Abstract
The aim of this study was to assess the major risk factors for venous thromboembolism (VTE) in Chinese patients with ovarian cancer and to explore optimal methods of prophylaxis and treatment.
A retrospective analysis of patients from Qilu Hospital of Shandong University was conducted from January 1, 2014, to January 1, 2017. We analyzed 388 patients who underwent surgery with a final diagnosis of ovarian cancer, of whom 35 developed VTE. Risk factors for preoperative and postoperative VTE were investigated. Preoperative patients with VTE were treated with anticoagulant therapy; chemotherapy with carboplatin paclitaxel was administered for 2 or 3 courses before cytoreductive surgery.
Fifteen patients were diagnosed with preoperative VTE and 20 with postoperative VTE. Eight of these 35 patients were also diagnosed with pulmonary embolism (PE), and 1 patient died. Univariate analysis showed differences in age, preoperative D-dimer value, platelet count, preoperative chemotherapy, operative time, and cardiovascular disease according to the presence or absence of VTE. In multivariate analysis, age 55 years and older, tumor diameter greater than 10 cm, preoperative platelet count greater than 300 × 109/L, and a D-dimer value greater than 0.5 μg/mL were independent risk factors for preoperative VTE, whereas a D-dimer value greater than 0.5 μg/mL and surgery time greater than 150 minutes were independent risk factors for postoperative VTE. Four preoperative patients with PE who underwent treatment with anticoagulant therapy and chemotherapy with carboplatin paclitaxel had disappearance of signs of PE and their ascites and mass sizes decreased substantially, leading to subsequent optimal cytoreduction.
Preoperative screening and perioperative preventive measures should be taken in gynecological oncology surgery, especially when patients have risk factors identified in this study. For patients with ovarian cancer who have been diagnosed with thrombosis before surgery, adjuvant chemotherapy and anticoagulant drugs can be used to control the progression of thrombosis and cancer.
Keywords: ovarian cancer, perioperative, risk factors, venous thromboembolism
1. Introduction
Venous thromboembolism (VTE), which comprises deep vein thrombosis (DVT) and its complication of pulmonary embolism (PE), is a multifactorial disease and is considered to be the second most common cause of death in patients with active cancer.[1,2] There is little information on how to treat perioperative events, especially in preoperative ovarian cancer patients with VTE.
The association between VTE and malignancy was first recognized by Trousseau, who described thrombophlebitis as the presenting sign of visceral malignancy. Since then, VTE has received growing attention as a potentially catastrophic event and one of the lethal perioperative complications. Unfortunately, despite increased monitoring and prevention of thrombosis in these tumor patients, the incidence of VTE has not been reduced but has increased over past years.[3]
Svendsen and Karwinski [4] found that only 12% of patients with malignant tumors survive for more than 1 year from the first diagnosis of VTE. There are currently no large-scale statistical data reported for the incidence of VTE after surgery for malignant ovarian tumors. Overall, the incidence of VTE is significantly increased after surgery in patients with gynecological malignancies. According to previous reports,[5,6] the rate of DVT in patients with gynecologic malignancy ranges from 7% to 45%, whereas the rate of PE ranges between 1% and 2.6%. Abdominal and pelvic tumors are closely linked with the occurrence of VTE.[7] A 14-year study[8] of male and female cancer patients hospitalized in the United Kingdom found that ovarian cancer patients with VTE account for approximately 4%, which was the second highest of all malignancies. Patients with ovarian cancer are particularly prone to thrombosis, due to late diagnosis, extensive spread, time-consuming surgery, intraoperative and postoperative immobilization, and extrinsic deformation of the vascular wall.[9,10]
There are many risk factors for VTE that have been reported in different studies,[11,12] such as increasing age, venous stasis with or without varicose veins, malignant disease, long-term immobility, history of VTE, severe infection, chronic renal failure, congestive heart failure, history of myocardial infarction, inflammatory bowel disease, more than 3 prior pregnancies, the peripartum period, hormone replacement therapy, the use of oral contraceptives, and obesity. In a word, previous studies, including the study from Ye et al[13] from China, almost focused on the high-risk factors, incidence, and timing of VTE. There is little information on how to treat preoperative ovarian cancer patients with VTE. The purpose of our present study was not only to identify specific risk factors for VTE in patients with ovarian cancer but also to explore how to treat VTE and prevent fatal complications during the perioperative period.
