Abstract
Excision repair cross-complementing group 1 (ERCC1) functions as a nucleotide excision repair (NER) enzyme. Altered ERCC1 expression or function is closely associated with cancer development and progression. This study determined the association of ERCC1 expression with survivin expression, clinicopathological characteristics, and survival of esophageal squamous cell carcinoma (ESCC) patients after postoperative concurrent chemoradiotherapy.
Tissue specimens from 102 resected ESCC patients were acquired for immunohistochemical analysis of ERCC1 and survivin protein expression.
ERCC1 expression was detected in 62.7% of ESCC tissues and in 9.8% of normal squamous epithelium tissues (P < .01), while survivin expression was detected in 60.8% of ESCC tissues and in 19.6% of normal squamous epithelia (P < .01). ERCC1 overexpression associated with advanced tumor clinical stage and lymph node metastasis (P < .05), but not with tumor size, depth of invasion, or differentiation (P > .05). ERCC1 overexpression was also associated with survivin levels (r = 0.42, P < .01) and worse progression-free survival of ESCC patients after concurrent chemoradiotherapy. Multivariate analysis data revealed that ERCC1 and survivin protein expression were independent predictors of overall survival of ESCC patients after chemotherapy and/or radiotherapy (P < .05).
ERCC1 overexpression is an important phenotype that is associated with ESCC lymph node metastasis and advanced tumor clinical stages. ERCC1 expression may also inhibit ESCC cell apoptosis via regulating survivin expression, and ERCC1 and survivin overexpression are independent predictors of prognosis for ESCC patients who receive chemotherapy and/or radiotherapy.
Keywords: ERCC1, esophageal squamous cell cancer, prognosis, survivin
1. Introduction
Esophageal cancer is a significant health problem in the world and in China.[1] Histologically, esophageal cancer occurs as either squamous cell carcinoma (ESCC) or adenocarcinoma. ESCC is the most common type of esophageal cancer in China, whereas adenocarcinoma is more prevalent in western countries, such as the United States of America.[1–3] Clinically, esophageal cancer is usually diagnosed at the advanced stages of disease, at which point surgery is not a viable option, and the effectiveness of chemotherapy and radiation therapy alone is limited. Thus, the overall survival rate of individuals diagnosed with esophageal cancer still remains very low. Thus, early detection, effective control of esophageal cancer progression, and prediction of prognosis and treatment responses could help medical oncologists to better treat this now deadly disease.
During esophageal carcinogenesis, there is altered expression, mutation, and/or epigenetically silencing of many genes.[3–5] The objective of this study was to evaluate useful biomarkers in predicting prognosis of esophageal cancer and response to chemotherapy and/or radiation therapy. In particular, we focused on a DNA repair enzyme as a biomarker, excision repair cross-complementing group 1 (ERCC1), which encodes a single-stranded DNA endonuclease that plays a crucial role in nucleotide excision repair (NER). Aberrant ERCC1 expression was associated with genomic instability and cancer development, as well as resistance to cancer chemotherapy and radiation therapy.[6–9] Thus, ERCC1 or pathway-related genes could be attractive anticancer targets.[9] Warnecke-Eberz et al[10] showed that ERCC1 expression was able to predict neoadjuvant chemoradiotherapy resistance and poor outcomes in esophageal cancer. Moreover, surviving, which belongs to the inhibitor of apoptosis (IAP) family, is able to inhibit caspase activation and suppress apoptosis, which is also an underlying mechanism of chemotherapy and radiation action. Thus, overexpression of survivin also leads to resistance of chemotherapy and radiation therapy in cancer cells.[11] The main function of survivin is to inhibit caspase-3 and caspase-7 activation and downregulate DNA repair enzymes during cell apoptosis. Thus, we measured expression of ERCC1 and survivin in esophageal cancer tissue specimens to determine whether these proteins are associated with clinicopathological characteristics and progression-free survival of ESCC patients who received postoperative concurrent chemoradiotherapy.
