Abstract
Ovarian masses are one of the most frequently identified entities in gynecological practice. Early differential diagnosis is a key factor in the medical management of each patient. Transvaginal ultrasound along with additional preoperative testing, such as serum cancer antigen 125 (CA-125) levels and the Risk of Ovarian Malignancy Algorithm (ROMA) score, usually provide sufficient information for a presumptive diagnosis. Minimally invasive surgery as a therapeutic approach is the standard procedure for uncomplicated and benign adnexal masses. Histopathological examination alone, or in conjunction with immunohistochemical testing establishes a more certain diagnosis in the final step of the patient management plan. We developed a retrospective descriptive observational study based on the evaluation of 107 patients admitted to the Department of Obstetrics and Gynecology at “Sf Pantelimon” Clinical Emergency Hospital in Bucharest between January 2000 and July 2017. Each patient was diagnosed with an ovarian mass and underwent laparoscopic surgery for treatment. All data underwent descriptive statistical analysis in order to establish correlations between preoperative test results and definitive diagnoses. The typical ultrasound findings of endometriotic cysts were histopathologically confirmed in 52.6% of the examined patients. Using ultrasound, benign teratomas were suspected in 66.6% of all documented dermoid cysts and 90% of the patients with a calculated ROMA score had corresponding values less than 15%. Mean CA-125 value was 26.58 U/mL. Laparoscopic surgery with ovarian cystectomy was performed for 78.5% of the cases. Histopathological examinations established endometriosis was present in 26.16% of cases. According to an independent samples t test (with 2 extreme values eliminated), patients in the premenopausal group had a significantly lower mean ROMA score than postmenopausal patients (6.87% vs 20.98%, respectively; P < .001). The groups had similar homogeneity (P = .131 according to the Levene test). Our results showed that transvaginal ultrasound established a presumptive diagnosis for almost half of our patients. Cystectomy was the main surgical procedure used for the management of benign ovarian masses. Endometriosis was the most common benign ovarian pathology evaluated and treated in our study.
Keywords: benign adnexal masses, histopathological diagnosis, laparoscopy, ROMA score, transvaginal ultrasound
1. Introduction
Ovarian tumors are among the most frequent pathophysiological conditions discovered in gynecological practice. Whether they are identified during a routine examination or as a consequence of presenting symptoms or complications, the primary symptoms are usually nonspecific. Pelvic examinations in conjunction with transvaginal ultrasound (TV-US) are the key elements of successful management before definitive diagnosis. An annual gynecologic examination and an annual pelvic examination are recommended for preventive health care.[1]
Worldwide, the number of newly diagnosed cases of ovarian cancer is approaching 250,000 per year.[2] Therefore, exclusion of a potentially malignant ovarian tumor is the main objective of every gynecologist. Borderline ovarian tumors, which are histologically defined by atypical epithelial proliferation without stromal invasion,[3] are between benign and malignant ovarian tumors and comprise approximately 15% to 20% of all epithelial ovarian malignancies[4]; therefore, a convergence of efforts to obtain a prompt diagnosis is required.
Patient outcomes have improved with each diagnostic decision that has been made objectively and in a stepwise manner. It has been shown that the International Ovarian Tumor Analysis (IOTA) simple rules (SR) for ultrasound are highly sensitive and specific for predicting ovarian malignancy preoperatively[5]; therefore, their utility in clinical practice has become increasingly significant. Characterization of ovarian pathology is often acquired through pelvic magnetic resonance imaging (MRI) or computerized tomography (CT). Although quality images can be easily obtained with CT and MRI, and although these images can simplify the diagnostic process, the use of these expensive testing modalities should be limited to their recommended indications. Usually, simple serous ovarian cysts do not require detailed investigations; however, other adnexal masses may require more extensive diagnostic imaging evaluations.
Cancer antigen 125 (CA-125) and the Risk of Ovarian Malignancy Algorithm (ROMA) score are extremely efficient tools for determining the nature of ovarian tumors in certain clinical backgrounds. Although CA-125 may be abnormally increased in approximately 80% of women with advanced ovarian cancer,[6] this test lacks diagnostic value due its limited specificity for only ovarian tissue. Excisional biopsy and histopathological (HP) examination are the gold standard procedures for diagnostic certainty.