2. Materials and methods
2.1. Data collection
A retrospective analysis of 388 patients with ovarian cancer who underwent surgical treatment in Qilu Hospital of Shandong University, from January 2014 to January 2017, was conducted. Preoperative clinical data collected included age, medical history, type of thrombosis, complications, laboratory tests such as D-dimer, platelet and cancer antigen 125 (CA 125), Federation International of Gynecology and Obstetrics (FIGO) stage, smoking status at diagnosis, and route of surgery (abdominal or laparoscopic). Preoperative or postoperative chemotherapy, operation time, intraoperative blood loss, postoperative pathological results, and any preventive methods were also analyzed, the latter of which included preoperative and postoperative application of low molecular weight heparin sodium injection (LMWH also named Qizheng produced by Qilu Pharmaceutical Co. Ltd). All the patients had expert pathology reviewed. Cancers were staged according to the 2014 FIGO guidelines. All patients were followed up for at least 6 months from the time of first diagnosis of ovarian cancer to the last chemotherapy treatment.
All patients with a likely diagnosis of ovarian cancer were routinely checked by ultrasound (Philips iU22 Ultrasound System) to exclude bilateral lower extremity DVT. Symptomatic patients underwent pulmonary computed tomography (CT) angiography to exclude PE. If a patient had pain in a lower extremity after operation, they underwent ultrasound examination. This study was approved by the Research Ethics Board of Qilu Hospital, Shandong University.
2.2. Treatment
Preoperative patients with VTE were treated with LMWH for 2 weeks followed by oral warfarin or rivaroxaban (Xarelto) for at least 1 month. Chemotherapy with carboplatin paclitaxel was recommended for 2 or 3 courses, after which cytoreductive surgery was performed.
Two patients were treated with inferior vena cava filter implantation before surgery, with the filter removed 3 months after surgery.
After 24 hours of cytoreductive surgery, a prophylatic VTE regimen was given to all patients without contraindications. They were given subcutaneous injections of 5000 u LMWH every 12 hours for at least 7 days.
2.3. Statistical analysis
Statistical analysis was performed using SPSS 22.0 (SPSS Inc., Chicago, IL). The differences between the 2 groups were compared using an independent-sample t test and the Chi-square test. Univariate analysis was performed to determine the association between different risk factors and VTE, and multivariate logistic regression analysis was used to identify significant risk factors for VTE. A probability value of < 0.05 was considered to indicate statistical significance.
3. Results
3.1. Risk factors for VTE in the patients with ovarian cancer
The clinical characteristics of the 2 groups are listed in Table 1. Among the 388 patients finally diagnosed with ovarian cancer, 35 were included in the VTE group and 335 patients in the non-VTE group. Eighteen patients were excluded due to lack of necessary clinical data. Fifteen events (42.9%) occurred in preoperative patients, and in 4 including PE, whereas 20 patients (57.1%) had postoperative events, including 4 that also included PE. Among the 8 patients with PE, 4 were PE only and the other 4 were PE merged with detectable DVT, including 1 patient with a subclavian vein thrombosis. The remaining 27 patients had a DVT in the left leg (n = 7), 8 in the right leg, and 12 in both legs.
Table 1.
Clinical factors of VTE group and non-VTE group.
