin-2 receptor and natural killer cell quantification confused with functional assays).
This study reports our single institutional experience with adult HLH with the aim to reduce erroneous testing with a quality improvement (QI) project, and to facilitate trigger discovery and mitigate hemorrhage. Provider education on HLH testing was the prospective intervention, followed by mistaken test removal. HLH triggers and diagnostic utility were determined by retrospective chart review. Risk factors for hemorrhage were determined by multivariable analysis.
Erroneous HLH testing was reduced from 74% to 24% of patients (P < .001) by the QI intervention. These changes were projected to save $11,700 yearly. The majority (64%) of patients evaluated for HLH were on non-hematology/oncology services, highlighting the need for vigilance in hematology consultation. Sixty-three patients met classic HLH-2004 criteria for HLH. Malignancy (38%), infection (27%), Epstein–Barr virus (EBV) (14%), or autoimmune disease (8%) triggered most HLH cases. HLH triggers were most commonly identified by serologic testing (27%) and bone marrow biopsy (19%). Biopsy of other affected organs based on PET-CT imaging after unsuccessful initial diagnostic measures was helpful, and focal fluorodeoxyglucose uptake was predictive of an underlying malignancy (likelihood ratio 8.3, P = .004). Major hemorrhage occurred in 41% of patients. On multivariable analysis the odds ratios (OR) for major hemorrhage were increased for patients with intensive care unit level care (OR 10.47, P = .005), and disseminated intravascular coagulation in the first week of admission (OR 10.53, P = .04).
These data are incorporated into a framework to encourage early HLH recognition with the HScore, facilitate trigger identification, identify those at risk for hemorrhage, and minimize low-yield or erroneous testing.
1. Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a frequently fatal, severe inflammatory syndrome arising from dysregulated macrophages and cytotoxic lymphocytes.[1,2] Patients with HLH are often critically ill and have diverse clinical presentations, but their hematologic abnormalities raise initial clinical suspicion of the illness. Fevers, cytopenias, coagulopathy, hepatosplenomegaly, hyperferritinemia, defects in natural killer cell (NK) function, and elevated soluble interlukin-2 receptor (sIL2R) characterize this syndrome.[3–5] Primary (genetic) and secondary (sporadic) forms of HLH have similar clinical presentations, and studies of primary HLH have been instrumental in elucidating the pathobiology of disease.[6,7] However, primary HLH is rare in adults and less is known about the biology and care of adults with HLH.[8,9]
Many case series and reviews have reported HLH etiologies.[2,3,10–18] Although HLH treatment is directed at the underlying illness, identifying this trigger is practically challenging and available data are inadequate to assist clinicians in patient care. Another clinical concern is delayed HLH diagnosis, as an adult patient may not meet the pediatric HLH-2004 criteria at presentation, and a clinician may discount the diagnosis of HLH due to representative bias. The HScore was specifically developed to facilitate timely HLH recognition in adults; however, clinical uncertainty still exists in adult patients around identifying triggers and interpreting diagnostic data.[19–21] For example, hemophagocytosis is common in HLH, yet this finding is neither sensitive nor specific for HLH, and it does not predict disease severity.[22–24] Similarly, ferritin is a helpful test but it is not specific for HLH; likewise, failure to realize that one-third of HLH patients have a ferritin less than 3000 ng/mL could lead to diagnostic error if a clinician is expecting only massive ferritin elevations.[15,25] In addition, NK cell functional assays and sIL2R testing are unique to HLH evaluations and providers may not be familiar with their use.
HLH has long been associated with increased propensity for hemorrhage,[13] but studies reporting hemorrhagic risks are notably absent in the literature with the exception of a recent study in intensive care patients.[26] In addition to the severe cytopenias found in HLH, the coexistence of disseminated intravascular coagulation (DIC), hepatic dysfunction, hypofibrinogenemia, and the release of plasminogen activators from activated macrophages produce a multi-factorial bleeding diathesis.[26,27] Coagulopathy, critical illness, and need for invasive procedures represents a confluence of hemorrhagic risk that may be underappreciated in the acute clinical setting.
Although HLH is far too rare to warrant its own Choosing Wisely recommendation,[28] we undertook a similar quality improvement (QI) approach to reduce erroneous testing and improve awareness of HLH evaluation after test misinterpretation, use of HLH-2004 criteria, and cognitive bias resulted in diagnostic errors affecting 2 consecutive HLH patients. With limited data available in the literature for diagnostic guidance, we developed an internal knowledge base by retrospective review. Here we summarize our institutional HLH experience to guide clinicians evaluating patients with suspected HLH, with objectives of reducing errors in testing, identifying triggers, and identifying patients at risk of major hemorrhage.
2. Methods
This single-center prospective quality-improvement intervention with planned observational cohort retrospective review was modeled on that used previously.[29] The primary aim was to reduce mistaken testing in HLH evaluations from cognitive error and systems factors,[30–32] and the secondary aims were to reduce the number of patients with delays in attaining sIL2R from erroneous interleukin-2 (IL2) test ordering, and to encourage use of sIL2R testing. The tertiary aim was to provide information to assist clinicians in HLH clinical evaluations. The Johns Hopkins Hospital (JHH) is a tertiary referral hospital located in Baltimore, MD, with approximately 1100 inpatient beds for inpatient general medicine and specialty services.
We observed incorrect HLH diagnostic testing where IL2 was confused with sIL2R, and NK quantification was confused with NK functional tests (see Additional Methods, Supplemental Digital Content 1, which describes the errors and analytic approach). This was discovered after incorrect testing contributed to diagnostic error in two consecutive HLH cases (see Figure, Supplemental Digital Content 2, which illustrates how mistaken testing impelled the project). The quality improvement intervention consisted of targeted provider education, followed by systems changes to remove the mistaken tests (see Figure, Supplemental Digital Content 2, for intervention timeline). Patients evaluated for HLH between January 1, 2009 and July 30, 2016 at JHH were identified from billing and laboratory test result databases and were followed through November 1, 2017. The retrospective cohort review was conducted after approval of the Johns Hopkins Institutional Review Board. Patients were included in the study if they were evaluated for suspected HLH during hospitalization by their treating clinicians. Those patients age < 18 years at the time of evaluation, evaluated as outpatients, and not evaluated for HLH were excluded based on clinical documentation (see Figure, Supplemental Digital Content 2, for patient flowchart).
The HScore was developed and validated in adults, yet most adult HLH series use the HLH-2004 diagnostic criteria. We report both criteria for clinical applicability, but present analysis with HLH-2004 criteria for comparison to prior literature. Patients with HLH fulfilled ≥5 of the 8 HLH-2004 diagnostic criteria.[33] Patients with potential HLH had an HScore of ≥169 during the first week of hospitalization, but satisfied <5 HLH-2004 criteria.[19,20] Outcomes for retrospective review included erroneous testing, HLH triggers, trigger identification method, survival, hemorrhage, laboratory values, and imaging findings. HLH trigger classifications were mutually exclusive and based on those reported previously.[12,34,35] Detailed methods are further reported (see Additional Methods, Supplemental Digital Content 1, for detailed criteria and analytic variables).