2. Materials and methods
2.1. Patients and specimens
In this study, we collected 102 tissue samples from ESCC patients from the Department of Thoracic Surgery, People's Hospital of Linyi (Shandong, China) between August 2010 and August 2012. Patient inclusion criteria were family members or patients provided consent before surgery, there were no other malignancies or ESCC distant metastasis, pathologically confirmed esophageal squamous cell carcinoma, and no patient received chemoradiotherapy or other anticancer therapy before surgery. The patient exclusion criteria were ESCC was not diagnosed pathologically, other malignancies and distant metastasis, and received anticancer therapy before surgery. All patients were subjected to total esophagectomy and radical lymph node dissection. Forty-six patients also received concurrent chemoradiotherapy with a radiotherapy dose of 50 Gy and standard cisplatin and 5-fluorouracil chemotherapy. These 102 surgically resected ESCC specimens contained pairwise normal esophageal mucosae and ESCC for immunohistochemistry analyses. The laboratory protocol of this study was approved by the Human Ethics Committee of Shandong Linyi People Hospital with the access number of KY2015028, and all participants provided written informed consent before being enrolled into this study.
All ESCCs were diagnosed according to the World Health Organization's classification for esophageal cancer[12] and ESCC staging was done according to the TNM classification of the American Joint Committee on Cancer.[13] Normal esophageal mucosae were taken from more than 5 cm away from tumor lesions, and there were no detectable cancer cells in the nontumor control samples.
2.2. Immunohistochemistry
Paraffin-embedded ESCC tissue blocks, together with paratumor normal esophageal mucosa blocks, were cut into 4 to 5 μm thin sections. For immunohistochemistry, the sections were deparaffinized with xylene and rehydrated in graded concentrations of ethanol. Antigen retrieval was conducted according to the vendor's instructions for the primary antibodies, that is, sections were cooked in a pressure cooker with 0.01 M sodium citrate solution (pH 6.0) for approximately 3 minutes. Next, the sections were incubated in 3% hydrogen peroxide (H2O2) solution for 10 minutes to block endogenous peroxidase activity and then washed with tap water and phosphate buffered saline (PBS) 3 times for 2 minutes each. Afterwards, the sections were incubated with a normal serum from the Polink-1 PV6000 secondary antibody detection kit (Zhongshan Goldenbridge Co., Ltd., Beijing, China) and then further incubated with 50 μL of a monoclonal anti-ERCC1 (Origene Biotechnology Co., Ltd., Rockville, MD) or anti-survivin (Epi Biotechnology Co. Ltd., San Francisco, CA) antibody at 37°C for 1 hour and subsequently with a secondary antibody from the detection kit according to the kit instructions. After washing with PBS 3 times, the sections were subjected to color reaction with 3, 3-diaminobenzidin (DAB) for approximately 10 minutes at room temperature in the dark, counterstained with Harris hematoxylin solution (Zhongshan Goldenbridge Biotechnology Co., Ltd.), dehydrated in gradient alcohol, cleared in xylene, and mounted with neutral gum. The negative control sections were stained with the same steps but replaced with the primary antibody with PBS only.
The immunostained sections were reviewed and scored under a light microscope by a pathologist who was blinded to the identity of the patients. Nuclear staining with a brown color was considered as positive staining for both ERCC1 and survivin proteins. Five fields of high power (400x) in each section were randomly selected and evaluated. The score criterion for staining intensity was 0 (negative), 1 (weak staining), 2 (moderate staining), and 3 (strong staining). The score criterion of percent cell staining was 0 (no staining), 1 (1–25% staining), 2 (26–50% staining), 3 (51–75% staining), and 4 (76–100% staining). The staining intensity and percent scores were added together to form a final assessment of immunostaining data. If the sum of these 2 scores was more than 4, ERCC1 and survivin expression was considered to be positive.[14]
2.3. Statistical analysis
We utilized the SPSS 21.0 software (SPSS, Chicago, IL) for all statistical analyses. The Chi-square test or Fisher exact probability test was performed to associate expression of ERCC1 and survivin proteins with various clinicopathological variables, while the Spearman test was used to analyze association of ERCC1 with survivin expression. The Kaplan–Meier curves and the log rank test were performed to determine the associations of ERCC1 and survivin proteins with overall and progression-free survival of patients. A P value equal to or less than .05 was considered statistically significant.
