Abstract
Rationale:
Clear cell sarcoma of tendon and aponeurosis (CCSTA) or soft parts is a rare malignant melanin producing tumor entity that is derived from the neural crest cells originating from soft tissues displaying melanocytic differentiation. Diagnosis of CCSTA is difficult as it is dependent on age, size, location, necrosis, calcifications, cystic degeneration, and local to distant metastatic deposits. These tumors have very poor prognosis with a survival rate of 5–10 years because of local recurrence, early to late metastasis to lymph nodes, lungs, bones, and liver.
Patient concerns:
A 30-year-old Asian male has presented with a painful mass in the posterior aspect of the right ankle. He recalled of noticing an increase in the size of the lump after a traumatic insult 3 months ago. Physical examination revealed a mass of size 9x4 cm in the posterior ankle with no cutaneous ulcerative lesions. There is no history of any longstanding illness or malignancy.
Diagnoses:
Clear cell Sarcoma of Tendon and Aponeurosis (CCSTA) or CCS of Soft parts.
Interventions:
Conventional radiography demonstrated merely a soft tissue mass in the posterior compartment of the right ankle and significant calcaneal bone erosion with the sparse trabecular pattern. Plain conventional tomography showed a well-defined soft tissue heterogeneous mass with a hypoattenuating osteo-destructive focal lesion in the calcaneus. Magnetic resonance imaging (MRI) - T1 weighted imaging (T1WI) revealed an iso-intense signal relative to adjacent muscle; heterogeneous high-signal intensity on fat saturated T2 weighted imaging (T2WI). On contrast examination, lesion on T1WI, showed a heterogeneous high signal intensity, central low signal intensity with peripheral and septal enhancement. The immune-histochemistry analysis was positive for HMB-45, S-100, myoD1 and Ki67 (30%). Correlating with imaging and immune-histochemistry, a confirmatory diagnosis of CCSTA was made.
Outcomes:
CCSTA is typically a slowly growing painless mass in the deep soft tissues of ear, pancreas, kidney, penis, abdomen, especially in the lower extremities- Achilles tendon and aponeurosis of the ankle or in foot of young adults. As, these tumors are highly malignant, difficult to diagnose, early recognition by imaging and surgical excision are the mainstay of management.
Lessons:
Our case emphasizes the importance of recognizing radiological characteristics of CCSTA, and its differentiation from other soft tissue tumors, when presenting atypically. MRI plays a significant role in the diagnosis supported by histopathology and immune-histochemistry. So, radiologists should be familiar about this presentation that could guide other personnel for early detection of soft tissue tumors while including CCSTA into differential diagnosis for evaluation.
Keywords: Achilles tendon, case report, clear cell sarcoma of soft parts, HMB-45, malignant melanoma, MRI imaging diagnosis, synovial sarcoma
1. Introduction
Clear cell sarcoma of soft parts (tendon and aponeurosis)—CCSTA is a rare melanin producing malignant soft tissue sarcoma or malignant melanoma (MM) of soft parts, which is derived from the neural crest cells originating from soft tissue displaying melanocytic differentiation due to intralesional deposition of melanin.[1] Occurrence of tumor is almost equal in both genders,[2] although some reported cases showed that there was slight female predominance (male over female ratio was 0.8). The tumor has a peak incidence of between 20 and 40 years of adolescent and young adults, ranging from 7 years to 83 years (median age of 27 years). The tumor is typically small (<5 cm), relatively slow growing, well defined, deep soft tissue lesion, often juxtaposed to tendons, fascia, or aponeuroses.[3] CCS can also be seen in various anatomical sites like ear, bone, penis, kidney, pleura, pancreas, and gastrointestinal tract.[4–6] The prognosis of clear cell sarcoma of soft tissue is poor and it is mostly dependent on the size of the tumor, necrosis, and the presence of metastasis. The presence of melanin pigment with clear cell in cytoplasm, positive expression of S-100 protein and HMB-45 on histopathology, immune-histochemistry, and clinical characteristics also support the diagnosis of the CCSTA.