Statistical methods: Descriptive statistics were computed with percentages, frequencies, medians, and means, as indicated. Overall survival was defined as date of initial hospital admission at JHH for HLH symptoms until death. Kaplan–Meier survival analysis and the log rank test were used to compare survival between groups where indicated. Positron emission tomography-computed tomography (PET-CT) utility was determined with likelihood ratio χ2 analysis. Odds ratios (OR) for univariate risk factor associations were determined with logistic regression. Univariate risk factors with P < .1 were included in the multivariable regression models. For assessment of QI interventions, HLH evaluations between January 2014 and December 2015 were pre-intervention and the active intervention period was December 2015 through July 2016. This pre-intervention interval was selected to reduce confounding from other institutional practices that likely changed over time, that is, order interface changes, paper ordering, and so on. QI outcomes were analyzed with the test of proportions or unpaired t test for continuous variables as indicated. All tests were 2-sided and performed at .05 level of statistical significance. Computations utilized STATA15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX).
3. Results
3.1. Patient demographics
During the retrospective study period, 86 patients were evaluated for HLH. The majority of patients undergoing evaluation (64.0%) were admitted to non-hematology/oncology services including medical intensive care unit (33.7%), general internal medicine (23.3%), rheumatology (3.5%), infectious diseases (2.3%), and liver transplant (1.2%). Sixty-three patients fulfilled HLH-2004 diagnostic criteria (Table 1), and 13 additional patients had potential HLH (see Table, Supplemental Digital Content 3, for clinical features of potential HLH). For comparison with prior studies we report further analysis on patients meeting HLH-2004 diagnostic criteria. Median HLH patient follow-up was 99 days (range, 1–2428 days) and confirmed all-patient mortality was 27.4% and 37.3% at 30 and 60 days, respectively. Markers of hemostatic perturbations were common, including severe renal dysfunction (38.1%), DIC (73.7%), moderately elevated D-dimer (71.9%), and markedly abnormal prothrombin (PT), or activated partial thromboplastin times (aPTT) (25.4%–46%) (see table for threshold values).
Table 1.
Characteristics of HLH cases.
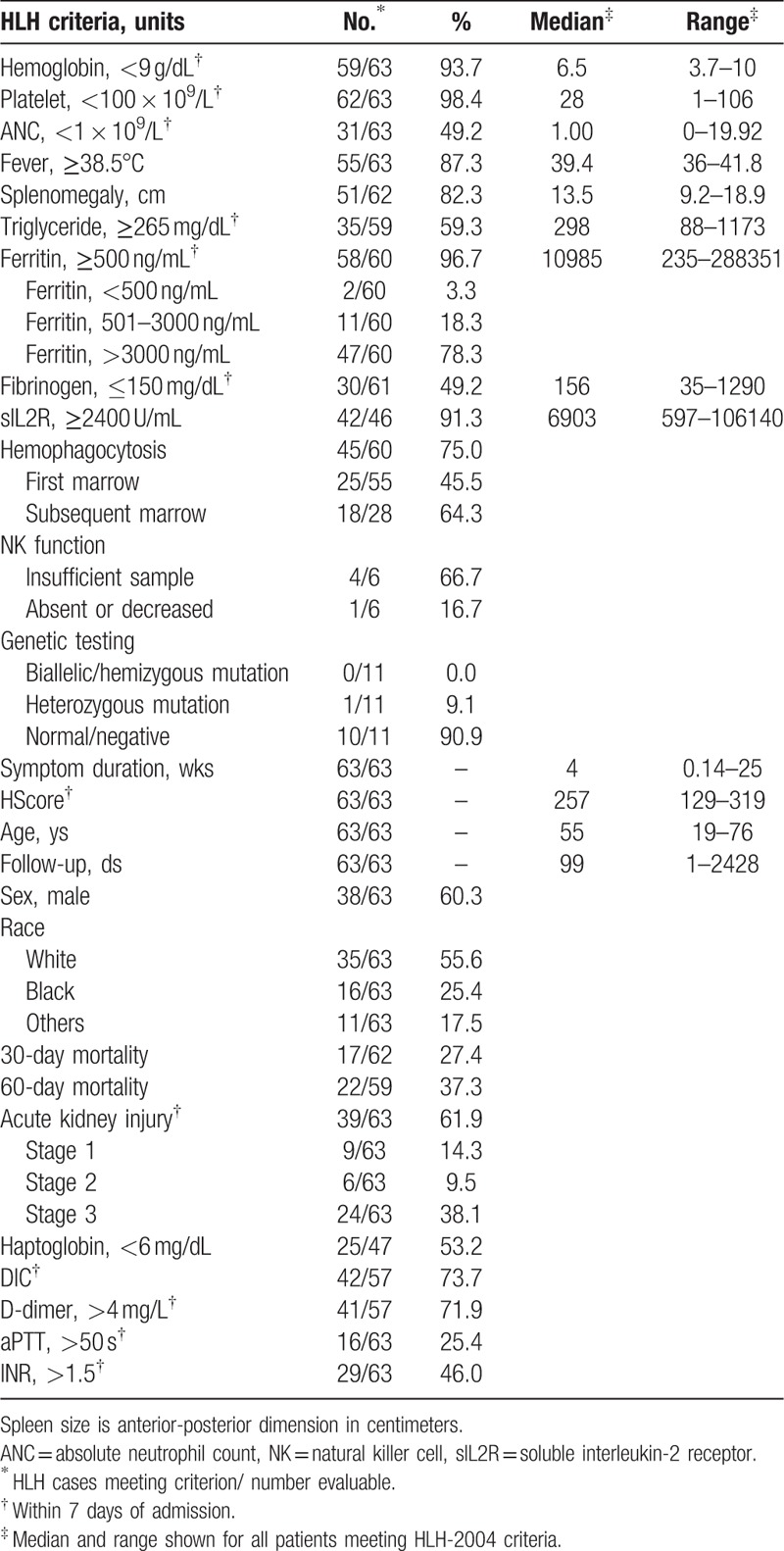
The number of HLH evaluations and cases increased with time. In 2009, there were 0.33 HLH evaluations and 0.33 HLH cases per month. In 2016, there were 2.9 HLH evaluations and 1.9 HLH cases per month. Relevant to our proposed diagnostic approach presented later, 21.6% of HLH patients had ferritins ≤3000 ng/mL during the first week, and 45.5% of cases demonstrated hemophagocytosis in the initial bone marrow sample. These data show that HLH patients present to several medical services, yet many may lack features often associated with HLH at presentation.
3.2. Errors in HLH testing
Over the entire study period, 54.2% of patients evaluated for HLH had errors in testing (Table 2). Although sIL2R is useful,[36–40] it may not be available in some regions, and send-out test turnaround time is a concern. Median sIL2R turnaround time was 10 days (range, 2–52 days). Patients with erroneous IL2 testing were significantly more likely to have delays or failure to obtain the sIL2R test (70.8% vs 34.1%, P = .0053) than patients without erroneous IL2 testing. Over the course of this study, erroneous NK and IL2 tests cost an estimated $22,348. Although such NK testing was reported in 42% of patients in recent German and Chinese series,[17,41] the frequency of erroneous IL2 testing at other centers is unknown.
Table 2.
The quality improvement initiative reduced test errors.