3. Results
3.1. Patient characteristics
Patients’ ages ranged between 42 and 78 years with a median age of 62 years. Histologically, among the 102 ESCC tumor specimens, 20 tumors infiltrated the muscularis propria and 82 tumors infiltrated the adventitia; 33 tumors were well differentiated, 55 moderately differentiated, and 14 poorly differentiated. Forty-eight tumors were at stage II, 54 at stage III, and 46 cases had lymph node metastasis, but 56 cases did not have tumor lymph node metastasis.
After patients were discharged from the hospital, they were followed regularly, every 3 months for the first 2 years and every 6 months thereafter. The total follow-up period of time was assessed as the time from diagnosis to the date of death or the last follow-up. All 102 patients were followed up and included in data analysis in this study. The last follow-up occurred in February 2015 with the median follow-up period of time for 30 months (range between 3 and 53 months).
3.2. Differential expression of ERCC1 and survivin proteins in ESCC tissue specimens
Immunohistochemistry revealed that ERCC1 and survivin were expressed in the nuclei of ESCC cells (Fig. 1). ERCC1 protein was expressed in 62.7% (64/102) of ESCC tissues compared with 9.8% (10/102) of normal squamous epithelia (χ2 = 61.84, P < .01). We also found that survivin protein was expressed in 60.8% (62/102) of ESCC tissues compared with 19.6% (20/102) of normal squamous epithelia (χ2 = 42.54, P < .01).
Figure 1.
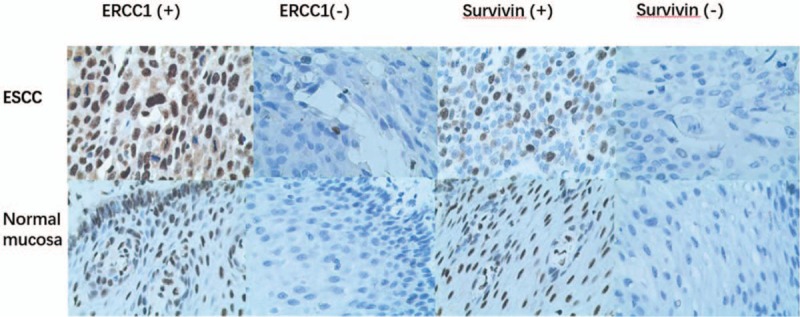
Differential expression of ERCC1 and survivin in ESCC tissues detected by immunohistochemistry. ESCC and normal squamous mucosae were immunostained with ERCC1 or survivin antibodies. All magnifications are at x400.
3.3. Associations of ERCC1 and survivin proteins with clinicopathological parameters from ESCC patients
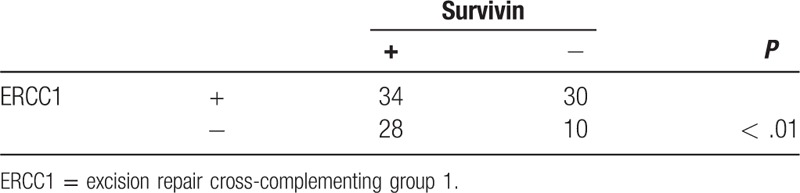
We next associated ERCC1 or survivin protein expression with clinicopathological variables in ESCC patients and found that ERCC1 overexpression was associated with advanced clinical stage and lymph node metastasis of ESCC patients (P < .05), but there was no association of ERCC1 expression with tumor size, invasion depth, or differentiation (P > .05). Survivin expression was associated with advanced clinical stage and lymph node metastasis (P < .05; Table 1). ERCC1 overexpression was also associated with survivin levels in ESCC tissues (r = 0.42, P < .01; Table 2).
Table 1.
Associations of ERCC1 and survivin expression with clinicopathological parameters from ESCC patients.