Histopathology, immune-histochemistry, cytogenetical analysis, reverse transcriptase polymerase chain reaction (RT-PCR), and fluorescent in situ hybridization (FISH study) which is presumed to be tumor specific[7] helps to differentiate CCSTA from other tumors of soft parts. It is associated with a distinct balanced reciprocal chromosomal translocation of t (12;22) (q13; q12) resulting in the formation of fusion protein,[8] EWS-AFT1 gene, BREF gene kinase region which is observed in more than 90% of cases with CCS. These tumors are highly malignant and difficult to diagnose, early recognition by imaging and surgical excision are the main stay of management.
2. Patient information
A 30-year-old male presented to our hospital with a painful swelling in the posterior aspect of the right ankle after a traumatic insult to ankle 3 months ago. However, his general well being deprived within 2 months duration for which he has been admitted into our hospital for further evaluation and management. The patient did not have any family history of long-standing illness or malignancy. Other history was unremarkable. There were no other abnormalities in the clinical examination other than a mass in the right ankle.
2.1. Imaging examination
All the material and methods were carried out in accordance with guidelines and regulations set by the First Affiliated Hospital of Dali University. All the protocols were approved by the Department of Radiology and Medical Imaging of Affiliated Hospital of Dali University. The patient provided the written consent on standard forms. Conventional radiography followed by computed tomography (CT) and magnetic resonance imaging (MRI) was done. CT image series were acquired and postprocessing was done as required. MRI series were acquired in different anatomical planes as per requirement in coronal, axial, and sagittal planes respectively.
2.2. Diagnostic assessment
X-ray revealed merely a soft tissue mass of size 9.6 cm × 4.2 cm × 3.5 cm in the posterior compartment of the right ankle with no calcaneal spur or calcifications in the overlying bursa. There is evidence of calcaneal bone erosion suggestive of secondary osteolytic focal lesion and sparse bony trabecular pattern (Fig. 1). Plain CT demonstrated a well-defined bulky soft tissue mass measuring 9.6 × 4.2 × 3.5 cm with a heterogeneous density in the posterior compartment of the right ankle (Fig. 2A) along with a hypoattenuating—focal osteo- destruction of calcaneus with sparse trabecular pattern (Fig. 2B).
Figure 1.
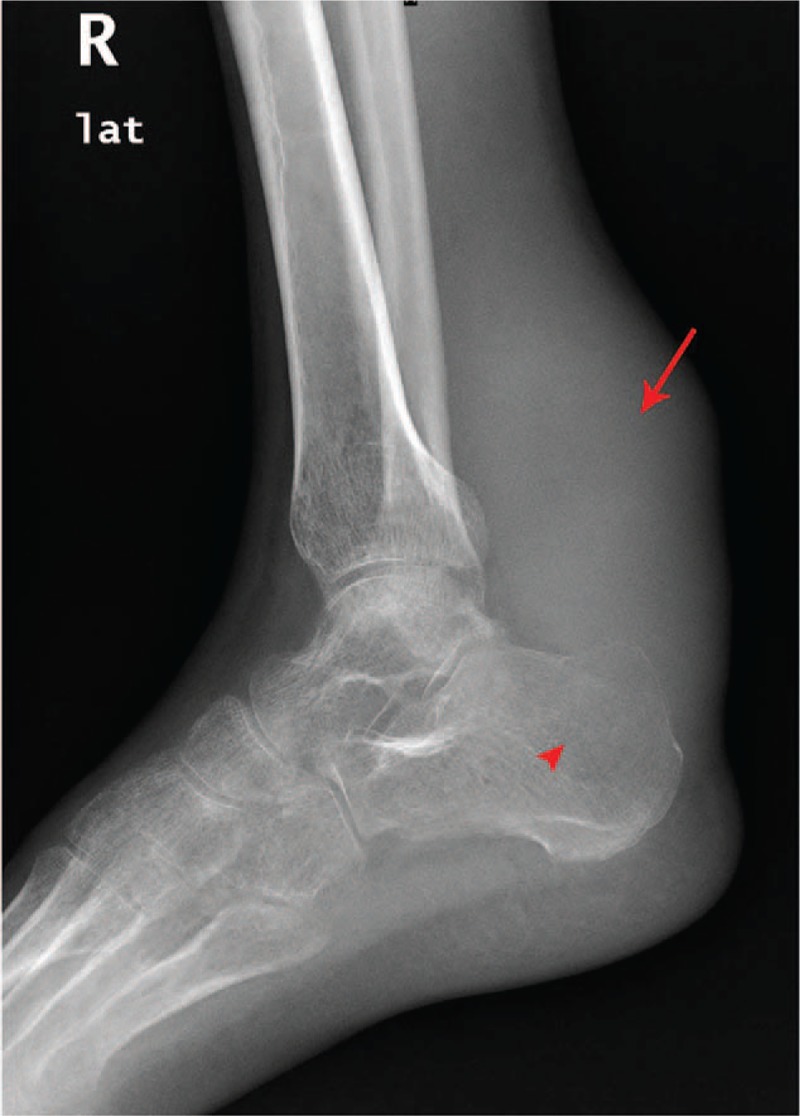
Conventional radiography of right ankle—lateral view showing a soft tissue swelling (red arrow) and ill defined, osteolytic lesion in the superio-posterior edge of the calcaneal bone (arrow head). Cortical thinning and sparse trabecular pattern can be appreciated.