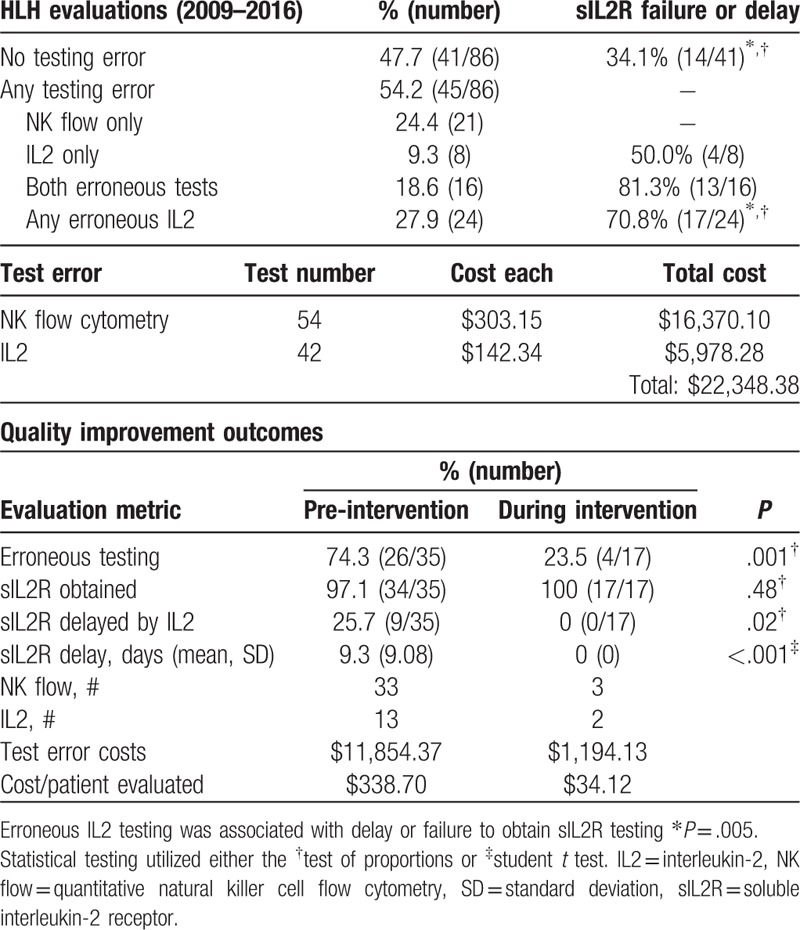
The QI intervention met the primary outcome of reducing erroneous testing. In the designated run up period before the QI intervention, 74.3% of patients had erroneous testing compared to 23.5% of patients during the intervention period (P = .001). The intervention met 1 of 2 secondary outcomes. The proportion of patients with delays in obtaining sILR2 due to IL2 testing decreased (25.7% vs 0%, P = .02), as did the mean delay (9.3 days vs 0 days, P < .01). The secondary outcome of increasing sIL2R utilization was not met, as baseline testing was common (97% vs 100%, P = .48). Although the time from admission to HLH trigger treatment decreased from 14.41 days to 7.27 days with the intervention, this benefit was not statistically significant (P = .20).
The intervention reduced but did not eliminate erroneous testing. We developed an electronic HLH orderset and decision support tool based on the HScore to increase the effect and make the effort durable. However, these approaches faced barriers to implementation. After discussions with our laboratory, a simpler option was to remove mistaken tests from the electronic test catalog since neither test had clinical utility. Extrapolating from the 2016 HLH evaluation frequency and pre-intervention erroneous test frequencies (0.94 NK test and 0.34 IL2 test/evaluation), these engineering interventions are predicted to save $11,700 per year by eliminating these 2 errors in testing. The patient-level events from test error that prompted the QI intervention did not recur during the intervention period.
3.3. HLH trigger determines treatment and survival
The HLH trigger was malignancy in 38.1%, infection (non-EBV) in 27.0%, Epstein–Barr virus (EBV) in 14.3%, idiopathic in 11.1%, and autoimmune in 7.9% of patients (Fig. 1A). Potential-HLH was triggered by autoimmune disease in 46.1% and infection in 38.4% (see Table, Supplemental Digital Content 4, for individual triggers and treatments). Survival with HLH was poor, but varied greatly by etiology. Median overall survival was 206 days for EBV and 45 days for malignancy-triggered HLH (Fig. 1B). In contrast, median survival was not reached for HLH triggered by autoimmune disease or infection. Others have shown that EBV-HLH has poor survival and warrants aggressive therapy,[42–45] yet many case series do not further specify HLH etiology in survival analyses. Thus, EBV-HLH may confound the survival of otherwise favorable HLH triggered by non-EBV infection. Consistent with this, survival for EBV or malignancy (adverse HLH) was inferior to autoimmune or non-EBV infection (favorable HLH) by log rank test (P < .01, Fig. 1C). There were no differences in survival between HLH from non-EBV infection and autoimmune etiologies (P = .51) or between HLH from EBV and malignancy (P = .30).
Figure 1.
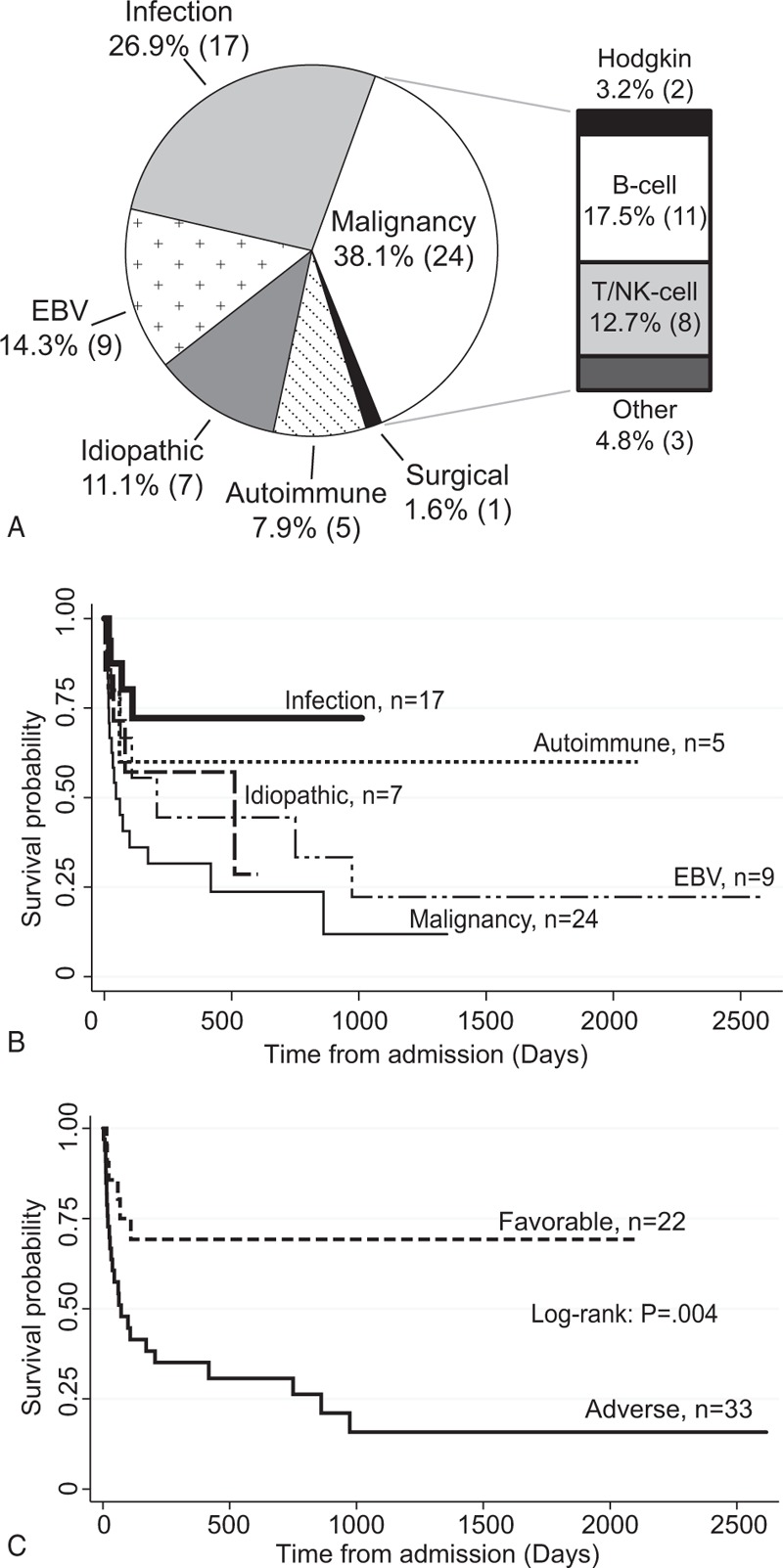
HLH triggers and overall survival. A, HLH triggers observed in the cohort. B, Kaplan-Meier overall survival from admission stratified by trigger. C, Kaplan-Meier overall survival was significantly higher for favorable HLH (autoimmune or infection triggered) compared to adverse HLH (EBV or malignancy triggered) by log-rank test. B-cell = B-cell lymphoma, EBV = Epstein Barr virus, T/NK-cell = T cell or natural killer cell lymphoma/leukemia.