Using evidence-based medicine, the approach to each clinical case depends on the patient's menopausal status. Menopausal state directs the physician's decision regarding the best method of evaluation. The decision to remain limited to clinical examination and TV-US testing with serum biomarker screening or to proceed directly to surgical exploration will depend mainly on the clinical context. Each of the former clinical investigative processes could be considered controversial depending on when each evaluation method should best be used.
The aim of this study was to emphasize the role of preoperative investigations and the unique indications for the different testing methods to determine a diagnosis for ovarian tumors. Another aim was to detect correlations between the preoperative diagnostic tests and the final HP diagnosis. We also reviewed the minimally invasive techniques used to provide therapeutic management for our study group at our clinic.
2. Methods
2.1. Study population
During this retrospective, observational study, we evaluated the diagnostic and therapeutic management of a group of patients with adnexal masses who were admitted to the Department of Obstetrics and Gynecology at “St Pantelimon” Clinical Emergency Hospital in Bucharest between January 2000 and July 2017. We developed our patient database by reviewing the electronic medical records and surgical protocol registries with a special focus on our basic inclusion criteria.
Patients were included if they were diagnosed with an ovarian mass by physicians at our clinic between 2000 and 2017 and if conventional laparoscopy was used for disease management. Exclusion criteria comprised a confirmed diagnosis of polycystic ovary syndrome and ovarian mass that was managed with abdominal surgery
2.2. Data collection
The electronic medical system record used by the hospital (Info World platform), provided the demographic and clinical data we used for the study and included patient age, reason for hospital admission, medical history, menopausal status, and ovarian tumor diagnosis according to the HP examination. The main preoperative data collected were TV-US results, CA-125 levels, and ROMA scores, which served as the quantitative and qualitative variables for statistical evaluation. TV-US examination and its pattern of recognition formed the presumptive diagnosis, which were counterbalanced by the certainty of diagnosis provided by the HP diagnosis. The data collection process also included an analysis of the surgical protocol registries, which included records of the patients’ main surgical procedures and hospital stay lengths. This study was retrospective in nature and was approved by the local ethics committee. Informed consent was provided by each patient, which included permission to use their data. This study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference for Harmonization.
2.3. Statistical analysis
Given the study design, the main potential sources of bias included imprecision during initial data input and human error.
The study population consisted of 107 patients who met the aforementioned inclusion criteria. The present report is based on descriptive statistics, statistical tests, and bivariate correlations between certain variables. The reported descriptive statistics comprised average values as well as minimum and maximum values and percentiles. For our statistical analyses, we used independent samples t test (difference between the mean values of 2 groups) and the Levene test (difference between the variances of 2 groups). Given the numeric variables from our database, we also used the Pearson correlation coefficient. P values < .001 were considered statistically significant and the associated confidence level was 99.9%.
3. Results
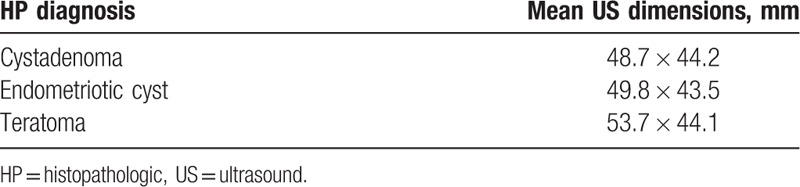
For this study, we evaluated 107 patients aged 15 to 74 years (mean age was 32 years). We divided the patients into 2 groups based on their menopause status. As such, 98 (91.5%) patients comprised the premenopausal group (mean age was 30 years), while 9 (8.4%) patients comprised the postmenopausal group (mean age was 58 years). The difference between the mean of ages between these 2 groups was found to be statistically significant using the t test for independent samples. The Levene test showed the equality between the 2 variances (P = .553), indicating that the groups varied significantly in different intervals, but had similar homogeneity. The majority of the patients were admitted with pelvic or abdominal pain. After a compulsory gynecological examination, 79 (73.8%) patients underwent a TV-US examination, which is the diagnostic imaging tool of choice at our clinic for the evaluation of adnexal masses. The TV-US provided dimensions for the ovarian structures in 50.6% of the cases. Table 1 presents the correlation between the mean ovarian structure dimensions on TV-US and the HP diagnosis. Of the 54 (50.6%) patients with documented ovarian structure dimensions data, 18 patients were confirmed to have an ovarian endometriotic cyst, 14 had a cystadenoma (serous or mucinous), and 8 had a mature ovarian teratoma or dermoid cyst.