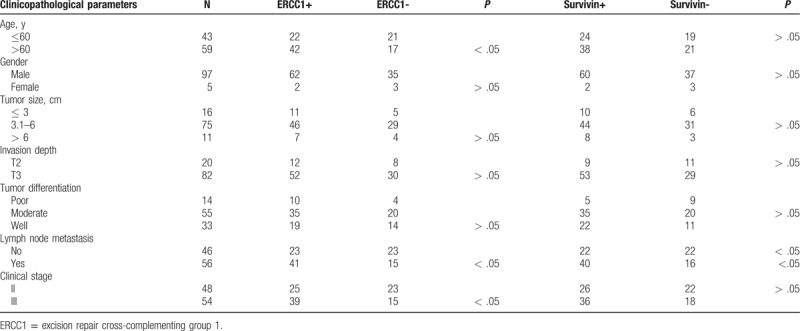
Table 2.
Associations of ERCC1 with survivin expression in ESCC tissue samples.
3.4. Associations of ERCC1 and survivin expression with worse overall (OS) and progression-free survival (PFS) of ESCC patients
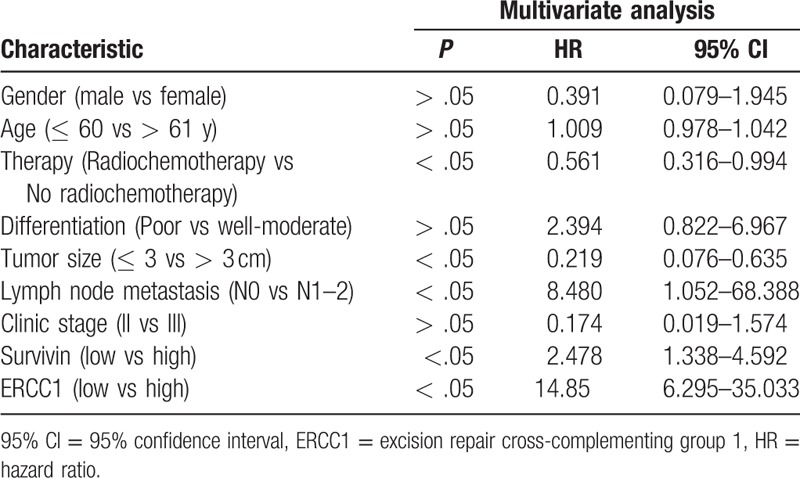
Expression of ERCC1 (t = 8.896, P < .05) and survivin proteins (t = 2.12, P < .05) was associated with poor PFS. Moreover, expression of ERCC1 (χ2 = 41.814, P < .05) and survivin (χ2 = 3.414, P < .05) were associated with poor OS (Fig. 2). Multivariate analysis indicated that both ERCC1 [P < .05; hazard ratio (HR) = 14.85] and survivin (P < .05, HR = 2.478) expression were independent prognostic factors for ESCC (Table 3).
Figure 2.
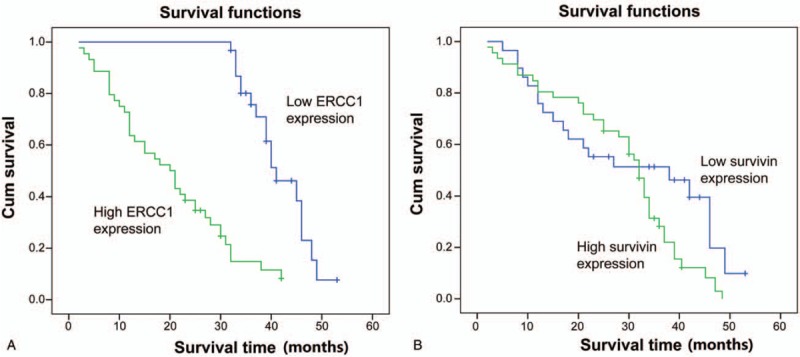
Kaplan–Meier curve analysis of overall survival stratified by ERCC1 and survivin expression. (A) ERCC1. (B) Survivin.
Table 3.
Multivariate analysis of overall survival of ESCC patients.