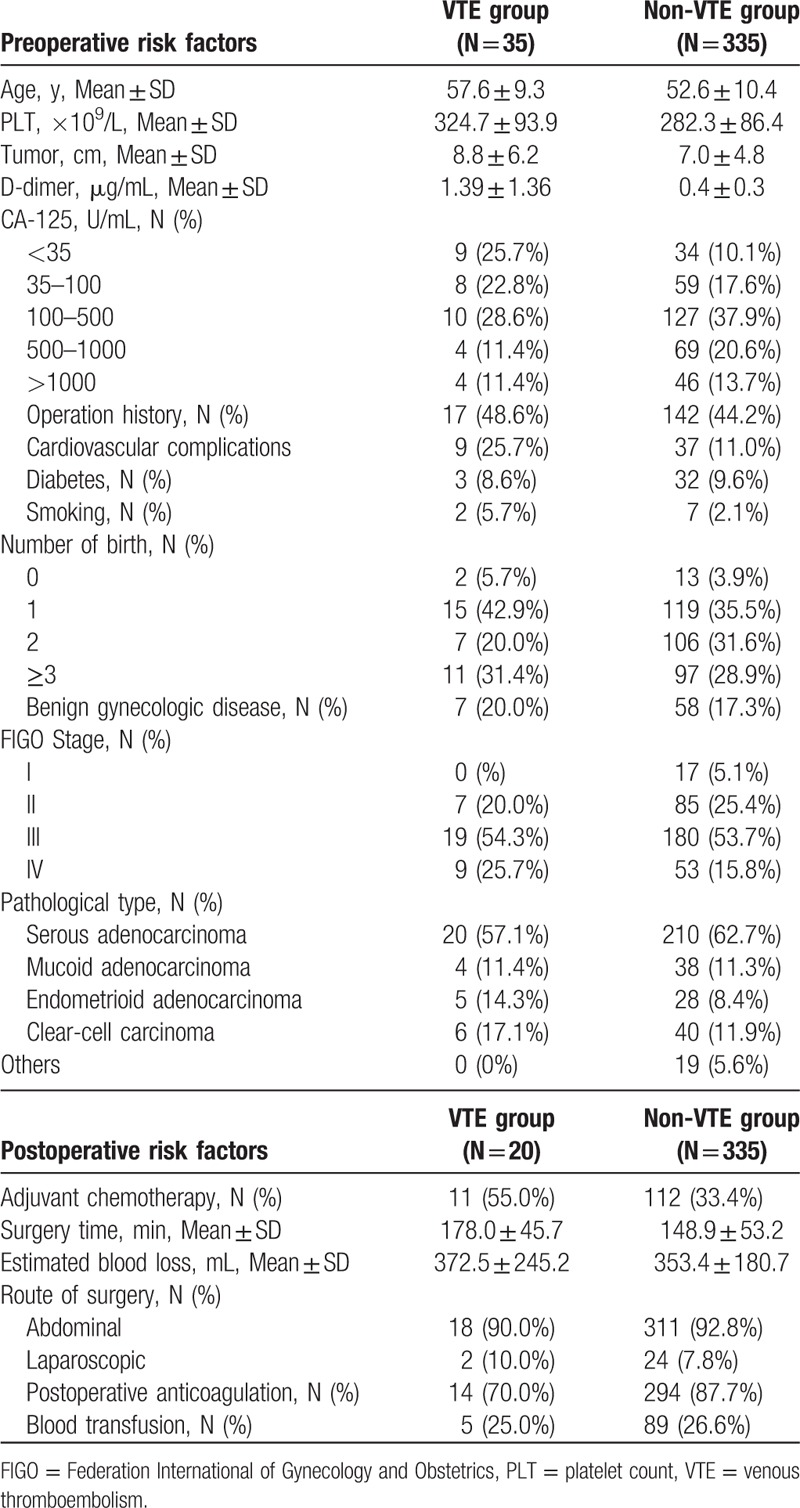
Twenty cases were serous adenocarcinoma, 4 mucinous adenocarcinoma, 5 endometrioid adenocarcinoma, and 6 were clear cell carcinoma. The average patient age was 57.6 years. The clinical data of the 2 groups are listed in Table 1. The median D-dimer value in VTE group is 1.39 μg/mL.
