To the Editor: Capecitabine (Xeloda, Roche) is an oral fluoropyrimidine prodrug which is a valuable substitute for parenteral 5-flourouracil (5-FU) for the treatment of colon cancer and metastatic breast cancer.1 In comparison with intravenous 5-FU, capecitabine was associated with less frequent diarrhea (47.7% vs 58.2%), neutropenia and stomatitis.2 These features have contributed to the increased use of capecitabine. There are two published case reports describing small bowel toxicity associated with capecitabine.3,4 We report a patient with terminal ileitis secondary to the use of this drug. This is the first reported case which was reversible, isolated and proven by endoscopy and histology.
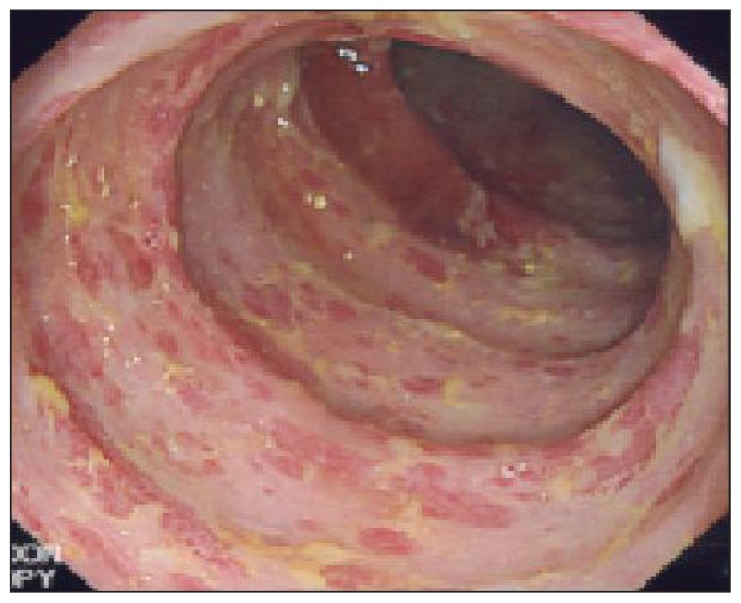
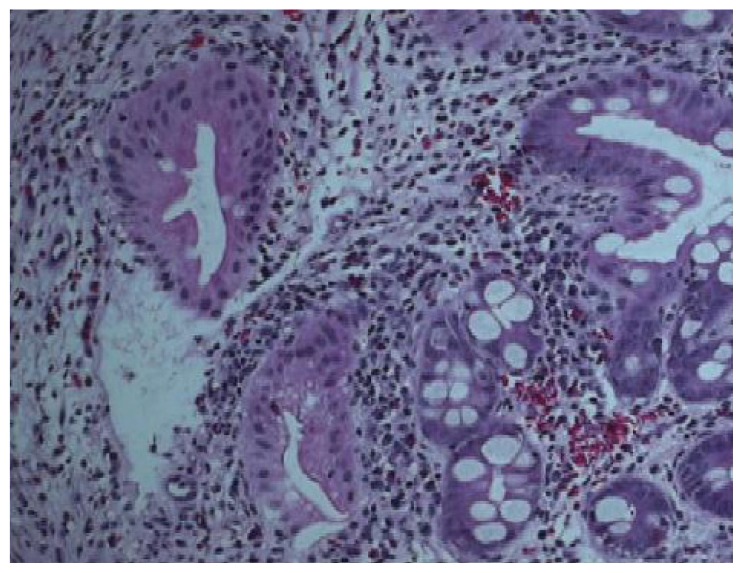
The patient was a 65-year-old Saudi male diagnosed with locally advanced and metastatic adenocarcinoma of the rectum. He received pelvic radiation therapy of 30 Gy given in 10 fractions over 2 weeks to control his pelvic disease. He was subsequently started on XELOX (capecitabine plus oxaliplatin) chemotherapy 4 weeks after finishing the radiation therapy. The dose of capecitabine was 1500 mg orally twice daily for 14 days every 21 days.5 This was equivalent to almost 2/3 of the standard dose. Twelve days after the start of this chemotherapy, he was admitted with fever, abdominal pain, vomiting and diarrhea. Initially, he was treated with broad spectrum IV antibiotics and fluids. Capecitabine was discontinued. Stool for Closteridium difficile, parasites and culture were all negative. His course was prolonged and colonoscopy showed an isolated ulceration in the terminal ileum (Figure 1). The histopathology was consistent with inflammatory changes and eosinophilic infiltrate but no evidence of malignancy or granulomas (Figure 2). His condition improved with conservative treatment. The dihydropyrimidine dehydrogenase (DPD) mutation test was negative. A repeat colonoscopy 2 months later did not show any residual abnormality. He subsequently received a second cycle of XELOX chemotherapy 5 weeks after this first cycle, but the dose of capecitabine reduced to 1000 mg twice daily. He tolerated this well with no recurrence of gastrointestinal toxicity. He continued to respond and tolerated the treatment well.