4. Discussion
ERCC1 is a key enzyme for the NER, and downregulation of ERCC1 expression is associated with genomic instability and cancer development. However, upregulation of ERCC1 expression in tumor tissues could contribute to tumor resistance to chemotherapy and radiation therapy.[7–9] NER is a multifunctional DNA repair mechanism that excises damaged nucleotides in the genomic DNA. Failure of NER leads to genetic instability and tumor development. In cancer patients, enhanced DNA repair capacity could lead to tumor resistance to chemotherapy and radiation therapy because most traditional chemotherapeutic agents and radiation damage genomic DNA and, therefore, kill tumor cells.[15,16] In this study, we found that ERCC1 protein expression was significantly upregulated in esophageal carcinoma (62.7%) compared with normal esophageal mucosa (9.8%; P < .05), suggesting that overexpression of ERCC1 is an important phenotype of ESCC. Upregulated expression of ERCC1 was associated with advanced ESCC clinical stage, lymph node metastasis, and poor PFS of patients. Moreover, survivin, an apoptosis-resistant gene that promotes tumor cell survival, was also upregulated in ESCC tissue specimens. Survivin protein expression was associated with invasion depth and ESCC lymph node metastasis, although this association did not reach statistical significance for PFS of ESCC patients. Thus, our current data suggest that ERCC1 and survivin may be useful biomarkers to predict the effects of chemotherapy and radiotherapy in ESCC patients. Further studies will assess whether targeting these proteins will enhance the effects of chemotherapy and radiotherapy in ESCC patients.
The literature demonstrates that ERCC1 protein overexpression is associated with progression of ovarian cancer, nasopharyngeal carcinoma, and gastric cancer.[17–19] Survivin is reported to be an important gene in ESCC, and overexpression of survivin is associated with poor OS and prognosis of ESCC patients.[16,20] Another study suggested that targeting nuclear survivin expression could be a therapeutic strategy for ESCC patients.[21] Our current data are consistent with these previously published studies and further support their conclusions.
Furthermore, our current study demonstrates that ERCC1 protein expression is associated with survivin expression in ESCC tissues, which supports the possibility that ERCC1 might modulate survivin-related tumor cell survival. Tumor cell apoptosis is an important aspect of tumor development and progression, as well as tumor cell resistance to chemotherapy and radiotherapy. The main function of survivin is to suppress caspase-3 and caspase-7, which are important apoptosis induction molecules. During cell apoptosis, caspase-3 and caspase-7 are activated and downregulate their DNA repair enzyme substrates, a process that is inhibited by survivin. The main biological effect of DNA injury is associated with cell death and gene mutation, whereby DNA damage can induce cell death or apoptosis. When DNA is damaged, the cell activation reaction pathways and cell cycle are paused. Thus, if the damaged DNA is beyond repair, the cells will commit to apoptosis; however, if the damaged DNA is not repaired or the cells do not undergo apoptosis, cells could undergo malignant transformation and cancer development. In contrast, during cancer chemotherapy, cisplatin, one of mostly used anticancer agents in solid tumors, induces DNA intrastrand crosslink to trigger a series of intracellular events, leading to cancer cell death. However, the DNA intrastrand crosslink could be repaired by the NER pathway. Thus, the NER capacity has a huge impact on chemotherapy resistance, normal tissue tolerance, and treatment outcome and prognosis. Bellmunt et al[22] showed that ERCC1 protein expression could predict survival of patients with bladder cancer after platinum-based therapy. ERCC1 was reported to be a novel target for melanoma and appeared to be a useful biomarker for prediction of response to neoadjuvant therapy in esophageal cancer patients.[23,24] Altered expression of various genes that are associated with prognosis of esophageal squamous cell carcinoma, such as FOXM1 and EGFR, can affect cell cycle, proliferation, and differentiation.[25,26] ERCC1 affects apoptosis via regulating survivin, which affects the prognosis of ESCC and also affects radiotherapy and chemotherapy. Clinically, in spite of radical tumor resection and extensive lymph node removal, most ESCC patients still die of tumor recurrence or distant metastasis. Such recurrence occurs in 27% to 52% of surgery patients, and 41.5% to 55% of patients have locoregional recurrences.[27] Thus, early and frequent occurrence of distant metastases generally leads to poor survival of esophageal cancer patients.[28] Chemoradiotherapy can reduce locoregional recurrence and improve outcomes after the surgery. The adjuvant chemotherapy of esophageal cancer could potentially target micro-metastatic disease, thus decreasing the risk of tumor cell distant spread.[29] Our study outcomes are limited by small sample size, but future study with a larger sample size from a multicenter could confirm our current data.
