Abstract
Background:
Much controversy persists regarding the role of statins therapy in patients with sepsis. This systematic review and meta-analysis of randomized trials aimed to evaluate the clinical efficacy of statin therapy on mortality from sepsis.
Methods:
We comprehensively searched PubMed, Embase, and Cochrane controlled trials register for relevant clinical studies. Randomized controlled trials reporting the effect of statin therapy on mortality in septic patients were included. Pooled risk estimates were calculated with a fixed-effects model. Heterogeneity was determined by Cochran chi-square test and the I2 statistic.
Results:
Nine trials with a total of 2333 patients were included. Seven randomized trials were eligible for the in-hospital mortality analysis. There were 257 deaths among 1078 individuals in the statin treatment group and 268 deaths among 1081 individuals in the control group. There was no statistically significant difference (RR, 0.96; 95% CI, 0.83–1.11). Five randomized trials reported data on 28 day-hospital mortality. There were 123 deaths among 613 individuals in the statin treatment group and 141 deaths among 633 individuals in the control group. There was no statistically significant difference (RR, 0.90; 95% CI, 0.73–1.11).
Conclusion:
This systematic review and meta-analysis of randomized trials indicates that statin therapy has no effect on mortality outcomes in patients with sepsis compared with placebo.
Keywords: meta-analysis, mortality, sepsis, statins
1. Introduction
Severe sepsis is a common intensive care unit (ICU) admission diagnosis and consumes vast amounts of precious healthcare resources.[1] Despite improvements in resuscitation techniques, antibiotic therapy, and source control measures, mortality rates associated with severe sepsis rates still remains unacceptably high.[2]
Severe sepsis is characterized by a marked systemic inflammatory response syndrome (SIRS) to infection.[3] Animal studies and observational human studies have indicated that statins have anti-inflammatory, antioxidant, and immune-modulating effects,[4] which may attenuate the inflammatory response to sepsis.[5] Since 2009, several small published randomized controlled trials have explored the potential beneficial effects of statins on mortality rates in patients with sepsis, with controversial results. In addition, some previous systematic reviews and meta-analysis have summarized the findings of relevant studies on the association between statin use and outcome of septic patients.[6–9] However, these studies used different inclusion criteria and sample size, and thus the results were also controversial.
Recently, several new randomized control trials on this topic have been reported. Therefore, we performed the present updated systematic review and meta-analysis of all available placebo-controlled randomized trials with the primary objective of summarizing evidence about the effect of statin therapy on mortality from sepsis.
2. Methods
2.1. Search strategies
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.[10] A comprehensive searching was performed in the following electronic databases: PubMed, Embase, and Cochrane controlled trials register. The dates searched were from the inception of each database to December 2017. The following keywords and their combinations were used as search strategy: (“statin” or “hydroxymethylglutarylcoenzyme A reductase inhibitors” or “anticholesteremic agents” or “simvastatin” or “rosuvastatin” or “pravastatin” or “atorvastatin” or “fluvastatin” or “cerivastatin” or “pitavastatin” or “lovastatin”) and (“infection” or “sepsis” or “bacteremia” or “pneumonia”) and (“randomized” or “randomization” or “random” or “randomness”). The bibliographies of relevant articles and reviews were also checked for additional studies. There were no limitations for language or publication date. This is a systematic review and meta-analysis, which was based on previous published studies and did not have original data. Therefore, no ethical approval and patient consent are required.
2.2. Inclusion and exclusion criteria
Included studies met all the following criteria: randomized trial; evaluated the effect of statin therapy on mortality in patients with sepsis; drop-out rate was <20%; provided enough data to calculate relative risk (RR) and its 95% confidence interval (CI). Letters to the editor and conference abstracts were excluded from this study because of insufficient description of study methodology. Study selection was independently performed by 2 authors (MC and MJ), with divergences resolved by consensus.
2.3. Data extraction
Data extraction from each included article was performed by the same 2 independent authors (MC and MJ) with a predesigned data collection form. The following information was recorded: study design, study population/setting, primary outcome, duration of follow-up, type of statin and dose. The primary outcome of this study was 28-day mortality. The second outcome was in-hospital mortality.
2.4. Evaluation of study quality
The quality evaluation was performed with a 5-item instrument proposed by Jadad et al.[11] Each item represents one point. A study with ≤ 2 points was considered low quality and > 2 points was considered high quality. The quality assessment was completed by 2 independent authors (MC and MJ). Any disagreements were resolved by consensus.