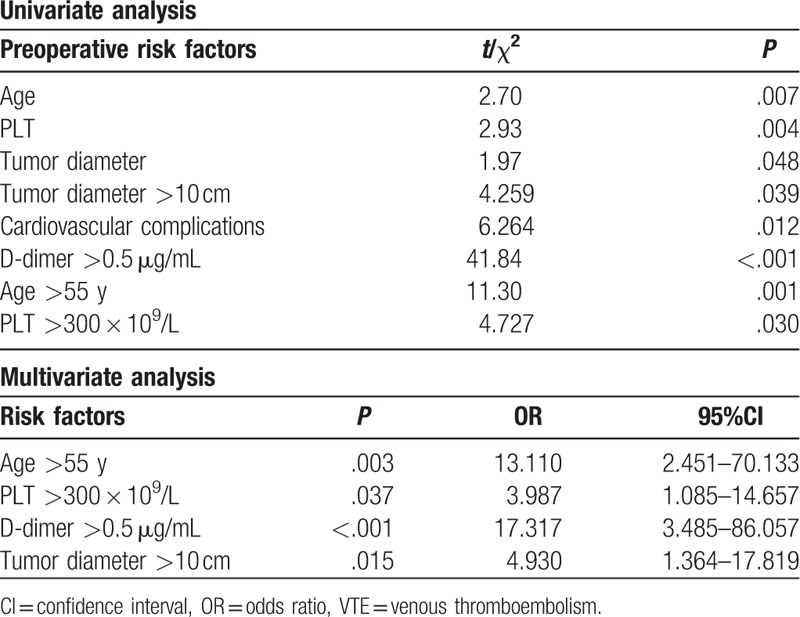
The results of univariate and multivariate analysis of preoperative risk factors for VTE are listed in Table 2. There was a significant difference in age, cardiovascular comorbidity, tumor diameter, platelet count (PLT), and D-dimer on univariate analysis, while on multivariate analysis, there was a significant association with age >55 years, PLT >300 × 109/L, D-dimer >0.5 μg/mL, and tumor diameter >10 cm, with the risk ratios being 13.11, 3.987,17.317, and 4.930, respectively (Table 2).
Table 2.
Risk factors for preoperative VTE.
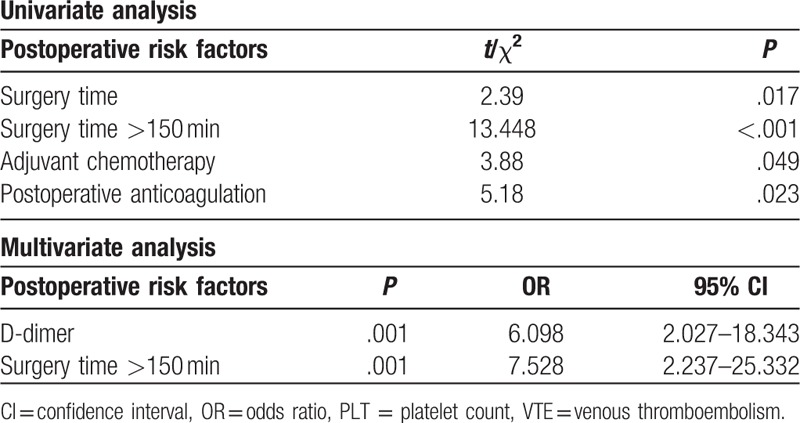
In addition, we also analyzed perioperative risk factors of VTE. Length of surgery, use of chemotherapy, and no use of postoperative anticoagulation were significantly related to postoperative VTE on univariate analysis (Table 3), but there was no significant difference according to estimated blood loss (EBL), type of surgery, and need for blood transfusion. Multivariate analysis revealed that a D-dimer >0.5 μg/mL and an operative time >150 minutes were independent risk factors for postoperative VTE with the risk ratios being 6.098 and 7.528 (Table 3).