Table 1.
Correlation between histopathological diagnosis and mean ultrasound dimensions (mm).
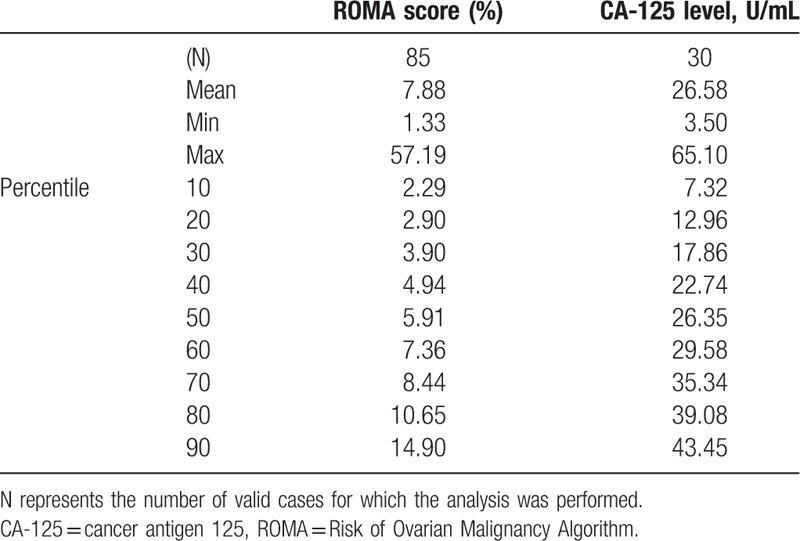
Descriptive statistics including the ROMA scores and CA-125 levels are summarized in Table 2. The ROMA scores had a wide range of values from 1.33% to 57.19%, with a mean value of 7.88%. In addition, 90% of patients whose screening included ROMA testing had corresponding scores less than 15%. The CA-125 levels also had a wide range of values from 3.5 to 65.1 U/mL with a mean value of 26.58 U/mL.
Table 2.
Descriptive statistics regarding Risk of Ovarian Malignancy Algorithm scores and Cancer Antigen 125 levels.
Mean ROMA score was 6.87% in the premenopausal group, while in the postmenopausal group, mean value of ROMA score was 20.98%. According to the independent samples t test (with 2 extreme values being eliminated), the postmenopausal group had a significantly greater ROMA score than the premenopausal group (P < .001) and the groups had similar homogeneity (P = .131 according to the Levene test). Further, 90% of the patients in the premenopausal group had a ROMA score less than 12%.
The CA-125 means and variations were similar between the premenopausal and postmenopausal groups (independent samples t test: P = .677; Levene test: P = .456). The afferent CA-125 levels of 30 patients indicated that the premenopausal group had CA-125 levels between 3.5 and 65.1 U/mL, and that those in the postmenopausal group had CA-125 levels between 15.3 and 46.1 U/mL.
The 2 paraclinical investigations are described in relation to menopause status in Fig. 1.
Figure 1.