2.5. Statistical analysis
The combined RR with its 95% CI was calculated with a Mantel–Haenszel fixed-effects model with a value of < 1 favoring statins.[12] Heterogeneity across included trials was evaluated using the Cochran chi-square test and the I2 statistic.[13] Publication bias was assessed with a visual plot. Statistical analyses were performed using STATA 10.0 (StataCorp, College Station, TX) with two-sided P values (set at .05).
3. Results
3.1. Study selection
The detailed process of study searching is outlined in Figure 1. We identified 285 potentially eligible studies. Most of them were excluded after reading titles and/or abstracts. 46 studies were further evaluated by full-text reading. Ultimately, 9 of the 46 studies were included in our study; of those not included, 35 were excluded because the study design was observational study, 2 because ward patients were included, and one because it included patients without sepsis.
Figure 1.
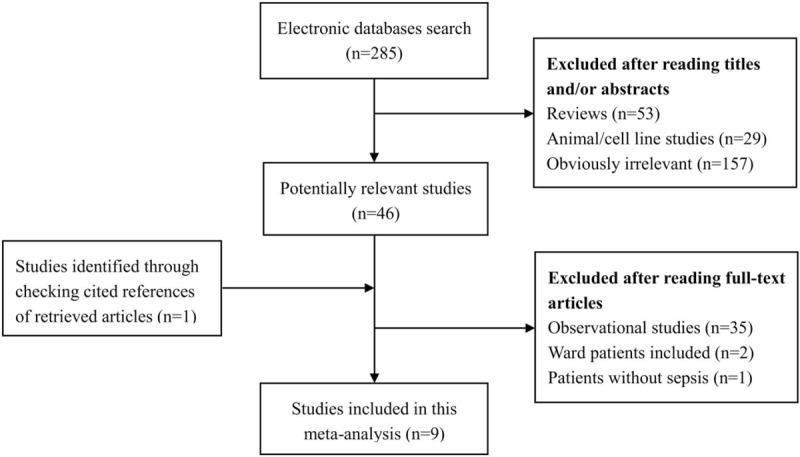
Flow of study selection.
3.2. Study characteristics and quality
All the included studies were published as peer-reviewed articles and all of them were in English.
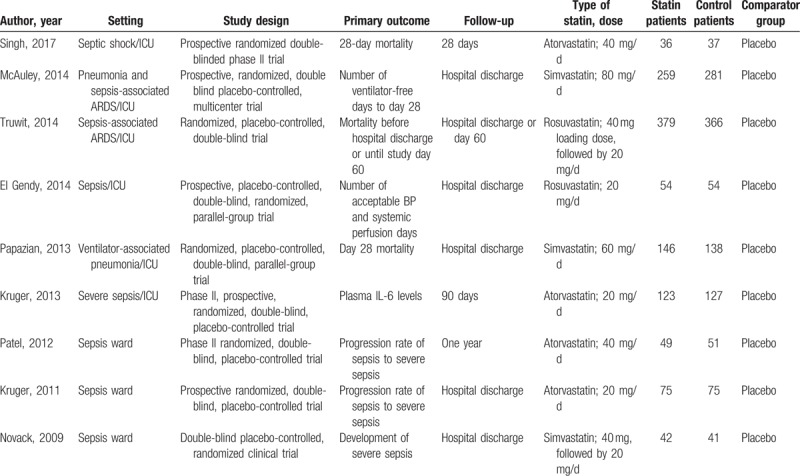
A total of 2333 sepsis patients were recruited to these 9 trials;[14–22] 1163 were randomized to the statin group and 1170 to the control group. All trials were placebo controlled. All included studies defined sepsis according to the American College of Chest Physicians/Society of Critical Care of Medicine Consensus Conference definitions (Sepsis Definition-1).[23] All the trials recruited sepsis patients without recent statin usage. Of the 9 trials, patients in 4 trials received atorvastatin,[14,15,19,22] patients in 3 trials received simvastatin,[16–18] and patients in 2 trials received rosuvastatin.[20,21] Four trials reported in-hospital mortality,[14,17,20,21] 2 trials reported 28-day mortality,[19,22] and 3 trials reported both as outcome of interest.[15,16,18] Detailed characteristics of the included studies are presented in Table 1.
Table 1.
Basic characteristics of included randomized controlled trials.