Figure 2.
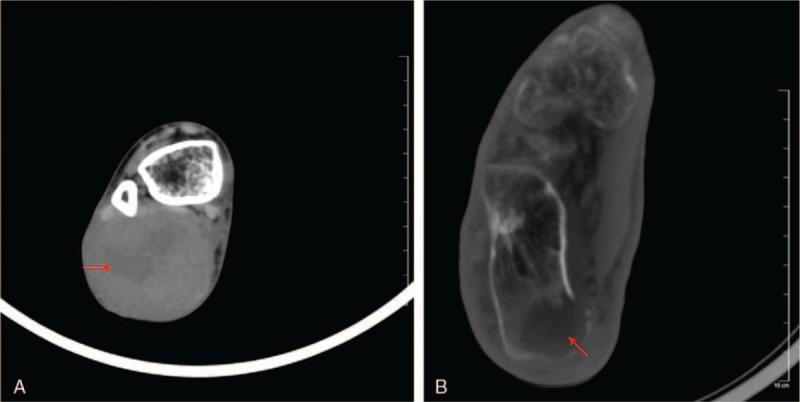
(A) CT axial view—soft tissue window showing a heterogeneous density within a well-defined mass in the posterior compartment of the right ankle (red arrow). (B) Axial view—bone window demonstrating a hypodense osteolytic lesion in the calcaneal bone (red arrow). CT = computed tomography.
Noncontrast T1 weighted imaging (T1WI) revealed, iso-intense signal relative to adjacent muscle (Fig. 3A). Proton density coronal image demonstrated multiple lobulated mass separated by septa (Fig. 3B). T2 weighted-imaging (T2WI) with fat saturation (fs) demonstrated a heterogeneous high-signal intensity (Fig. 3C and D). After contrast with Gadolinium, on T1WI, lesion showed heterogeneous high-signal intensity (Fig. 3E and F). The lesion also showed some central low-signal intensity with peripheral and septal enhancement, suggestive of necrotic tissue with loculated fluid.
Figure 3.