Table 3.
Risk factors for postoperative VTE.
3.2. Outcomes in preoperative patients with PE after treatment with anticoagulation therapy and chemotherapy with carboplatin paclitaxel
The 4 preoperative patients with PE received 2 courses of chemotherapy and anticoagulation with LMWH. All 4 patients’ PE disappeared on imaging, and their ascites and mass sizes also decreased. Furthermore, they were all treated with cytoreductive surgery with satisfactory results.
4. Discussion
VTE prophylaxis is particularly important for surgical oncologists given the high rate of DVT in patients with malignancy. When compared with cervical cancer and endometrial cancer, ovarian cancer patients are more likely to have VTE.[8,14] In our present study, the incidence of VTE during the perioperative period for ovarian cancer was 9.02%, which was lower than that reported previously,[15–17] possibly due to the use of LMWH in all patients during the perioperative period and not including asymptomatic thrombosis diagnosed on routine testing.
Many risk factors that increase the occurrence of VTE have been reported,[11,12] such as age, a history of VTE, more than 3 previous pregnancies, and cardiovascular disease. We found that age more than 55 years, larger tumors, high PLT, and high D-dimer levels were independent risk factors for the formation of VTE. D-dimer levels is not only in correlation with ovarian cancer prognosis but also with VTE.[18] A high D-dimer level is helpful in screening preoperative VTE, especially silent VTE. If a patient's D-dimer value greater than 0.5 μg/mL needs to be vigilant about VTE risk rather than diagnosis of VTE according to our univariate and multivariate analysis, the cutoff value of D-dimer for predictive VTE is still controversial. Ay et al[19] suggested the cutoff for elevated D-dimer was greater than 1.44 μg/mL for various cancers; however, Satoh et al[20] and Kawaguchi et al[21] recommended that the cutoff value could be greater than 1.5 μg/mL in ovarian cancer. In our present study, we found that the median value of D-dimer was 1.39 μg/mL. Our result is almost the same as mentioned above.
Larger tumors compress the pelvic vasculature, impacting pelvic and lower limb blood circulation, thereby increasing blood viscosity, resulting in VTE. Surprisingly, both diabetes and smoking were not significant risk factors for VTE. This may be because there were few diabetic patients and few smokers in our study. The stage of tumor also had some influence on the incidence of VTE in our study. In a study of 641 cases,[22] clear cell and high-grade undifferentiated adenocarcinomas were the most likely to result in VTE, the former related perhaps to lymph node spread and hypercoagulability.
For the treatment-related factors, in our study, adjuvant chemotherapy was found to be an independent risk factor for VTE. Chemotherapy has played a significant role in improving the prognosis of ovarian cancer, but it is also a risk factor for VTE. Cisplatin is known to be associated with a variety of toxic effects, including VTE, hypertension, myocardial infarction, and stroke. The mechanism of VTE related to chemotherapy is not very clear, but some of the effects of chemotherapy have been described.[23] Ratib et al[8] found patients who received chemotherapy had a higher incidence of VTE than those who did not. The 4 PE preoperative patients who received chemotherapy with cisplatin paclitaxel for 2 courses, and anticoagulation with LMWH in our study all had complete disappearance of their PE, their ascites and mass sizes decreased, and underwent optimal cytoreductive surgery, with no new VTE events. Large tumors or massive ascites in ovarian cancer may compress the intrapelvic veins and enhance the risk of DVT even before surgery.[20] Thus, we recommend that any patient who is diagnosed preoperatively should be treated with chemotherapy and anticoagulation before cytoreductive surgery.