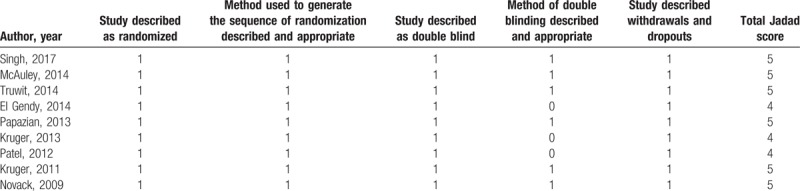
The quality of the included studies was assessed by Jadad scores, and the median Jadad score of the included studies was 5 (range, 4–5). Methods of randomization and allocation concealment were adequately addressed in the majority of trials. All 9 included studies were double-blind. All trials clearly stated withdrawals and dropouts (Table 2).
Table 2.
Jadad scoring of randomized controlled trials.
3.3. Data synthesis
3.3.1. In-hospital mortality
Seven randomized trials were eligible for the in-hospital mortality analysis. There were 257 deaths among 1078 individuals in the statin treatment group and 268 deaths among 1081 individuals in the control group. There was no statistically significant difference (RR, 0.96; 95% CI, 0.83–1.11) (Fig. 2). There was no significant heterogeneity of findings across the studies (I2 = 20%, P = .28).
Figure 2.
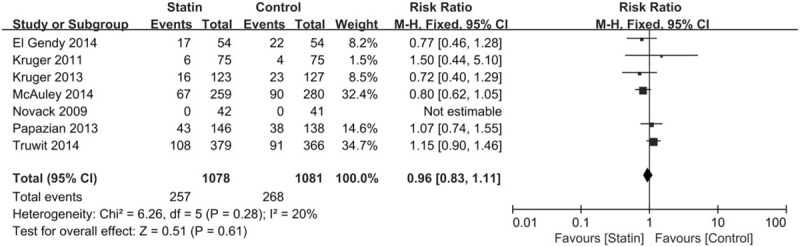
Summary effects of statins versus placebo on changes in in-hospital mortality.
3.3.2. 28-day hospital mortality
Five randomized trials reported data on 28 day-hospital mortality. There were 123 deaths among 613 individuals in the statin treatment group and 141 deaths among 633 individuals in the control group. There was no statistically significant difference (RR, 0.90; 95% CI, 0.73–1.11) (Fig. 3). No significant heterogeneity was observed among the studies (I2 = 29%, P = .23).
Figure 3.
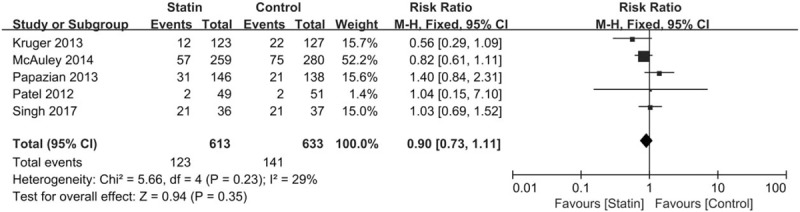
Summary effects of statins versus placebo on changes in 28-day mortality.
3.4. Publication bias
Visual inspection of funnel plot did not identify a skewed or asymmetrical shape, excluding the presence of small publication bias (Fig. 4).
Figure 4.
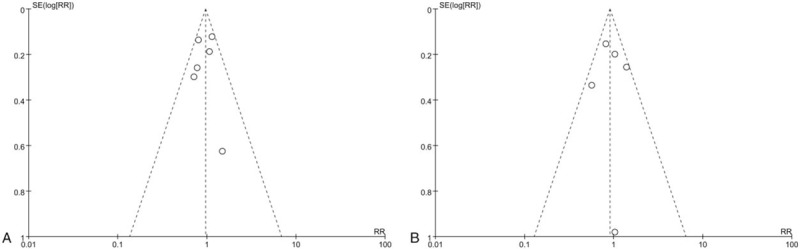
Funnel plot for the risk of (A) in-hospital mortality and (B) 28-day mortality.
4. Discussion
The present meta-analysis summarizes the results of 9 randomized trials, including 2333 sepsis patients. To the best of our knowledge, this is the largest meta-analysis of clinical trials evaluating the effect of statin therapy on mortality in patients with sepsis. The results indicated that statin use did not improve mortality outcomes in septic patients compared with placebo.