Cytotoxic chemotherapy was commonly used for malignancy (75.0%), idiopathic (71.4%), and EBV-triggered HLH (66.7%). However, some patients were not candidates for such treatment due to goals of care or death before the trigger was identified. In contrast, cytotoxic chemotherapy was used less frequently when HLH was triggered by infection (5.9%) or autoimmune (20%) conditions. A common treatment strategy was initiation of corticosteroids and/or intravenous gamma globulin to stabilize the patient during the acute period. Once the trigger was identified, treatment was tailored to the trigger. The 2 patients receiving bone marrow transplantation for HLH (1 each autoimmune and EBV) were young adults and transplantation occurred on the pediatric services. Of note, although published data are limited, at our institution bone marrow transplantation is not a common therapy for adults with secondary HLH because of historically poor outcomes consistent with a recent multi-center report.[46]
3.4. HLH trigger identification
Data to guide HLH evaluations are lacking in the literature beyond reports of trigger type. The HLH trigger was established in 79.4% of cases with biopsy, laboratory test, or imaging (Table 3). Serologic testing had the highest diagnostic yield (27.0%), followed by bone marrow biopsy (19.0%), and other methods. However, in 19.0% of HLH cases the trigger was determined clinically after exclusion of other causes. The triggers of potential-HLH were similarly identified (see Table, Supplemental Digital Content 4, for potential HLH trigger identification methods). If initial evaluation was unrevealing, biopsy of an affected organ was useful to establish the trigger. Imaging or other findings (proteinuria, rash) were used to inform biopsy site selection.
Table 3.
Diagnostic methods identifying the trigger in HLH patients.
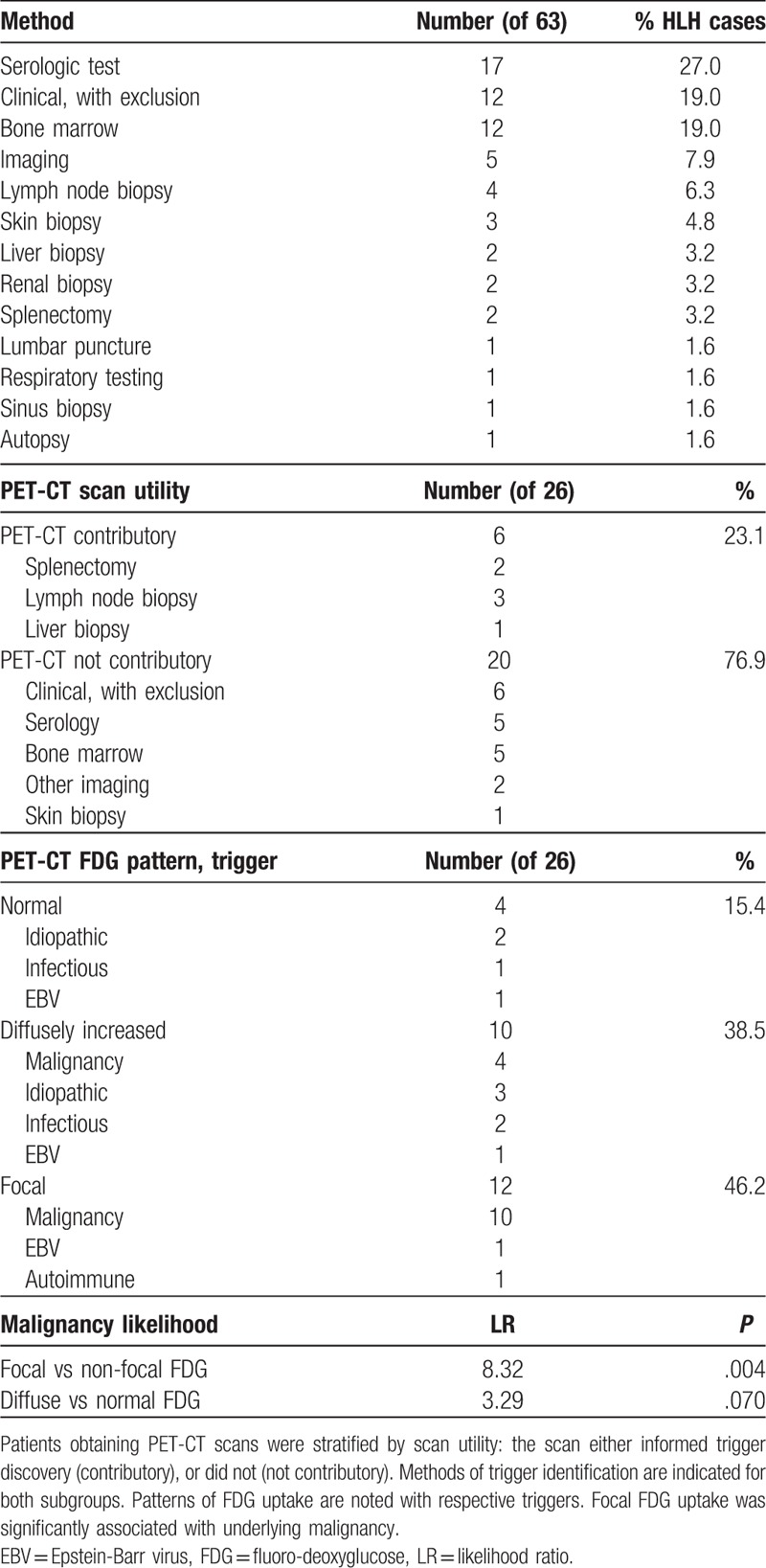
Zheng et al[47] recently reported that PET-CT was helpful to identify HLH triggers by determining biopsy location. In our series, 36.5% of HLH patients (26/63) underwent PET-CT before HLH trigger was evident. In 23.0% (6/26) of patients, the PET-CT findings contributed to trigger identification by directing biopsy location, and all such triggers were malignancies with therapeutic implications. In the remaining 76.9% (20/26) of patients where PET-CT did not contribute to trigger identification, method of trigger identification is listed (Table 3). Three patterns of scan avidity were noted in HLH patients: normal uptake (n = 4), diffusely increased uptake (n = 10), and focal increased uptake (n = 12). Focal increased uptake demonstrated a likelihood ratio (LR) of 8.32 for underlying malignancy compared to normal or diffuse uptake (P = .004). Although PET-CT is expensive and clearly not necessary for every patient, it may be helpful to determine biopsy site after an unremarkable initial HLH evaluation.