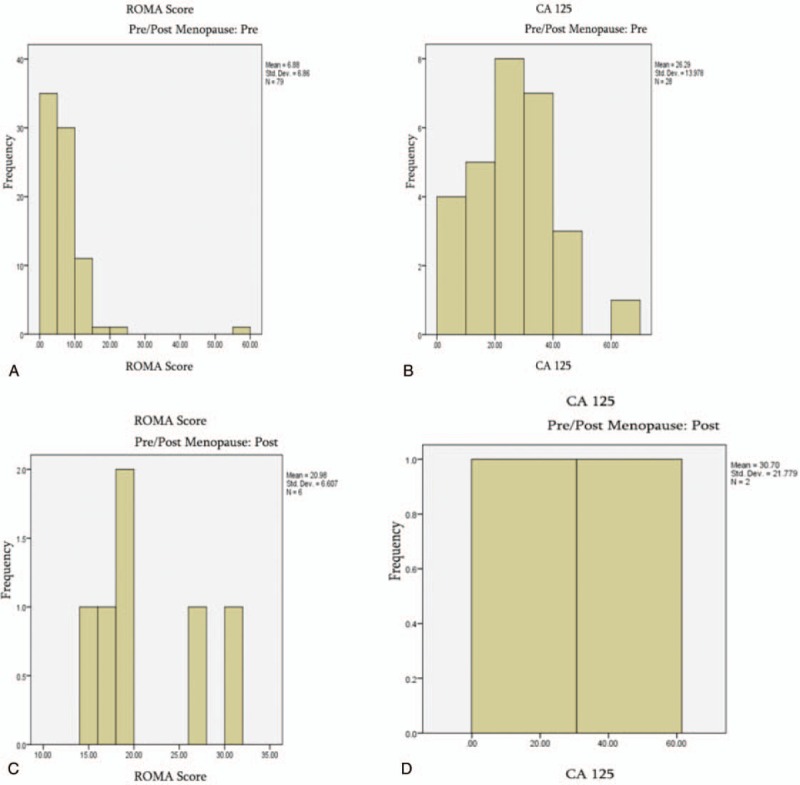
Histograms of risk of ovarian malignancy algorithm scores and cancer antigen 125 levels for pre/postmenopausal groups. ROMA score for premenopausal (A) and postmenopausal (C) patients. CA-125 levels of premenopausal (B) and postmenopausal (D) patients. CA-125 = cancer antigen 125, ROMA = Risk of Ovarian Malignancy Algorithm.
Details of the laparoscopic management of the 107 study patients are presented in Fig. 2. The main surgical procedures were unilateral right or left ovarian cystectomies (identified in 42 clinical records). Two methods of minimizing surgery were noted: replacement of classic surgery with the laparoscopic approach, and ovarian cystectomy performed at the same time, when applicable, instead of partial ovarian resection or oophorectomy.
Figure 2.
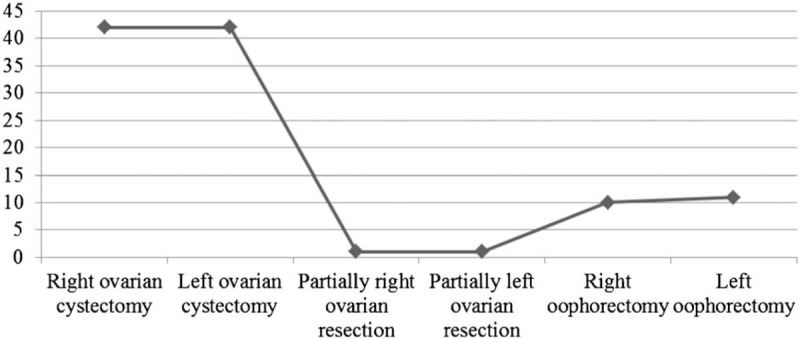
Laparoscopic procedures applied in the management of ovarian masses.
The evaluation of HP diagnoses confirmed that endometriosis was the most frequent ovarian pathology in our study sample with 28 (26.16%) cases registered. Twenty-one patients (19.6%) were diagnosed with ovarian endometriotic cysts and 7 (6.5%) patients were diagnosed with ovarian endometriotic foci. In the remaining cases, cystadenomas were diagnosed as follows: 8 (7.4%) serous, 5 (4.6%) mucinous, and 4 (3.7%) unspecified; mature teratomas were identified in 10 (9.3%) cases. Only 1 case of borderline ovarian tumor was identified and there were no cases of ovarian malignancies. Other benign entities such as follicular cysts, ovarian tissue, or yellow body ovarian cysts accounted for 33 (30.8%) cases. Of the 7 patients who had multiple benign ovarian pathologies that were clinically described as masses, 1 patient was specifically noted to have a bilateral ovarian pathology that included a mature teratoma on the left ovary and an endometriotic cyst on the right ovary.