Figure 1.
Colonoscopy showed isolated terminal ulceration.
Figure 2.
The histopathology was consistent with inflammatory changes and eosinophilic infilterate but no evidence of malignancy, granulomas or CMV (cytomegalovirus).
Most patients tolerate capecitabine well. However, a number of patients develop severe and sometimes life-threatening toxicity after standard doses of capecitabine (which is 1250 mg/m2 orally twice daily for 14 days every 21 days).5 The major gastrointestinal manifestation of 5-FU cytotoxicity is diarrhea, which may be severe as well as dose-dependent and schedule dependant. However, in the patient described in this report, the colon appeared to be normal with toxicity limited to the the terminal ileum. This toxicity appeared to be dose dependant as there was resolution of the initial complaints and the patient was able to tolerate the treatment without suffering with an appropriate dose reduction. Radiation-induced ileitis was believed less likely to be the cause as the field of radiation exposure was away from the terminal ileum. In addition, there was a gap of 6 weeks between the end of radiation and the onset of the symptoms and a relatively low dose of radiation was utilized (30 Gy over 2 weeks). Finally the symptoms correlated with the dose of capecitabine.
There are very limited reports describing ileitis caused by capecitabine. We found two cases reported separately.3,4 The first was of a patient receiving single agent capecitabine in the adjuvant setting. Similar to our patient, ileitis was confirmed by endoscopy and histologically. The second patient received XELOX and bevacizumab in the metastatic setting. Terminal ileitis was diagnosed clinically and radiologically without endoscopy examination. To our knowledge, our report describes for the first time a reversible capecitabine-induced terminal ileitis that was confirmed endoscopically and histologically. This complication was successfully prevented from recurrence by reduction of the capecitabine dose in the subsequent cycles. Physicians should be aware of this side effect, which could be life threatening but appears to be dose dependant.
REFERENCES
- 1.Miwa M, Ura M, Nishada M, Sawada N, Ishikawa T, Mori K, Shimma N. Design of a novel oral fluoropyrimidine, capecitabine, which generates 5-fluorouracil selectively in tumors by enzymes concentrated in human liver and cancer tissue. Eur J cancer. 1998 Jul;34(8):1274–81. doi: 10.1016/s0959-8049(98)00058-6. [DOI] [PubMed] [Google Scholar]
- 2.Cassidy J, Twelves C, Van Cutsem E. First-line oral capecitabine therapy in metastatic colorectal cancer: A favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol. 2002;13:566–575. doi: 10.1093/annonc/mdf089. [DOI] [PubMed] [Google Scholar]
- 3.Barton Debora, Brazil, Cruz Marcelo, Brazil, Schraibman Vladimir. Ulcerative Ileitis Secondary to Adjuvant Capecitabine for Colon Cancer: A Case Report. UICC world Cancer Congress 2006)–abstract. [Google Scholar]
- 4.Bouma G, Imholz A. Ileitis following capecitabine use. NedTijdschr Source :DeventerZiekenhuis, afd. InterneGeneeskunde, Deventer, the Netherlands. Geneeskd. 2011;155:A3064. [PubMed] [Google Scholar]
- 5.Hyodo I, Shirao K, Doi T, Hatake K, Arai Y, Yamaguchi K, Tamura T. A phase II Study of the global dose and schedule of capecitabine in Japanese patients with metastatic colorectal cancer. Jpn J ClinOncol. 2006;36(7):410–7. doi: 10.1093/jjco/hyl058. [DOI] [PubMed] [Google Scholar]