Unidentified triggers and the potential for empiric treatment to obscure triggers (trigger masking) are practical concerns for care. Excluding the 7/63 patients with idiopathic HLH, the median time from admission to trigger identification was 5 days (range, 0–238 days). Delayed trigger identification (>30 days) was observed in 12.5% (7/56) of HLH patients, 6 triggers were malignancies and one was infectious. Delayed triggers were found by splenectomy (patient 1, day 36; and patient 2, day 49), lymph node biopsy (patient 3, day 45), mycobacterial bone marrow culture (patient 4, day 54), skin biopsy (patient 5, day 56), a third liver biopsy (patient 6, day 109), and a fourth bone marrow biopsy (patient 7, day 238). Two of the 7 patients with delayed trigger identification possibly had trigger masking from empiric therapy. Of the idiopathic HLH cases, 4/7 had possible trigger masking from receipt of empiric therapy within 30 days of hospitalization. Accounting for all cases of possible trigger masking and assuming that either none or all of the 4 possible idiopathic cases were masked by treatment, HLH trigger masking may have occurred in 3.2% (2/63) to 9.5% (6/63) of patients. The theoretical risk of trigger masking is below the risk of early mortality or major hemorrhage, as discussed below.
In contrast to the observed utility of the methods discussed, no patient had HLH etiology determined by HLH-specific mutation testing. We expressly report this because HLH genetic testing is expensive ($4000), frequently requested by primary teams, and takes 6 to 8 weeks for results. Of the 11 patients with genetic testing, (median age 38 years vs 55 years for the HLH cohort) only 1 heterozygous mutation in LYST was found. This was of unclear clinical significance as the patient recovered fully after chemotherapy for the trigger (lymphoma). Although laboratory-based series report rare adults with compound or homozygous HLH-associated mutations,[8,9] and an expert opinion includes upfront genetic testing,[5] our data do not support routine genetic testing. To our knowledge, we here report the largest collection of adult HLH cases with genetic testing and patient level data.
3.5. Hemorrhage risk factors in HLH
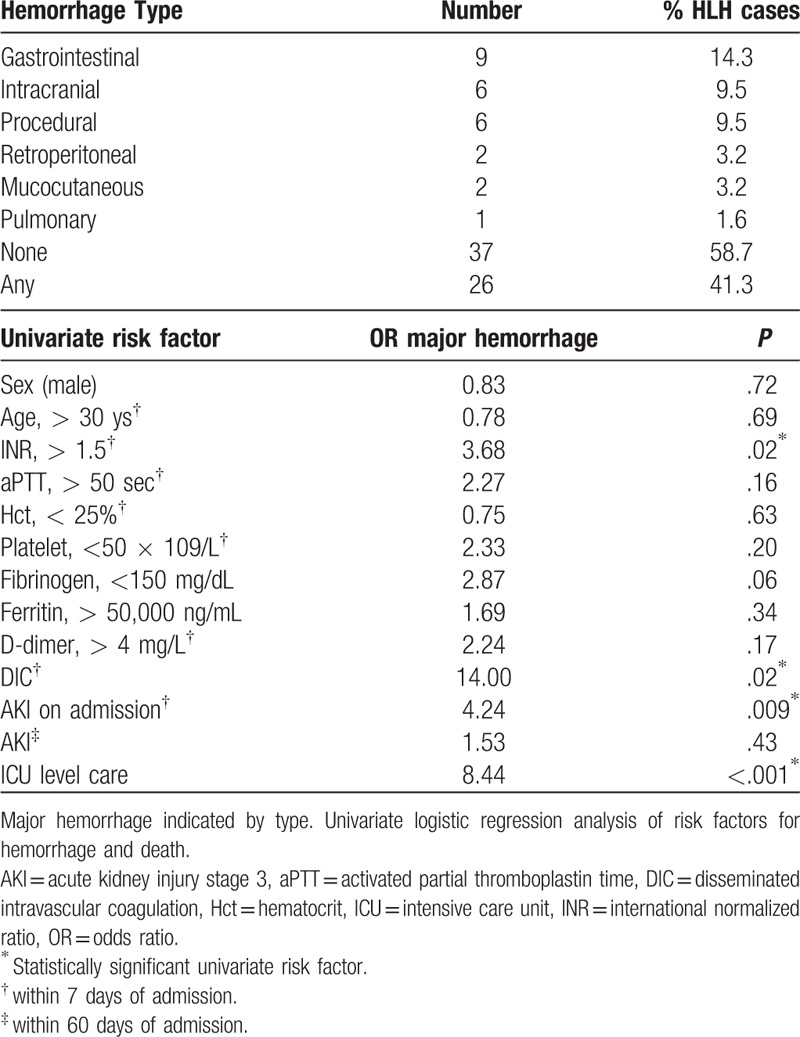
Major hemorrhage occurred in 41.3% (26/63) of HLH patients (Table 3). Two patients (2/26, 7.7%) had hemorrhage during relapse (both EBV-HLH at day 82 and 729, respectively), all other hemorrhages occurred during the initial HLH episode at our hospital. Median time from initial JHH admission to hemorrhage was 10 days (range, 0–729 days), and 4 patients had hemorrhage before transfer. Gastrointestinal, intracranial, and procedural hemorrhage were the most common hemorrhage types and occurred in 14.3%, 9.5%, and 9.5% of patients, respectively. Anticoagulant use within 24 hours of hemorrhage was noted in 13.6% (1 warfarin, 2 heparin). A known risk for hemorrhage in HLH (fibrinogen <200 mg/dL)[26] was temporally associated with hemorrhage in 64.7% of major hemorrhage episodes. Disseminated intravascular coagulation (DIC) was present in 83.3% of patients at the time hemorrhage. Procedures associated with major hemorrhage included: splenectomy (2), dialysis access placement (2), cystogastrostomy (1), and sinus biopsy (1).
Univariate analysis was conducted with factors known or suspected to influence hemorrhagic risk from prior studies (cytopenias, coagulation abnormalities, renal dysfunction), and markers of HLH activity available to clinicians early in the disease course (ferritin, fibrinogen, ICU level care) to determine odds ratios (OR) for subsequent major hemorrhage (Table 4).[26,48–51] We analyzed ICU level care preceding the hemorrhagic event to avoid possible confounding of ICU admissions occurring because of hemorrhage. The previously reported fibrinogen threshold of 200 mg/dL[26] is within our normal reference range (150–450 mg/dL), we therefore used 150 mg/dL as the threshold in our analyses. In a sensitivity analysis, we explored thresholds of age, fibrinogen, and ferritin with no change in univariate risk significance (see Additional Methods, Supplemental Digital Content 1, for sensitivity analysis variables). On multivariable analysis, intensive care unit (ICU) level care (OR 10.47, P = .005), and DIC in the first week of admission (OR 10.53, P = .04) were significant risk factors for subsequent major hemorrhage. Risk factors for death on multivariable analysis were limited to fibrinogen <150 mg/dL (OR 3.84, P = .03).
Table 4.
Bleeding complications and risk factors in HLH patients.
4. Discussion
Hemophagocytic lymphohistiocytosis is a highly morbid syndrome of severe inflammation that presents primarily to non-hematology/oncology services. A growing body of literature illustrates the differences between primary HLH found predominantly in young children and the secondary HLH observed in adults.[12,15,17,18,27,52–54] Here we report our results of a QI initiative to improve evaluation by reducing mistaken testing, and frame our institutional experience to specifically aide clinicians in future HLH evaluations. Our goals with this report are to reduce erroneous testing, facilitate trigger evaluations in clinical practice, and mitigate hemorrhage risk.