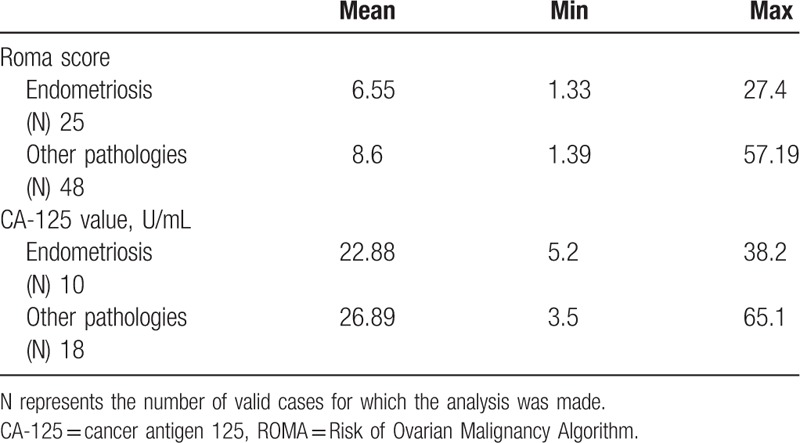
Patients who were confirmed to have endometriosis of both aforementioned forms were compared to those with different diagnoses based on their ROMA scores and CA125 values. Table 3 presents the means and variances for both biomarkers, and shows that they differed insignificantly, mostly within the range of their afferent values.
Table 3.
Descriptive statistics in patients diagnosed with endometriosis regarding their Risk of Ovarian Malignancy Algorithm scores and Cancer Antigen125 levels.
A similar type of analysis was used to determine the means and variances in patients diagnosed with a mature teratoma. This analysis showed that both biomarkers insignificantly varied.
In all patients, hospitalization length varied between 1 and 14 days, with an average of 4 days.
4. Discussion
The incidence of ovarian cancer increases with age (0.2–1.4 per 100,000 women aged 20–29 years and 1.8–2.2 per 100,000 women aged 30–39 years). The lifetime risk of developing ovarian cancer is currently 1.4%.[7] In the current study, the average age of patients was 32 years: therefore, compared with the cited lifetime risk, there was a slightly higher risk of developing ovarian malignancy in our study group. Fortunately, there were no cases of malignant ovarian tumors in our patient group study: 97 patients were diagnosed with benign tumors and one was diagnosed with a borderline tumor. In addition, 79 ultrasound US examinations were performed, but the presumptive diagnoses were seldom specific. However, a multicenter study concluded that up to 90% of extrauterine masses could be correctly classified as benign or malignant by an experienced ultrasonographer.[8] Consequently, it is important to take into account the US characteristics indicating benign or malignant appearances for presumptive diagnosis.
IOTA recommendations regarding indications for SR, Risk of Malignancy Index (RMI), logistic regression models [logistic regression model 1 (LR1) and logistic regression model 2 (LR2)], and expert US evaluation are strictly stated in the literature. Ultrasound evaluation is frequently used for diagnosis in cases of uncomplicated benign ovarian tumors such as serous cysts due its ease of use and relatively low cost for TV-US. Furthermore, according to the SR, reuniting variables such as the M features for malignant tumors and B features for benign tumors facilitates the orientation of a certain diagnosis. When the SR are not applicable, RMI LR1, or LR2 can be used. When none of the above indices are sufficient, expert evaluation is required.
In our report, the typical US appearance of “ground glass” inside a hypoechoic cystic structure was strongly suggestive of ovarian endometriotic cysts and this US finding was observed in 52.6% of the examined patients. Teratoma was suspected whenever thick-walled cysts with hyperechoic nonhomogeneous contents were identified using US alone; this was prevalent in 66.6% of all biopsy-confirmed cases of dermoid cysts.