Recently, the potential relationship between statin therapy and mortality in patients with sepsis has captured attention and published results on this association have been contradictory. A meta-analysis performed by Janda et al[24] included 20 studies (only 1 randomized control trial) and suggested a protective effect for statins in patients with sepsis. Another meta-analysis of observational studies by Tleyjeh et al[25] also indicated that statin use might be associated with a beneficial effect in treating and preventing various infections. A study by Wan et al[9] summarized the evidence from 5 clinical trials and 27 observational studies. They found a survival advantage in meta-analysis of observational studies but not in randomized controlled trials. Rothenberg et al[7] systematically reviewed of statin in critically ill patients and concluded that aspirin might play a role in reducing mortality in non-cardiac critically ill patients. Quinn et al[6] found that statins use did not significantly improve either in-hospital mortality or 28-day mortality in patients with sepsis with a systematic review and meta-analysis. Deshpande et al[26] and Pasin et al[8] also performed a meta-analysis of 7 trials and 5 trials, respectively. They concluded that treatment with statin could not improve a septic patient's outcome. Up to now, several new randomized control trials on this topic have been performed. Our meta-analysis included the more recent studies and thereby had larger sample size; potentially improved statistical power. In addition, our study only included high quality randomized control trials, which reduced the heterogeneity across studies (no obvious heterogeneity was observed in this study) and enhanced the robustness of our findings.
The main findings from the present meta-analysis of clinical trials were inconsistent with those of observational studies, which may be due to the methodological differences between clinical trials and observational studies. Observational studies can’t completely avoid unknown or unquantified confounders, which are inherent to the study design. In particular, “healthy user” effect is one major source of such potentially confounding factors.[27] Another limitation of meta-analysis based on observational studies was the significant heterogeneity across studies. By contrast, our meta-analysis of clinical trials is able to control the “healthy-user” effect. In addition, no obvious heterogeneity is detected in our study.
There are several important limitations should be considered in interpreting the results of our meta-analysis. First, although visual inspection of funnel plot did not identify a skewed or asymmetrical shape, some inevitable publication bias may exist. Second, the overall sample size of the contributing studies in specific outcome analysis was still small. Third, despite generally similar demographic characteristics and no significant evidence of heterogeneity across these clinical trials, some confounding factors such as difference in statin type and dose, early definition, and early antimicrobial therapy might affect the results of statin therapy and thus cause potential bias. A meta-analysis is unable to solve problems with confounders that could be inherent in the original studies.[28] Finally, because most included studies did not provide risk estimates according to different dose, we were not able to evaluate the effect of different dose on mortality outcomes in septic patients.
Our study also had some important strength. To date, little is known about the effects of statin therapy on mortality in patients with sepsis, although observational studies have suggested a protective effect. Several randomized trials have been performed to evaluate the relationship between statin use and the mortality outcome in septic patients, but the results were inconsistent and conflicting. As individual studies may have insufficient statistical power, the current systematic review and meta-analysis of 9 randomized trials involving relatively large samples of sepsis patients enhanced the statistical power to detect an important difference and provided more reliable estimates.
5. Conclusion
The present meta-analysis based on 9 prospective randomized trials indicated that statin therapy could not improve mortality in septic patients compared with placebo-controls.
Author contributions
Conceptualization: Mengyan Chen, Mingxia Ji, Xiaoshui Si.
Data curation: Mengyan Chen, Mingxia Ji, Xiaoshui Si.
Formal analysis: Mengyan Chen, Mingxia Ji, Xiaoshui Si.
Funding acquisition: Mengyan Chen, Mingxia Ji.
Investigation: Mengyan Chen, Mingxia Ji, Xiaoshui Si.
Methodology: Mengyan Chen, Mingxia Ji, Xiaoshui Si.
Project administration: Mengyan Chen, Mingxia Ji.
Resources: Mengyan Chen, Mingxia Ji, Xiaoshui Si.
Software: Mengyan Chen, Mingxia Ji.
Supervision: Mengyan Chen, Mingxia Ji.
Validation: Mengyan Chen, Mingxia Ji, Xiaoshui Si.
Visualization: Mengyan Chen, Mingxia Ji.
Writing – original draft: Mengyan Chen, Mingxia Ji.
Writing – review & editing: Mengyan Chen, Mingxia Ji, Xiaoshui Si.
Footnotes
Abbreviations: CI = confidence interval, ICU = intensive care unit, RR = relative risk, SIRS = systemic inflammatory response syndrome.
This study was supported by grants from the public welfare science and technology projects of Yiwu Science and Technology Bureau (2016-S-15).
The authors have no conflicts of interest to disclose.