The electronic medical record and provider unfamiliarity contributed to test errors. Our QI effort attained its narrow goal of reducing errors and delays in testing. Erroneous testing is not unique to our institution, and recent series suggest it may be quite common at other centers.[17,41] Our efforts to eliminate inappropriate tests from the electronic test catalog are projected to save over $11,000 yearly. Although often delayed, before our QI project we observed high rates of sIL2R testing compared to other reports.[12,15,25,55] This likely contributed to the failure of one secondary aim to increase sIL2R testing. While sIL2R has diagnostic limitations, including elevations in other conditions, [56–59] it is useful in adults to diagnose HLH and monitor response to therapy and we found it was be readily obtainable.[36,38,39]
The clinical implications of test errors were more concerning than cost–mistaken tests contributed to diagnostic error. Since no other HLH-related efforts were active when the QI project was underway, we assume the improvements were causal. Other steps that may improve the use of diagnostic testing include a description of the test in the electronic ordering system, and we are currently using this to reduce errors around other orders. The educational and systems interventions can be adapted at other centers and had no upfront costs beyond clinician time. However, achieving this narrow goal has been slow. Our initial plan to use a decision support tool was ultimately not practical. Although the patient-level events that inspired our QI project have not recurred, use of hard-wired systems controls to minimize mistaken testing can ensure such events remain exceptionally rare.
HLH evaluations are understandably difficult. The HLH-2004 diagnostic criteria were developed for use in children, have many limitations in adults (reviewed in[4]), and can lead to delays in HLH diagnosis, as we observed. Use of the HScore and a systematic approach of testing can facilitate timely trigger discovery and minimize delays. We derived an HLH diagnostic approach from our data (Fig. 2). This approach addresses trigger identification, bleeding risk, and use of empiric therapy. Our lower observed PET-CT utility compared to others (23% vs 65%) is likely a consequence of fewer malignancy HLH cases in our cohort (38% vs 72% of cases).[47] We did not observe management changes from HLH mutational testing; however, selectively evaluating adult patients at high pre-test probability of primary HLH may be worthwhile to inform management. Although evidence to support HLH mutational testing is limited to 3 patients in similar retrospective series,[12,15,55] 2 subsequent young adults evaluated for HLH at our center who were found to have primary immune deficiencies from genetic testing not included on the available HLH gene panel (heterozygous RAS and CTLA-4 mutations, respectively). This proposed framework could streamline evaluation and would be easily adaptable at other centers, with modifications to account for local trigger frequencies.
Figure 2.
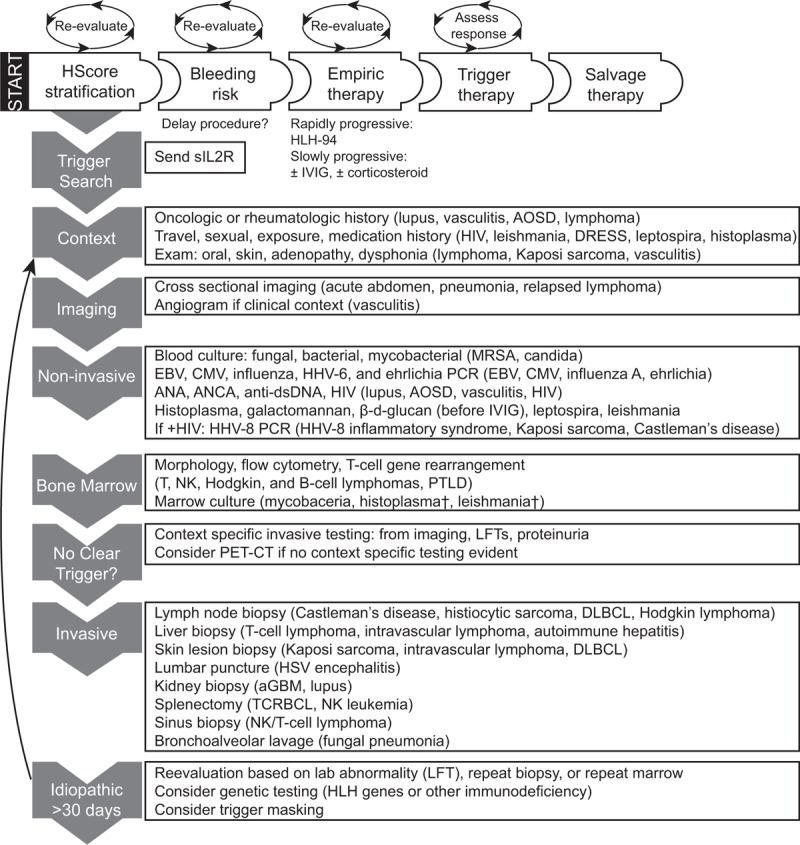
Evidence-based diagnostic and therapeutic strategies for adult HLH. Evaluation begins with HScore stratification. Assessment for bleeding risk, empiric therapy, and triggers occur simultaneously. Periodic reassessment of each factor is warranted as clinical course develops, as indicated by circular arrows. Trigger search proceeds through high-yield, low risk interventions to interventions with lower utility and increased risk of bleeding (grey arrows on left sidebar). Contributory findings are listed with triggers from our cohort (63 cases by HLH-2004 criteria, 13 cases by HScore). If no trigger is found, reassessment is warranted with consideration of expanded testing and repeat biopsy (black arrow). Note that IVIG leads to false positive β-d-glucan testing that may lead to prolonged antifungal therapy if misinterpreted. †Subsequently detected by non-invasive testing. aGBM = anti-glomerular basement membrane disease, AOSD = adult onset Still disease, CMV = cytomegalovirus, DLBCL = diffuse large B-cell lymphoma, DRESS = drug reaction with eosinophilia and systemic symptoms, EBV = Epstein–Barr virus, HHV = human herpes virus, HIV = human immunodeficiency virus, HSV = herpes simplex virus, IVIG = intravenous gamma globulin, LFT = liver function tests, MRSA = methicillin resistant staphylococcus aureus, NK = natural killer cell, PCR = polymerase chain reaction, PTLD = post-transplant lymphoproliferative disease, sIL2R = soluble interleukin-2 receptor, TCRBCL = T-cell rich large B-cell lymphoma.
Hemorrhage in HLH has been described since the 1980 s in children and adults.[13,60] Higher platelet goals in HLH have been proposed empirically,[51] but studies on hemorrhagic risks are limited. We observed nearly twice as many major hemorrhages as reported from our institution previously and elsewhere (22%).[13,26] Clinically apparent risk factors for hemorrhage were identifiable and could improve management. We speculate that patients at increased risk of hemorrhage may benefit from institution of empiric HLH treatment, or from postponing high-risk procedures until after DIC has improved, however each patient care scenario is unique. Although this idea is best tested prospectively, this would be difficult given the rarity of HLH and the paucity of prospective trials.
This study has several limitations. Patients may have been missed for inclusion in this single center study if the clinical team did not suspect HLH and begin an evaluation. The prospective QI intervention focused on reducing errors in testing and was not capable of testing our proposed evaluation strategy, since this strategy was developed from our retrospective analysis. Finally, the QI outcomes were assessed across the institution, but the interventions may not be applicable at other institutions depending on test workflow and ordering practices. We expect that the education-based QI effect will diminish with time and staff turnover, and we are monitoring our evaluations to maintain the gains achieved.
Successful HLH therapy requires prompt HLH recognition and trigger identification. We found that diagnostic errors and delays were surprisingly common, and we changed our systems and workflow to prevent these errors. Early evaluation for common infectious and malignancy triggers is paramount. We analyzed our experience to develop a practical approach for HLH evaluation to further guide clinicians when this initial evaluation is unrevealing. We currently use this approach in clinical practice, and with it, HLH loses most of its mystique originating from the uncertainties of evaluation.