The analysis of the 107 study patients into the 2 subgroups of premenopausal and postmenopausal women was based on the scientific idea that the postmenopausal ovary continues to produce cysts.[9] A meta-analysis of 2 multicenter cohorts that were divided based on menopausal status proved that IOTA SR for US examinations had the same Se between groups: 0.93, 95% confidence interval (95% CI) = 0.84–0.97 for premenopausal patients and 0.93, 95% CI = 0.88–0.96 for postmenopausal patients. However, for premenopausal patients, Se was significantly higher than those detected using other US algorithms. For example, in premenopausal patients, LR2 had a Se of 0.85 and RMI had a Se of 0.44, while in postmenopausal patients, LR2 had a Se of 0.94 and RMI had a Se of 0.79, thereby reflecting the efficiency of SR and LR2 in optimizing pretherapeutic management.[10]
Overall, in our study, 73.46% of premenopausal women and 77.77% of the postmenopausal women underwent TV-US evaluation. A previous study showed that the diagnostic accuracy of TV-US is significantly higher for premenopausal women than for postmenopausal women (97% vs 85%, respectively; P < .05).[11] In a prospective study of 1066 patients with persistent adnexal masses, US pattern recognition was shown to be superior to serum CA-125 for the discrimination between benign and malignant adnexal masses.[12]
Although the practice guidelines recommend that ovarian cancer screening should begin at age 35,[13] our analysis of the clinical and radiologic imaging examinations indicated the need for CA-125 levels in 19 patients younger than 35 years. On the basis of data from a meta-analysis of 6 studies, a CA-125 level greater than 35 U/mL has a Se of 69% to 97% and a Sp of 81% to 93% for the diagnosis of ovarian cancer.[14] Laparoscopy is feasible and should be performed for ovarian masses whenever the preoperative US examination has not revealed findings suspicious for ovarian malignancy.[8]
All 107 patients included in this study underwent minimally invasive laparoscopic surgery for the treatment of ovarian masses. Laparoscopic ovarian cystectomy was the preferred type of surgical procedure. For most benign adnexal tumors, the most common surgery performed is laparoscopy,[8] especially for women with fertility issues. Laparoscopy is often chosen due to its advantages, such as allowing an examination of the entire abdominal cavity to visualize the important pelvic pathologies thereby leading to acute modifications of the therapeutic strategy.[15]
Endometriosis and teratomas are relatively frequent gynecological pathologies, but their coexistence is very rare,[16] especially when both tumors affect the same ovary. In these cases, the literature cites the classic open abdominal surgical approach, which preserves part of the affected ovary and ultimately, its function.[16] In our study, only 1 patient had an HP diagnosis of a dermoid cyst on the left ovary, and a simultaneous endometriotic cyst on the right ovary. Even though the patient underwent laparoscopic surgery instead of an open procedure, ovarian tissue could not be preserved; therefore, menopause was a result of this procedure. Moreover, another case of interest in our study is that of a 31-year-old patient diagnosed with a borderline ovarian tumor via a frozen section procedure. Pelvic MRI was performed before the intervention. Laparoscopic right ovarian cystectomy was initially attempted because of the patient's desire for future fertility. However, because of the long-term postoperative complications, a laparoscopic right adnexectomy and peritoneal biopsies were performed according to an established protocol.[17] As the opportunity to obtain a frozen section is not always available, immunohistochemistry (IHC) techniques as part of the HP evaluation have become one of the main paraclinical examinations for establishing a definitive diagnosis, because each borderline tumor categorys has IHC features.[18] IHC is also applicable for differential diagnoses. For example, in a rare and challenging case documented in October 2015 at our clinic, based on the fact that the Ki67 antigen is positive in less than 2% of borderline tumors, the detection of a Ki67 proliferation index in 60% of the nuclei of tumor cells was extremely helpful in establishing the diagnosis of synchronous serous carcinoma of the ovary.[19]
The absence of ovarian function at follow-up is one of the limitations of this study. However, the literature presents conflicting and controversial data regarding this aspect. A recently published prospective case–control study referred to the short-term impact of laparoscopic cystectomy on the ovarian function of 67 patients who were previously diagnosed with endometrioma or nonendometriotic cysts. This study found that ovarian function test levels decreased similarly in both groups after cystectomy.[20] The importance of laparoscopy has also been described for the optimal assessment of adnexa associated with congenital anomalies.[21] A 15-month prospective case–control study based on 70 patients diagnosed with benign ovarian cysts who underwent the same type of minimally invasive surgical treatment concluded that there was no detectable difference from baseline values with respect to ovarian reserve marker levels at 6 and 12 months after surgery,[22] irrespective of the initial diagnosis. Our study showed that postoperative follow-up of patients, especially during the short term, should include biological investigation of ovarian function.
The present study had several limitations. First, it was a retrospective study based on medical records. Second, only 1 patient from our study group underwent pelvic MRI, thus limiting our reportable findings regarding potential alternative imaging tests that were useful for the evaluation of suspicious ovarian masses. Third, postoperative follow-up of patients, especially in the short-term, should have included a biological investigation of ovarian function. Finally, there were 106 cases of benign ovarian tumors and 1 borderline tumor case, which made it difficult to extrapolate our results to a nonhomogenous population in which malignancies occur more frequently. Although not a limitation, that our specialists who performed the TV-US examinations for all the patients were not US experts, but obstetric gynecological practitioners who evaluate many different gynecological pathologies daily, not just ovarian masses.