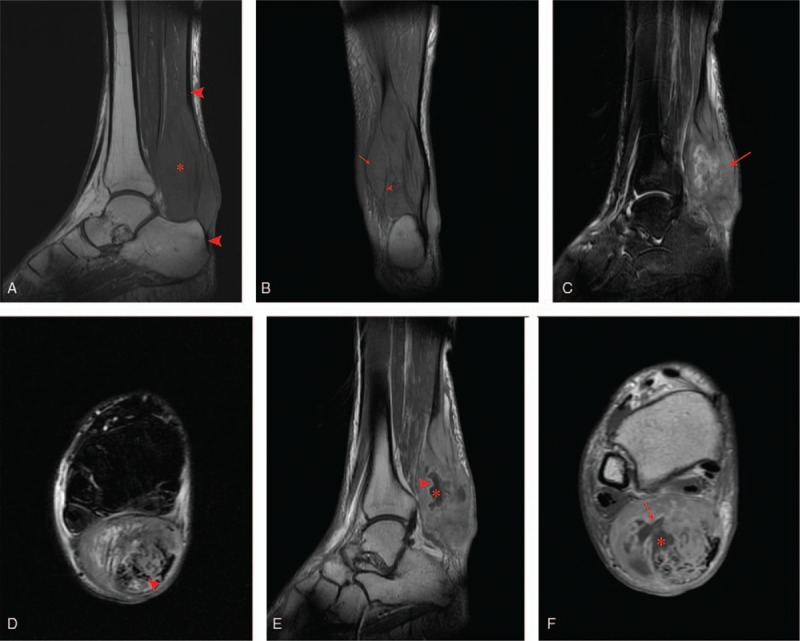
(A) Sagittal T1-weighted image showing ill-defined soft tissue mass with iso-intense signal compared with adjacent muscles (asterisks), arrow heads represent the Achilles tendon. (B) Coronal proton density (PD) image showing multiple lobulated mass (red arrow) separated by septa (arrow head). (C) Sagittal (D) axial T2-wieighted fat-saturation image showing high-signal intensity mass (red arrow) with rupture Achilles tendon (arrow head). (E) Sagittal (F) axial T1-weighted image after contrast (gadolinium) administration, showing heterogeneously enhanced high-signal intensity mass with central necrosis (asterisks) and peripheral enhancement (red arrow). PD = proton density.
2.3. Histopathology and immune-histochemistry
After interpretation of the images, the patient was treated surgically. A small tissue was extracted for further histopathology and immune-histochemistry analysis. Postoperative microscopic histopathological examination revealed few melanin granules and moderate amount of clear to eosinophilic cytoplasm. Round to oval-shaped tumor cells with clear or eosinophilic cytoplasm growing in sheets, nested to fascicular pattern with thin fibrous septa. Tumor cells possess prominent nucleoli, and a moderate amount of eosinophilic to clear cytoplasm (Fig. 4A). Immunohistochemistry revealed positive for HMB-45 (Human Melanoma Black-45) (Fig. 4B), S-100, myoD1, Ki67 (+30%), and negative for epithelial membrane antigen (EMA), muscle-specific actin (MSA) and CD99, and smooth muscle actin (SMA).
Figure 4.
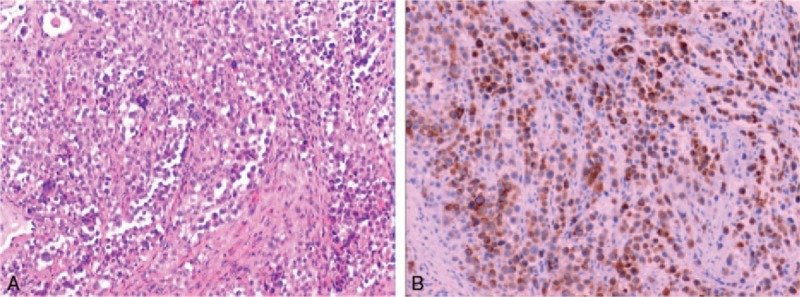
A. Round to oval-shaped tumor cells, nests of epithelioid cells with moderate amount of eosinophilic to clear cytoplasm (hematoxylin–eosin staining ×200). (B) Neoplasmatic cells with positive expression of HMB-45 (Human Melanoma Black-45 staining ×100). HMB-45 = human melanoma black-45.
With the support of MRI, which revealed characteristic features and as the immunohistochemistry analysis was positive for HMB-45, S-100, myoD1, and Ki67 (30%), a definitive diagnosis of clear cell sarcoma of tendon and aponeurosis (CCSTA) or CCS of soft parts was made.
3. Discussion
CCS was first described by Enzinger in 1965 as “soft tissue sarcomas that are typically malignant, which affects the tendon and aponeurosis and occurring mainly in the lower extremities, especially in the foot and ankle regions of young adults”. The incidence is <1% accounting for all malignancies of the musculoskeletal system.[1] Soft tissues of lower extremities, foot and ankle are the most common sites and gastrointestinal tract are the rarest site.[9] CCS can metastasize to lymph nodes, the lungs, skin, bone, liver and brain with 84% chance of local recurrence, 63% chance of late metastasis, and 30% chance of early metastasis. However, no metastatic depositions are detected in our case. One study showed that 5-year and 10-year survival rate was approximately 67% and 33%, respectively.[10]
Medical imaging plays a very important role for the evaluation of primary and secondary soft tissue tumors. Conventional plain radiography, CT, and MR imaging modalities (both non-contrast and contrast) are very useful for analyzing the tumor specific characteristics to differentiate CCSTA from other pathologies.