Acknowledgments
The authors acknowledge assistance for clinical data coordination from the Center for Clinical Data Analytics, supported in part by the Johns Hopkins Institute for Clinical and Translational Research (ICTR). We thank Drs. Thomas Kickler, Richard Ambinder, and Michael Borowitz for their helpful discussions. We thank Ximin Li from the ICTR for statistical consultation. We would like to acknowledge support for the Johns Hopkins Institute for Clinical and Translational Research (ICTR)statistical consultation from the National Center for Research Resources and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health through Grant Number 1UL1TR001079.
Author contributions
Conceptualization: Samuel A. Merrill.
Data curation: Samuel A. Merrill.
Formal analysis: Samuel A. Merrill.
Funding acquisition: Robert A. Brodsky.
Investigation: Samuel A. Merrill.
Methodology: Samuel A. Merrill, Michael B. Streiff, Evan M. Braunstein, Alison M. Moliterno, Robert A. Brodsky.
Project administration: Samuel A. Merrill, Rakhi Naik, Robert A. Brodsky.
Resources: Samuel A. Merrill, Rakhi Naik, Robert A. Brodsky.
Supervision: Satish Shanbhag, Sophie Lanzkron, Evan M. Braunstein, Alison M. Moliterno, Robert A. Brodsky.
Validation: Samuel A. Merrill.
Visualization: Samuel A. Merrill.
Writing – original draft: Samuel A. Merrill.
Writing – review & editing: Samuel A. Merrill, Rakhi Naik, Michael B. Streiff, Satish Shanbhag, Sophie Lanzkron, Evan M. Braunstein, Alison M. Moliterno, Robert A. Brodsky.
Supplementary Material
Footnotes
Abbreviations: DIC = disseminated intravascular coagulation, EBV = Epstein–Barr virus, HLH = hemophagocytic lymphohistiocytosis, ICU = intensive care unit, IL2 = interleukin-2, JHH = Johns Hopkins Hospital, LR = likelihood ratio, NK = natural killer cell, OR = odds ratio, PET-CT = positron emission tomography-computed tomography, PT = prothrombin time, PTT = partial thromboplastin time, QI = quality improvement, sIL2R = soluble interleukin-2 receptor.
SAM was supported by National Institutes of Health, National Heart, Lung, and Blood Institute hematology fellowship training grant T32 HL007525. This work was made possible by a generous gift from the Strome Family Foundation. This publication was made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR) which is funded in part by Grant Number UL1TR001079 from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
The authors declare no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Henter JI, Elinder G, Soder O, et al. Incidence in Sweden and clinical features of familial hemophagocytic lymphohistiocytosis. Acta Paediatr Scand 1991;80:428–35. [DOI] [PubMed] [Google Scholar]
- [2].Hayden A, Park S, Giustini D, et al. Hemophagocytic syndromes (HPSs) including hemophagocytic lymphohistiocytosis (HLH) in adults: A systematic scoping review. Blood Rev 2016;30:411–20. [DOI] [PubMed] [Google Scholar]
- [3].Ramos-Casals M, Brito-Zeron P, Lopez-Guillermo A, et al. Adult haemophagocytic syndrome. Lancet 2014;383:1503–16. [DOI] [PubMed] [Google Scholar]
- [4].Machowicz R, Janka G, Wiktor-Jedrzejczak W. Similar but not the same: differential diagnosis of HLH and sepsis. Crit Rev Oncol Hematol 2017;114:1–2. [DOI] [PubMed] [Google Scholar]
- [5].Schram AM, Berliner N. How I treat hemophagocytic lymphohistiocytosis in the adult patient. Blood 2015;125:2908–14. [DOI] [PubMed] [Google Scholar]
- [6].Stepp SE, Dufourcq-Lagelouse R, Le Deist F, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science 1999;286:1957–9. [DOI] [PubMed] [Google Scholar]
- [7].Feldmann J, Callebaut I, Raposo G, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell 2003;115:461–73. [DOI] [PubMed] [Google Scholar]
- [8].Zhang K, Jordan MB, Marsh RA, et al. Hypomorphic mutations in PRF1, MUNC13-4, and STXBP2 are associated with adult-onset familial HLH. Blood 2011;118:5794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang K, Chandrakasan S, Chapman H, et al. Synergistic defects of different molecules in the cytotoxic pathway lead to clinical familial hemophagocytic lymphohistiocytosis. Blood 2014;124:1331–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Takahashi N, Chubachi A, Kume M, et al. A clinical analysis of 52 adult patients with hemophagocytic syndrome: the prognostic significance of the underlying diseases. Int J Hematol 2001;74:209–13. [DOI] [PubMed] [Google Scholar]
- [11].Shabbir M, Lucas J, Lazarchick J, et al. Secondary hemophagocytic syndrome in adults: a case series of 18 patients in a single institution and a review of literature. Hematol Oncol 2011;29:100–6. [DOI] [PubMed] [Google Scholar]
- [12].Schram AM, Comstock P, Campo M, et al. Haemophagocytic lymphohistiocytosis in adults: a multicentre case series over 7 years. Br J Haematol 2016;172:412–9. [DOI] [PubMed] [Google Scholar]
- [13].Reiner AP, Spivak JL. Hematophagic histiocytosis. A report of 23 new patients and a review of the literature. Medicine (Baltimore) 1988;67:369–88. [PubMed] [Google Scholar]
- [14].Park HS, Kim DY, Lee JH, et al. Clinical features of adult patients with secondary hemophagocytic lymphohistiocytosis from causes other than lymphoma: an analysis of treatment outcome and prognostic factors. Ann Hematol 2012;91:897–904. [DOI] [PubMed] [Google Scholar]
- [15].Parikh SA, Kapoor P, Letendre L, et al. Prognostic factors and outcomes of adults with hemophagocytic lymphohistiocytosis. Mayo Clin Proc 2014;89:484–92. [DOI] [PubMed] [Google Scholar]
- [16].Machaczka M, Vaktnas J, Klimkowska M, et al. Malignancy-associated hemophagocytic lymphohistiocytosis in adults: a retrospective population-based analysis from a single center. Leuk Lymphoma 2011;52:613–9. [DOI] [PubMed] [Google Scholar]
- [17].Li J, Wang Q, Zheng W, et al. Hemophagocytic lymphohistiocytosis: clinical analysis of 103 adult patients. Medicine (Baltimore) 2014;93:100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Riviere S, Galicier L, Coppo P, et al. Reactive hemophagocytic syndrome in adults: a retrospective analysis of 162 patients. Am J Med 2014;127:1118–25. [DOI] [PubMed] [Google Scholar]
- [19].Debaugnies F, Mahadeb B, Ferster A, et al. Performances of the H-Score for diagnosis of hemophagocytic lymphohistiocytosis in adult and pediatric patients. Am J Clin Pathol 2016;145:862–70. [DOI] [PubMed] [Google Scholar]
- [20].Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol 2014;66:2613–20. [DOI] [PubMed] [Google Scholar]
- [21].Otrock ZK, Daver N, Kantarjian HM, et al. Diagnostic challenges of hemophagocytic lymphohistiocytosis. Clin Lymphoma Myeloma Leuk 2017;17S:S105–10. [DOI] [PubMed] [Google Scholar]
- [22].Goel S, Polski JM, Imran H. Sensitivity and specificity of bone marrow hemophagocytosis in hemophagocytic lymphohistiocytosis. Ann Clin Lab Sci 2012;42:21–5. [PubMed] [Google Scholar]
- [23].Ho C, Yao X, Tian L, et al. Marrow assessment for hemophagocytic lymphohistiocytosis demonstrates poor correlation with disease probability. Am J Clin Pathol 2014;141:62–71. [DOI] [PubMed] [Google Scholar]
- [24].Lim SH, Park S, Jang JH, et al. Clinical significance of bone marrow hemophagocytosis in adult patients with malignancy and non-malignancy-induced hemophagocytic lymphohistiocytosis. Ann Hematol 2016;95:325–35. [DOI] [PubMed] [Google Scholar]