In the present study, the surgical time of the VTE group was significantly longer than that of the non-VTE patients, which was in keeping with other studies.[24,25] There are several differences between gynecologic tumor surgery and general surgical procedures with the former patients often requiring special procedures, such as dissection of lymph nodes, and pelvic and peritoneal metastases, resulting in prolonged operative time, and an increased incidence of VTE. The primary mechanism of VTE in this situation is probably vascular injury caused by the dissection with exposed vascular endothelial cells and factor III leak, leading to the release of cytokines, thereby activating the coagulation process.[26–30]
Standard techniques for prevention of VTE include the prophylactic use of LMWH, intermittent calf compression after surgery, and early ambulation.[31,32]
5. Conclusion
VTE may be prevented by careful assessment of ovarian cancer patients with the risk factors identified in this study. Ovarian cancer patients who are older than 55 years have cardiovascular complications, who receive adjuvant chemotherapy, and have high D-dimer values and high PLTs should undergo further investigations to exclude VTE. Furthermore, the use of perioperative anticoagulants, as well as a shortened surgery time, should reduce the incidence. For patients with ovarian cancer who have been diagnosed with thrombosis before surgery, adjuvant chemotherapy and anticoagulant drugs can be used to control the extension and spread of both the thrombosis and the cancer.
Acknowledgment
We thank Professor Michael Quinn for help with editing.
Author contributions
Conceptualization: Hongyan Cheng, Guiyu Zhang.
Data curation: Xiaofei Liu, Zhaojie Yang, Guiyu Zhang.
Formal analysis: Wentong Zhang, Guiyu Zhang.
Funding acquisition: Guiyu Zhang.
Investigation: Wentong Zhang, Xiaofei Liu, Hongyan Cheng, Guiyu Zhang.
Methodology: Guiyu Zhang.
Footnotes
Abbreviations: CT = computerized tomography, DVT = deep vein thrombosis, EBL = estimated blood loss, LMWH = low molecular weight heparin, PE = pulmonary embolism, PLT = platelet count, VTE = venous thromboembolism.
Funding/support: This work was supported by Key research and development project of Shandong Province Science and Technology (2015GSF118068).
The authors have no conflicts of interest to disclose.
References
- [1].Cohen AT, Nandini B, Wills JO, et al. VTE prophylaxis for the medical patient: where do we stand? A focus on cancer patients. Thromb Res 2010;125:21–9. [DOI] [PubMed] [Google Scholar]
- [2].Oranratanaphan S, Termrungruanglert W, Khemapech N. Incidence and clinical characteristic of venous thromboembolism in gynecologic oncology patients attending King Chulalongkorn Memorial Hospital over a 10 year period. Asian Pac J Cancer Prev 2015;16:6705–9. [DOI] [PubMed] [Google Scholar]
- [3].Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis 2016;41:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Svendsen E, Karwinski B. Prevalence of pulmonary embolism at necropsy in patients with cancer. J Clin Pathol 1989;42:805–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Clarkpearson DL, Delong E, Synan IS, et al. A controlled trial of two low-dose heparin regimens for the prevention of postoperative deep vein thrombosis. Obstet Gynecol 1990;75:684–9. [PubMed] [Google Scholar]
- [6].Einstein MH, Pritts EA, Hartenbach EM. Venous thromboembolism prevention in gynecologic cancer surgery: a systematic review. Gynecol Oncol 2007;105:813–9. [DOI] [PubMed] [Google Scholar]
- [7].Andersen TF. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846–50. [DOI] [PubMed] [Google Scholar]
- [8].Ratib S, Walker AJ, Card TR, et al. Risk of venous thromboembolism in hospitalised cancer patients in England—a cohort study. J Hematol Oncol 2016;9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dewdney SB, Benn T, Rimel BJ, et al. Inferior vena cava filter placement in the gynecologic oncology patient: a 15-year institutional experience. Gynecol Oncol 2011;121:344–6. [DOI] [PubMed] [Google Scholar]