In conclusion, in patients with suspected ovarian masses after clinical examination, TV-US, as a complementary approach, remains the main imaging method used in our clinic. Even though its role in the presumptive diagnosis of pelvic masses in some cases might be invaluable, pelvic MRI is still difficult to use as a first-line imaging approach.
Assessments using ovarian tumor biomarkers, such as ROMA scores and CA-125 levels, are important for paraclinical investigations and these tests have a remarkable value in providing key information for the clinicians when used under the right indications. The laparoscopic approach for the management of ovarian masses is now a standard in the surgical world. Our department offers minimally invasive surgical treatment whenever possible. In fact, cystectomy is currently the main surgical procedure currently used in our clinic for benign ovarian masses. In our patient population, endometriosis was the most common benign pathology evaluated and treated in our study. HP examinations offer definitive diagnoses of each ovarian tumors; therefore, new opportunities are available regarding the development of IHC techniques and their accessibility.
Acknowledgment
We would like to thank Editage (www.Editage.com) for English language editing
Author contributions
Conceptualization: Cringu Antoniu Ionescu, Dan Navolan.
Data curation: Alexandra Matei, Roxana Bohiltea, Adrian Neacsu, Corina Ilinca.
Formal analysis: Cringu Antoniu Ionescu, Alexandra Matei, Dan Navolan, Mihai Dimitriu, Roxana Bohiltea, Adrian Neacsu.
Investigation: Cringu Antoniu Ionescu, Alexandra Matei.
Methodology: Cringu Antoniu Ionescu, Alexandra Matei, Dan Navolan, Mihai Dimitriu, Liana Ples.
Software: Dan Navolan, Corina Ilinca.
Supervision: Cringu Antoniu Ionescu, Dan Navolan, Roxana Bohiltea, Corina Ilinca, Liana Ples.
Validation: Cringu Antoniu Ionescu, Alexandra Matei, Dan Navolan, Mihai Dimitriu, Roxana Bohiltea, Adrian Neacsu, Corina Ilinca, Liana Ples.
Visualization: Cringu Antoniu Ionescu, Alexandra Matei, Dan Navolan, Roxana Bohiltea, Adrian Neacsu, Liana Ples.
Writing – original draft: Cringu Antoniu Ionescu, Alexandra Matei.
Writing – review & editing: Cringu Antoniu Ionescu, Dan Navolan.
Author name: Orcid number:
ORCID Nr: 0000-0003-4533-6766
Footnotes
Abbreviations: CA-125 = cancer antigen 125, CT = computed tomography, HP = histopathological, IHC = immunohistochemistry, IOTA = International Ovarian Tumor Analysis, LR = logistic regression, MRI = magnetic resonance imaging, RMI = Risk of Malignancy Index, ROMA score = Risk of Ovarian Malignancy Algorithm score, Se = sensitivity, Sp = specificity, SR = simple rules of ultrasound, TV-US = transvaginal ultrasound.
CAI, AM, and DN contributed equally to this work.
The authors report no conflict of interest and no funding to disclose.