References
- [1].Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–10. [DOI] [PubMed] [Google Scholar]
- [2].Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med 2017;45:486–552. [DOI] [PubMed] [Google Scholar]
- [3].Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348:138–50. [DOI] [PubMed] [Google Scholar]
- [4].Blanco-Colio LM, Tunon J, Martin-Ventura JL, et al. Anti-inflammatory and immunomodulatory effects of statins. Kidney Int 2003;63:12–23. [DOI] [PubMed] [Google Scholar]
- [5].Mekontso-Dessap A, Brun-Buisson C. Statins: the next step in adjuvant therapy for sepsis? Intensive Care Med 2006;32:11–4. [DOI] [PubMed] [Google Scholar]
- [6].Quinn M, Moody C, Tunnicliffe B, et al. Systematic review of statins in sepsis: there is no evidence of dose response. Indian J Crit Care Med 2016;20:534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rothenberg FG, Clay MB, Jamali H, et al. Systematic review of beta blocker, aspirin, and statin in critically ill patients: importance of severity of illness and cardiac troponin. J Investig Med 2017;65:747–53. [DOI] [PubMed] [Google Scholar]
- [8].Pasin L, Landoni G, Castro ML, et al. The effect of statins on mortality in septic patients: a meta-analysis of randomized controlled trials. PLoS One 2013;8:e82775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wan YD, Sun TW, Kan QC, et al. Effect of statin therapy on mortality from infection and sepsis: a meta-analysis of randomized and observational studies. Crit Care 2014;18:R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. W64. [DOI] [PubMed] [Google Scholar]
- [11].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [12].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [13].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [14].Kruger PS, Harward ML, Jones MA, et al. Continuation of statin therapy in patients with presumed infection: a randomized controlled trial. Am J Respir Crit Care Med 2011;183:774–81. [DOI] [PubMed] [Google Scholar]
- [15].Kruger P, Bailey M, Bellomo R, et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am J Respir Crit Care Med 2013;187:743–50. [DOI] [PubMed] [Google Scholar]
- [16].McAuley DF, Laffey JG, O’Kane CM, et al. Simvastatin in the acute respiratory distress syndrome. N Engl J Med 2014;371:1695–703. [DOI] [PubMed] [Google Scholar]
- [17].Novack V, Eisinger M, Frenkel A, et al. The effects of statin therapy on inflammatory cytokines in patients with bacterial infections: a randomized double-blind placebo controlled clinical trial. Intensive Care Med 2009;35:1255–60. [DOI] [PubMed] [Google Scholar]
- [18].Papazian L, Roch A, Charles PE, et al. Effect of statin therapy on mortality in patients with ventilator-associated pneumonia: a randomized clinical trial. JAMA 2013;310:1692–700. [DOI] [PubMed] [Google Scholar]
- [19].Patel JM, Snaith C, Thickett DR, et al. Randomized double-blind placebo-controlled trial of 40 mg/day of atorvastatin in reducing the severity of sepsis in ward patients (ASEPSIS Trial). Crit Care 2012;16:R231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Truwit JD, Bernard GR, Steingrub J, et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med 2014;370:2191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].El Gendy HA, Elsharnouby NM. Safety and vasopressor effect of rosuvastatin in septic patients. Egypt J Anaesth 2014;30:311–7. [Google Scholar]
- [22].Singh RK, Agarwal V, Baronia AK, et al. The effects of atorvastatin on inflammatory responses and mortality in septic shock: a single-center, randomized controlled trial. Indian J Crit Care Med 2017;21:646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864–74. [PubMed] [Google Scholar]
- [24].Janda S, Young A, Fitzgerald JM, et al. The effect of statins on mortality from severe infections and sepsis: a systematic review and meta-analysis. J Crit Care 2010;25:656e7-22. [DOI] [PubMed] [Google Scholar]
- [25].Tleyjeh IM, Kashour T, Hakim FA, et al. Statins for the prevention and treatment of infections: a systematic review and meta-analysis. Arch Intern Med 2009;169:1658–67. [DOI] [PubMed] [Google Scholar]
- [26].Deshpande A, Pasupuleti V, Rothberg MB. Statin therapy and mortality from sepsis: a meta-analysis of randomized trials. Am J Med 2015;128:410.e1–7.e1. [DOI] [PubMed] [Google Scholar]
- [27].Majumdar SR, McAlister FA, Eurich DT, et al. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: population based prospective cohort study. BMJ 2006;333:999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA 2010;303:1077–83. [DOI] [PubMed] [Google Scholar]