If the patient has history of trauma, then mass may be confused with hematoma. So, clear cell sarcoma should be considered in the differential diagnosis accordingly, when a mass is detected in the posterior compartment of the ankle with or without trauma.
CCSTA on a CT shows only homogeneous density soft tissue mass lesion but heterogeneous when necrosis is involved, significant bone destruction can be appreciated. But CT does not provide adequate information for differentiation as there is no soft tissue contrast.
3.1. Magnetic resonance imaging (MRI) presentation
MRI provides an excellent soft tissue contrast resolution which makes it highly sensitive for detection of soft tissue lesions and has satisfactory multi-planar imaging capabilities. The melanin containing tumors appear hyper-intense on T1WI and hypo-intense on T2WI, which is a classical presentation because of the paramagnetic properties of melanin causing the shortening of T1 and T2 relaxation time.[11] So, melanin can be considered as the precursor for such signal intensities and that signal intensity depends on the amount of intratumoral/lesional melanin. Lesions containing less or no melanin will give unspecific signals as iso-intense signal. On contrast examination, CCS usually shows a strong enhancement.[12] Literature review of 31 CCSST cases, 19% of the cases showed iso-intense signal in T1WI, and 52% of the cases were hyper-intense signal in T2WI. Parameters like size, location, cellularity, signifies the variations in signal intensities, depending on melanin content, connective tissues, necrosis, and cystic degeneration. HMB-45 positivity plays an important role on variant signal intensities on T1WI.[12] Schnarkowski et al[13] study of CCSTA, MR image showed iso-intense signal in T1WI and heterogeneous high-signal intensity on T2-fat suppression image. A report by Gandolfo et al[14] study of CCSTA, MR image showed that high-signal intensity on postcontrast T1-weighted image and high-signal intensity on T2WI. In our case, T1WI showed iso-intense signal to adjacent muscle, high-signal intensity on postcontrast T1WI and high-signal intensity on T2WI-fat saturation image.
The iso-intense signal in T1WI in cases of CCSTA is probably due to less amount of melanin in the tumor. In our case, melanin content was not appreciated which might have led to showing iso-intense signal relative to the adjacent muscle, which explains the MR significance on diagnosis the CCS.
3.2. Differential diagnosis
So, when forming a diagnosis of clear cell sarcomas of soft tissues/tendon aponeurosis (CCSSTA), parameters like age, size, location, presence of skin lesions, necrosis, calcifications, cystic degeneration, invasion into surrounding tissues-metastatic deposits[12] should be considered accordingly. Typical pathologies like malignant melanoma, synovial sarcoma, rhabdomyosarcoma, fibrosarcoma, and histocytosarcoma show some similarities on radiographic presentations but can be differentiated as following:
3.2.1. Malignant melanoma
It is originated in the neural crest melanocytes. Wide spread metastatic changes can be observed around the site. The CT scan is manifested as unilateral irregular soft tissue mass, no cystic degeneration, necrosis and calcification, invasion of surrounding tissue, and even destruction of bone.[12] On MRI, T1WI has high signal while T2WI has low signal because the melanoma has a para-magnetic effect. This classical MR-imaging features are similar to CCS; however, the definitive diagnosis of malignant melanoma relies on FISH and RT-PCR technique.[9]
3.2.2. Synovial sarcoma
It is not formed from synovial differentiation but occurring from the undifferentiated mesenchymal cells. It occurs most frequently in young and adolescent groups, in the extremities, especially confined to the joints, tendon sheath, joint capsule and bursa of the lower limbs. On CT, a synovial sarcoma appears as a deep seated soft tissue heterogeneous mass with density similar to or slightly lower than muscle with cystic degeneration and marginal calcifications.[15] On MR, T1WI-synovial sarcoma appears as a prominently heterogeneous multilobulated soft tissue mass with signal intensity slightly higher or iso-intense to the adjacent muscle. On T2WI synovial sarcoma demonstrate heterogeneity with high-signal intensity.[16] Heterogeneity signal has been described as the “Triple sign” by Jones et al.[17] This triple signal sign is high-signal intensity of liquid due to hemorrhage or necrosis, intermediate signal intensity or slightly higher than fat medium signal is as a result of mixture of solid cellular component and low signal are due to calcified or fibrotic collagenized regions.