- [25].Schram AM, Campigotto F, Mullally A, et al. Marked hyperferritinemia does not predict for HLH in the adult population. Blood 2015;125:1548–52. [DOI] [PubMed] [Google Scholar]
- [26].Valade S, Azoulay E, Galicier L, et al. Coagulation disorders and bleedings in critically ill patients with hemophagocytic lymphohistiocytosis. Medicine (Baltimore) 2015;94:e1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shimazu H, Munakata S, Tashiro Y, et al. Pharmacological targeting of plasmin prevents lethality in a murine model of macrophage activation syndrome. Blood 2017;130:59–72. [DOI] [PubMed] [Google Scholar]
- [28].Hicks LK, Bering H, Carson KR, et al. Five hematologic tests and treatments to question. Blood 2014;124:3524–8. [DOI] [PubMed] [Google Scholar]
- [29].Merrill S, Stevens P, Verschraegen C, et al. Utility and costs of routine staging scans in early-stage breast cancer. Am J Heme/Onc 2016;12:9–16. [Google Scholar]
- [30].Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med 2003;78:775–80. [DOI] [PubMed] [Google Scholar]
- [31].Saposnik G, Redelmeier D, Ruff CC, et al. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak 2016;16:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Singh H, Graber ML, Kissam SM, et al. System-related interventions to reduce diagnostic errors: a narrative review. BMJ Qual Saf 2012;21:160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Henter JI, Horne A, Arico M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007;48:124–31. [DOI] [PubMed] [Google Scholar]
- [34].Wang Y, Huang W, Hu L, et al. Multicenter study of combination DEP regimen as a salvage therapy for adult refractory hemophagocytic lymphohistiocytosis. Blood 2015;126:2186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wang J, Wang Y, Wu L, et al. PEG-aspargase and DEP regimen combination therapy for refractory Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. J Hematol Oncol 2016;9:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tsuji T, Hirano T, Yamasaki H, et al. A high sIL-2R/ferritin ratio is a useful marker for the diagnosis of lymphoma-associated hemophagocytic syndrome. Ann Hematol 2014;93:821–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang L, Zhang S, Xu J, et al. Significance of soluble interleukin-2 receptor in patients with hemophagocytic lymphohistiocytosis. Leuk Lymphoma 2011;52:1360–2. [DOI] [PubMed] [Google Scholar]
- [38].Hayden A, Lin M, Park S, et al. Soluble interleukin-2 receptor is a sensitive diagnostic test in adult HLH. Blood Adv 2017;1:2529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schaer DJ, Schleiffenbaum B, Kurrer M, et al. Soluble hemoglobin-haptoglobin scavenger receptor CD163 as a lineage-specific marker in the reactive hemophagocytic syndrome. Eur J Hematol 2005;74:6–10. [DOI] [PubMed] [Google Scholar]
- [40].Lin M, Park S, Hayden A, et al. Clinical utility of soluble interleukin-2 receptor in hemophagocytic syndromes: a systematic scoping review. Ann Hematol 2017;96:1241–51. [DOI] [PubMed] [Google Scholar]
- [41].Birndt S, Schenk T, Brunkhorst FM, et al. Hemophagocytic lymphohistiocytosis in adults (aHLH): results from the German HLH Registry. Blood 2016;128:2523–12523. [Google Scholar]
- [42].Imashuku S, Kuriyama K, Sakai R, et al. Treatment of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis (EBV-HLH) in young adults: a report from the HLH study center. Med Pediatr Oncol 2003;41:103–9. [DOI] [PubMed] [Google Scholar]
- [43].Imashuku S, Kuriyama K, Teramura T, et al. Requirement for etoposide in the treatment of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. J Clin Oncol 2001;19:2665–73. [DOI] [PubMed] [Google Scholar]
- [44].Imashuku S, Tabata Y, Teramura T, et al. Treatment strategies for Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis (EBV-HLH). Leuk Lymphoma 2000;39:37–49. [DOI] [PubMed] [Google Scholar]
- [45].Li Z, Wang Y, Wang J, et al. Haploidentical hematopoietic stem cell transplantation for adult patients with Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Leuk Lymphoma 2017;1–8. [DOI] [PubMed] [Google Scholar]
- [46].Machowicz R, Suarez F, Jedrzejczak WW, et al. Allogeneic hematopoietic stem cell transplantation in hemophagocytic lymphohistiocytosis (HLH) in adults: a retrospective study of the chronic malignancies and inborn errors working parties (CMWP and IEWP) of the EBMT. Blood 2016;128:3490–13490. [Google Scholar]
- [47].Zheng Y, Hu G, Liu Y, et al. The role of 18F-FDG PET/CT in the management of patients with secondary haemophagocytic lymphohistiocytosis. Clin Radiol 2016;71:1248–54. [DOI] [PubMed] [Google Scholar]
- [48].Fernandez F, Goudable C, Sie P, et al. Low haematocrit and prolonged bleeding time in uraemic patients: effect of red cell transfusions. Br J Hematol 1985;59:139–48. [DOI] [PubMed] [Google Scholar]
- [49].Schulman S, Kearon C. Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Hemost 2005;3:692–4. [DOI] [PubMed] [Google Scholar]
- [50].Uhl L, Assmann SF, Hamza TH, et al. Laboratory predictors of bleeding and the effect of platelet and RBC transfusions on bleeding outcomes in the PLADO trial. Blood 2017;130:1247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jordan MB, Allen CE, Weitzman S, et al. How I treat hemophagocytic lymphohistiocytosis. Blood 2011;118:4041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cetica V, Sieni E, Pende D, et al. Genetic predisposition to hemophagocytic lymphohistiocytosis: Report on 500 patients from the Italian registry. J Allergy Clin Immunol 2016;137:188–96. e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Trottestam H, Horne A, Arico M, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood 2011;118:4577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Behrens EM, Canna SW, Slade K, et al. Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J Clin Invest 2011;121:2264–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Otrock ZK, Eby CS. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol 2015;90:220–4. [DOI] [PubMed] [Google Scholar]
- [56].Witkowska AM. On the role of sIL-2R measurements in rheumatoid arthritis and cancers. Mediators Inflamm 2005;2005:121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cho E, Lee JH, Lim HJ, et al. Soluble CD25 is increased in patients with sepsis-induced acute kidney injury. Nephrology (Carlton) 2014;19:318–24. [DOI] [PubMed] [Google Scholar]
- [58].Yasuda N, Takamatsu T, Kanoh T, et al. Serum levels of soluble interleukin 2 receptor in patients with non-haematological disorders. Br J Hematol 1988;69:573. [DOI] [PubMed] [Google Scholar]
- [59].Zambello R, Pizzolo G, Trentin L, et al. Evaluation of serum levels of soluble interleukin-2 receptor in patients with chronic lymphoproliferative disorders of T-lymphocytes. Cancer 1989;64:2019–23. [DOI] [PubMed] [Google Scholar]
- [60].Hadchouel M, Prieur AM, Griscelli C. Acute hemorrhagic, hepatic, and neurologic manifestations in juvenile rheumatoid arthritis: possible relationship to drugs or infection. J Pediatr 1985;106:561–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