- [10].Barber EL, Clarke-Pearson DL. Prevention of venous thromboembolism in gynecologic oncology surgery. Gynecol Oncol 2017;144:420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Heit JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med 2002;162:1245–8. [DOI] [PubMed] [Google Scholar]
- [12].Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius study. Arch Intern Med 2000;160:3415. [DOI] [PubMed] [Google Scholar]
- [13].Ye S, Zhang W, Yang J, et al. Pattern of venous thromboembolism occurrence in gynecologic malignancy: incidence, timing, and distribution a 10-year retrospective single-institutional study. Medicine 2015;94:e2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang X, Fu S, Freedman RS, et al. Venous thromboembolism syndrome in gynecological cancer. Int J Gynecol Cancer 2006;16:458–71. [DOI] [PubMed] [Google Scholar]
- [15].Rodriguez AO, Wun T, Chew H, et al. Venous thromboembolism in ovarian cancer. Gynecol Oncol 2007;105:784–90. [DOI] [PubMed] [Google Scholar]
- [16].Tateo S, Mereu L, Salamano S, et al. Ovarian cancer and venous thromboembolic risk. Gynecol Oncol 2005;99:119–25. [DOI] [PubMed] [Google Scholar]
- [17].Black D, Iasonos A, Ahmed H, et al. Effect of perioperative venous thromboembolism on survival in ovarian, primary peritoneal, and fallopian tube cancer. Gynecol Oncol 2007;107:66–70. [DOI] [PubMed] [Google Scholar]
- [18].Wu J, Fu Z, Liu G, et al. Clinical significance of plasma D-dimer in ovarian cancer: a meta-analysis. Medicine (Baltimore) 2017;96:e7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood 2010;116:5377–82. [DOI] [PubMed] [Google Scholar]
- [20].Satoh T, Oki A, Uno K, et al. High incidence of silent venous thromboembolism before treatment in ovarian cancer. Br J Cancer 2007;97:1053–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kawaguchi R, Furukawa N, Kobayashi H. Cut-off value of D-dimer for prediction of deep venous thrombosis before treatment in ovarian cancer. J Gynecol Oncol 2012;23:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bakhru A. Effect of ovarian tumor characteristics on venous thromboembolic risk. J Gynecol Oncol 2013;24:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hennessy B, O’Connor M, Carney DN. Acute vascular events associated with cisplatin therapy in malignant disease. Irish Med J 2002;95:145. [PubMed] [Google Scholar]
- [24].Swenson CW, Berger MB, Kamdar NS, et al. Risk factors for venous thromboembolism after hysterectomy. Obstet Gynecol 2015;125:1139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kim JY, Khavanin N, Rambachan A, et al. Surgical duration and risk of venous thromboembolism. JAMA Surg 2015;150:110. [DOI] [PubMed] [Google Scholar]
- [26].De CM. The prothrombotic state in cancer: pathogenic mechanisms. Crit Rev Oncol Hematol 2004;50:187–96. [DOI] [PubMed] [Google Scholar]
- [27].Suzuki N, Kataoka F, Higashiguchi A, et al. Intermittent pneumatic compression for prevention of pulmonary thromboembolism after gynecologic surgery. Thromb J 2005;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Di RF, Baiocchi G. Value of lymph node assessment in ovarian cancer: status of the art at the end of the second millennium. Int J Gynecol Cancer 2000;10:435. [DOI] [PubMed] [Google Scholar]
- [29].Oshita T, Itamochi H, Nishimura R, et al. Clinical impact of systematic pelvic and para-aortic lymphadenectomy for pT1 and pT2 ovarian cancer: a retrospective survey by the Sankai Gynecology Study Group. Int J Clin Oncol 2013;18:1107–13. [DOI] [PubMed] [Google Scholar]
- [30].Panici PB, Plotti F, Zullo MA, et al. Pelvic lymphadenectomy for cervical carcinoma: laparotomy extraperitoneal, transperitoneal or laparoscopic approach? A randomized study. Gynecol Oncol 2006;103:859–64. [DOI] [PubMed] [Google Scholar]
- [31].Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:381S–453S. [DOI] [PubMed] [Google Scholar]
- [32].Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007;25:5490–505. [DOI] [PubMed] [Google Scholar]