3.2.3. Rhabdomyosarcoma
Rhabdomyosarcoma is the most common childhood soft tissue sarcoma with a high degree of malignancy.[18] In contrast, rhabdomyosarcoma is rare in adults. They arise from immature mesenchymal cells. CT scan reveals that the tumors are iso-dense or slightly hyperdense to adjacent muscle, may enhance after contrast, adjacent bone destruction and hazy peripheral muscle gap in 20% of cases and show calcifications along long axis. Periosteal reaction is rare. On MRI, demonstrates iso-intensity, or heterogeneously hyper-intense on T1WI and heterogeneously hyper-intense on T2WI.[19] On contrast examination, T1WI enhances considerably.
4. Conclusion
Clear cell sarcoma of tendon and aponeurosis (CCSTA) is a slowly growing, relentless, highly malignant soft tissue sarcoma with a tendency of local recurrence and metastatic spread with predilection to lower extremities. Confirmatory diagnosis can be made by MR imaging characteristics, while associating with other clinical parameters, with further support of specific histological and immune-histochemical analysis. Radiologists should be familiar about this rare entity while including it into the differential diagnosis of soft tissue tumors.
Acknowledgments
The authors would like to express their sincere thanks to the Department of Pathology, The First Affiliated Hospital of Dali University, Dali, China, for their assistance in obtaining good quality images for histopathology study and suggestion to immune-histochemistry analysis.
Author contributions
Conceptualization: Kalyan Sharma, Bimbadhar Valluru, Ling Liu.
Data curation: Kalyan Sharma, Bimbadhar Valluru, Ling Liu.
Formal analysis: Kalyan Sharma, Bimbadhar Valluru.
Investigation: Kalyan Sharma, Bimbadhar Valluru, Ling Liu.
Methodology: Kalyan Sharma, Bimbadhar Valluru, Sudhir Kumar Yadav.
Project administration: Kalyan Sharma, Bimbadhar Valluru, Ling Liu.
Resources: Kalyan Sharma, Ling Liu, Sudhir Kumar Yadav.
Supervision: Kalyan Sharma, Ling Liu, Sudhir Kumar Yadav.
Validation: Kalyan Sharma, Bimbadhar Valluru, Ling Liu.
Visualization: Bimbadhar Valluru, Ling Liu, Sudhir Kumar Yadav.
Writing – original draft: Bimbadhar Valluru.
Writing – review & editing: Kalyan Sharma.
Footnotes
Abbreviations: CCSTA = clear cell sarcoma of tendon and aponeurosis, CT = computed tomography, MRI = magnetic resonance imaging, T1WI = T1 weighted imaging, T2WI = T2 weighted imaging.
KS and BV These authors contributed equally to this work.
Funding/support: This work was supported by Medical Imaging Department, the First Affiliated Hospital of Dali University, key subjects for medical imaging.
First Author- Kalyan Sharma Co-First Author- Bimbadhar Valluru.
The authors have no conflicts of interest to disclose.