5. Conclusion
ERCC1 overexpression is an important phenotype in ESCC and is also associated with advanced ESCC clinical stages, lymph node metastasis, and poor PFS. ERCC1 could block chemotherapeutic-induced tumor cell apoptosis via regulating survivin expression. ERCC1 and survivin expression are independent prognostic predictors for patients with resected ESCC who receive chemotherapy and (or) radiotherapy.
Acknowledgments
The authors would like to thank Mr Jinming Yu of Shandong Cancer Hospital (Shandong, China) for participation in the study design and help in performance of the statistical analysis, Mr Dianbin Mu of Shandong Cancer Hospital (Shandong, China) for help in reviewing and scoring of immunostained tissue sections, and all thoracic surgeons at Linyi People Hospital (China) for their patient care.
Author contributions
HYP reviewed and scored the immunostained tissue sections and drafted the manuscript. SBY prepared tissue sections and helped to review and score the immunostained tissue sections. YXZ and WHG collected the patients’ clinical data. QYD and ZYJ helped to draft the manuscript. LY designed the study and performed the statistical analyses. All authors read and approved the final version of the manuscript.
Conceptualization: Li Yan.
Data curation: Haiying Peng, Shaobo Yao, Qingyu Dong, Yanxia Zhang, Weihong Gong, Li Yan.
Formal analysis: Yanxia Zhang
Writing – original draft: Haiying Peng, Qingyu Dong, Zhongyao Jia, Li Yan.
Footnotes
Abbreviations: DAB = 3, 3-diaminobenzidin, ERCC1 = excision repair cross-complementing group 1, ESCC = esophageal squamous cell carcinoma, IAP = inhibitor of apoptosis, NER = nucleotide excision repair.
The study protocol was established according to the ethical guidelines of the Helsinki Declaration and approved by the Human Ethics Committee of Shandong Linyi People Hospital with access number of KY2015028. All participants signed a written informed consent form before enrolled into the study.
The authors declare that there is no conflict of interest in this work.
References
- [1].Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [2].Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- [3].Yoon MS, Nam TK, Lee JS, et al. VEGF as a predictor for response to definitive chemoradiotherapy and COX-2 as a prognosticator for survival in esophageal squamous cell carcinoma. J Korean Med Sci 2011;26:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hoshino M, Fukui H, Ono Y, et al. Nuclear expression of phosphorylated EGFR is associated with poor prognosis of patients with esophageal squamous cell carcinoma. Pathobiology 2007;74:15–21. [DOI] [PubMed] [Google Scholar]
- [5].Noguchi T, Takeno S, Shibata T, et al. VEGF-C expression correlates with histological differentiation and metastasis in squamous cell carcinoma of the esophagus. Oncol Rep 2002;9:995–9. [PubMed] [Google Scholar]
- [6].Lin Y, Totsuka Y, He Y, et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol 2013;23:233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li JS, Ying JM, Wang XW, et al. Promoter methylation of tumor suppressor genes in esophageal squamous cell carcinoma. Chin J Cancer 2013;32:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tepeli E, Caner V, Buyukpinarbasili N, et al. Expression of ERCC1 and its clinicopathological correlations in non-small cell lung cancer. Mol Biol Rep 2012;39:335–41. [DOI] [PubMed] [Google Scholar]
- [9].Gossage L, Madhusudan S. Current status of excision repair cross complementing-group 1 (ERCC1) in cancer. Cancer Treat Rev 2007;33:565–77. [DOI] [PubMed] [Google Scholar]
- [10].Warnecke-Eberz U, Metzger R, Miyazono F, et al. High specificity of quantitative excision repair cross-complementing 1 messenger RNA expression for prediction of minor histopathological response to neoadjuvant radiochemotherapy in esophageal cancer. Clin Cancer Res 2004;10:3794–9. [DOI] [PubMed] [Google Scholar]