References
- [1].ACOG Committee Opinion No 280. The role of the Generalist Obstetrician Gynecologist in the early detection of ovarian cancer. Obstet Gynecol 2002;100:1413–6. [DOI] [PubMed] [Google Scholar]
- [2].World cancer research fund international. Cancer facts and figures. http://www.wcrf.org/int/cancer-facts-figures/worldwide-data. Accessed April 17, 2017. [Google Scholar]
- [3].Seidman JD, Russell P, Kurman RJ. Kurman RJ. Surface epithelial tumors of the ovary. Blaustein's pathology of the female genital tract 5th ed.New York: Springer Verlag; 2002. 791. [Google Scholar]
- [4].Fischerova D, Zikan M, Dundr P, Cibula D. Diagnosis, treatment and follow-up of borderline ovarian tumors. Oncologist 2012;17:1515–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Garg S, Kaur A, Mohi J, Kanwal Sibia P. Evaluation of IOTA simple ultrasound rules to distinguish benign and malignant ovarian tumours. J Clin Diagn Res 2017;11:TC06–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Muto M. Patient education: Ovarian cysts (Beyond the Basics). Section Editors: Barbara Goff, William J Mann. Deputy Editor: Sandy J Falk. https://www.uptodate.com/contents/ovarian-cysts-beyond-the-basics. Accessed December 2017. [Google Scholar]
- [7].Springer Verlag, Andrade R, Tavares A, Mountzios G. International Manual of Oncology Practice (iMop) Principles of Medical Oncology. 2015;963–964. [Google Scholar]
- [8].Pleş L, Sima R, Burnei A, et al. The experience of our clinic in laparoscopy for adnexal masses and the correlation between ultrasound findings and pathological results. Rom J Morphol Embryol 2016;57:1337–41. [PubMed] [Google Scholar]
- [9].McDonald JM, Modesitt SC. The incidental postmenopausal adnexal mass. Clin Obstet Gynecol 2006;49:506–16. [DOI] [PubMed] [Google Scholar]
- [10].Kaijser J, Sayasneh A, Van Hoorde K, et al. TI Presurgical diagnosis of adnexal tumours using mathematical models and scoring systems: a systematic review and meta-analysis. Hum Reprod Update 2014;20:449–62. [DOI] [PubMed] [Google Scholar]
- [11].Strigini FA, Gadducci A, DelBravo B. Differential diagnosis of adnexal masses with transvaginal sonography, color flow imaging, and serum CA 125 assay in pre- and postmenopausal women. Gynecol Oncol 1996;61:68–72. [DOI] [PubMed] [Google Scholar]
- [12].Van Calster B, Timmerman D, Bourne T, et al. Discrimination between benign and malignant adnexal masses by specialist ultrasound examination versus serum CA-125. J Nat Cancer Inst 2007;99:1706–11. [DOI] [PubMed] [Google Scholar]
- [13].Genetic/Familial High-Risk Assessment: Breast and Ovarian Clinical Practice Guidelines. J Natl Compr Canc Netw 2006;4:156. DOI: 10.6004/jnccn.2006.0016. [Google Scholar]
- [14].Myers ER, Bastian LA, Havrilesky LJ, et al. Management of Adnexal Mass. Evidence Report/Technology Assessment No.130 (Prepared by the Duke Evidence-based Practice Center under Contract No. 290-02-0025). AHRQ Publication No. 06-E004, Agency for Healthcare Research and Quality, Rockville, MD February 2006. [Google Scholar]
- [15].Grigoriu R, Calin A, Arbune M, Mihalceanu E, Onofriescu M, Ionescu C. News in the ovarian drilling in the polycystic ovary syndrome. Rev Chim 2016;67:366–9. [Google Scholar]
- [16].Sindilar A, Socolov R, Socolov D, et al. Teratoma and endometriotic ovarian cysts on ipsilateral ovary occurring after ectopic pregnancy. case presentation of a rare association. Gineco ro 2011;7:208–9. [Google Scholar]
- [17].Bratila E, Manta AM, Strambu VDE, et al. Therapeutic conduct in borderline ovarian tumors in women at fertile age. Gineco ro 2016;11:28–32. [Google Scholar]
- [18].Bohîltea RE, Bacalbasa N, Turcan N, et al. Bilateral serous surface papillary borderline ovarian tumor in 19 years old patient: ultrasound, immunohistochemical and therapeutic particularities. Rom J Morphol Embriol 2017;58:989–95. [PubMed] [Google Scholar]
- [19].Ionescu C, Vladareanu S, Ples L, et al. Sinchronous bilateral primary ovarian carcinoma – case presentation. Rom J Morphol Embryol 2017;58:219–23. [PubMed] [Google Scholar]
- [20].Salihoglu KN, Dilbaz B, Cırık DA. Short-term impact of laparoscopic cystectomy on ovarian reserve tests in bilateral and unilateral endometriotic and nonendometriotic cysts. J Minim Invasive Gynecol 2016;23:719–25. [DOI] [PubMed] [Google Scholar]
- [21].Dragusin R, Tudorache S, Surlin V, et al. Importance of laparoscopic assesment of the uterine adnexa in a Mayer-Rokitansky-Kustner-Hauser syndrome Type 2. Curr Health Sci J 2014;40:144–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ding Y, Yuan Y, Ding J. Comprehensive assessment of the impact of laparoscopic ovarian cystectomy on ovarian reserve. J Minim Invasive Gynecol 2015;22:1252–9. [DOI] [PubMed] [Google Scholar]