References
- [1].Enzinger FM. Clear cell sarcoma of tendons and aponeuroses. An analysis of 21 cases. Cancer 1965;18:1163–74. [DOI] [PubMed] [Google Scholar]
- [2].Isoda H, Kuroda M, Saitoh M, et al. MR findings of clear cell sarcoma: two case reports. Clin Imaging 2003;27:229–32. [DOI] [PubMed] [Google Scholar]
- [3].Panagopoulos I, Mertens F, Isaksson M, et al. Absence of mutations of the BRAF gene in malignant melanoma of soft parts (clear cell sarcoma of tendons and aponeuroses). Cancer Genet Cytogenet 2005;156:74–6. [DOI] [PubMed] [Google Scholar]
- [4].Achten R, Debiec-Rychter M, De Wever I, et al. An unusual case of clear cell sarcoma arising in the jejunum highlights the diagnostic value of molecular genetic techniques in establishing a correct diagnosis. Histopathology 2005;46:472–4. [DOI] [PubMed] [Google Scholar]
- [5].Friedrichs N, Testi MA, Moiraghi L, et al. Clear cell sarcoma-like tumor with osteoclast-like giant cells in the small bowel: further evidence for a new tumor entity. Int J Surg Pathol 2005;13:313–8. [DOI] [PubMed] [Google Scholar]
- [6].Huang W, Zhang X, Li D, et al. Osteoclast-rich tumor of the gastrointestinal tract with features resembling those of clear cell sarcoma of soft parts. Virchows Arch 2006;448:200–3. [DOI] [PubMed] [Google Scholar]
- [7].Patel RM, Downs-Kelly E, Weiss SW, et al. Dual color, breakapart fluorescence in situ hybridization for EWS gene rearrangement distinguishes clear cell sarcoma of soft tissue from malignant melanoma. Mod Pathol 2005;18:1585–90. [DOI] [PubMed] [Google Scholar]
- [8].Langezall SM, Graadt van Roggen JF, Cleton-Jansen AM, et al. Malignant melanoma is genetically distinct from clear cell sarcoma of tendon and aponeurosis (malignant melanoma of soft parts). Bri J Cancer 2001;84:535–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ekfors TO, Kujari H, Isomäki M. Clear cell sarcoma of tendons and aponeuroses (malignant melanoma of soft parts) in the duodenum: the first visceral case. Histopathology 1993;22:255–9. [DOI] [PubMed] [Google Scholar]
- [10].Clark MA, Johnson MB, Thway K, et al. Clear cell sarcoma (melanoma of soft parts): The Royal Marsden Hospital experience. Eur J Surg Oncol 2008;34:800–4. [DOI] [PubMed] [Google Scholar]
- [11].Farrokh D, Fransen P, Faverly D. MR findings of a primary intramedullary malignant melanoma: case report and literature review. AJNR Am J Neuroradiol 2001;22:1864–6. [PMC free article] [PubMed] [Google Scholar]
- [12].De Beuckeleer LH, De Schepper AM, Vandevenne JE, et al. MR imaging of clear cell sarcoma (malignant melanoma of the soft parts): a multicenter correlative MRI-pathology study of 21 cases and literature review. Skeletal Radiol 2000;29:187–95. [DOI] [PubMed] [Google Scholar]
- [13].Schnarkowski P, Peterfy CG, Johnston JO, et al. Clear cell sarcoma mimicking peripheral nerve sheath tumor. Skeletal Radiol 1996;25:197–200. [DOI] [PubMed] [Google Scholar]
- [14].Gandolfo N, Martinoli C, Cafiero F, et al. Malignant melanoma of soft tissues (clear cell sarcoma) of the foot. Is MRI able to perform a specific diagnosis? Report of one case and review of the radiological literature. Anticancer Res 2000;20:3993–8. [PubMed] [Google Scholar]
- [15].Mark DM, Michael SG, Bryan TJ, et al. Imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics 2006;26:1543–65. [DOI] [PubMed] [Google Scholar]
- [16].Mahajan H, Lorigan JG, Shirkhoda A. Synovial sarcoma: MR imaging. Magn Reson Imaging 1989;7:211–6. [DOI] [PubMed] [Google Scholar]
- [17].Jones BC, Sundaram M, Kransdorf MJ. Synovial sarcoma: MR imaging findings in 34 patients. AJR Am J Roentgenol 1993;161:827–30. [DOI] [PubMed] [Google Scholar]
- [18].Kindblom L-G, Lodding P, Angervall L. Clear-cell sarcoma of tendons and aponeuroses. An immunohistochemical and electron microscopic analysis indicating neural crest origin. Virchows Arch 1983;401:109–28. [DOI] [PubMed] [Google Scholar]
- [19].Jing QiZhu, JianHuaZhang GuangYuTang. CT and MRI observations of rhabdomyosarcoma in the head and neck. Oncology letters 2014;8:155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