- [11].Ikeguchi M, Yamaguchi K, Kaibara N. Survivin gene expression positively correlates with proliferative activity of cancer cells in esophageal cancer. Tumour Biol 2003;24:40–5. [DOI] [PubMed] [Google Scholar]
- [12].Aaloten L. World Health Organization Classification of Tumours, Pathology and Genetics Tumour of the Digestive System. Lyon: IARC Press; 2000. [Google Scholar]
- [13].International Union Against Cancer. TNM Classification of the Esophagus. 6th ed.2002;New York: J Willy&Sons, 5–25. [Google Scholar]
- [14].Shen Z, Ren Y, Ye D, et al. Significance and relationship between DJ-1 gene and surviving gene expression in laryngeal carcinoma. Eur J Histochem 2011;55:e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xu H, Swoboda I, Bhalla PL, et al. Plant homologue of human excision repair gene ERCC1 points to conservation of DNA repair mechanisms. Plant J 1998;13:823–9. [DOI] [PubMed] [Google Scholar]
- [16].Rosato A, Pivetta M, Parenti A, et al. Survivin in esophageal cancer: an accurate prognostic marker for squamous cell carcinoma but not adenocarcinoma. Int J Cancer 2006;119:1717–22. [DOI] [PubMed] [Google Scholar]
- [17].Steffensen KD, Waldstrom M, Jeppesen U, et al. Prediction of response to chemotherapy by ERCC1 immunohistochemistry and ERCC1 polymorphism in ovarian cancer. Int J Gynecol Cancer 2008;18:702–10. [DOI] [PubMed] [Google Scholar]
- [18].Huang PY, Li Y, Mai HQ, et al. Expression of ERCC1 predicts clinical outcome in locoregionally advanced nasopharyngeal carcinoma treated with cisplatin-based induction chemotherapy. Oral Oncol 2012;48:964–8. [DOI] [PubMed] [Google Scholar]
- [19].Huang ZH, Hua D, Du X, et al. ERCC1 polymorphism, expression and clinical outcome of oxaliplatin-based adjuvant chemotherapy in gastric cancer. World J Gastroenterol 2008;14:6401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mega S, Miyamoto M, Li L, et al. Immunohistochemical analysis of nuclear survivin expression in esophageal squamous cell carcinoma. Dis Esophagus 2006;19:355–9. [DOI] [PubMed] [Google Scholar]
- [21].Grabowski P, Kuhnel T, Muhr-Wilkenshoff F, et al. Prognostic value of nuclear survivin expression in oesophageal squamous cell carcinoma. Br J Cancer 2003;88:115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bellmunt J, Paz-Ares L, Cuello M, et al. Gene expression of ERCC1 as a novel prognostic marker in advanced bladder cancer patients receiving cisplatin-based chemotherapy. Ann Oncol 2007;18:522–8. [DOI] [PubMed] [Google Scholar]
- [23].Song L, Ritchie AM, McNeil EM, et al. Identification of DNA repair gene Ercc1 as a novel target in melanoma. Pigment Cell Melanoma Res 2011;24:966–71. [DOI] [PubMed] [Google Scholar]
- [24].Brabender J, Vallbohmer D, Grimminger P, et al. ERCC1 RNA expression in peripheral blood predicts minor histopathological response to neoadjuvant radio-chemotherapy in patients with locally advanced cancer of the esophagus. J Gastrointest Surg 2008;12:1815–21. [DOI] [PubMed] [Google Scholar]
- [25].Hashimoto T, Yanaihara N, Okamoto A, et al. Cyclin D1 predicts the prognosis of advanced serous ovarian cancer. Exp Ther Med 2011;2:213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wang J, Yu J-M, Jing S-W, et al. Relationship between EGFR over-expression and clinicopathologic characteristics in squamous cell carcinoma of the esophagus: a meta-analysis. Asian Pac J Cancer Prev 2014;15:5889–93. [DOI] [PubMed] [Google Scholar]
- [27].Jingu K, Nemoto K, Matsushita H, et al. Results of radiation therapy combined with nedaplatin (cis-diammine-glycoplatinum) and 5-fluorouracil for postoperative locoregional recurrent esophageal cancer. BMC Cancer 2006;6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hurmuzlu M, Ovrebo K, Monge OR, et al. High-dose chemoradiotherapy followed by surgery versus surgery alone in esophageal cancer: a retrospective cohort study. World J Surg Oncol 2010;8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xu Y, Liu J, Du X, et al. Prognostic impact of postoperative radiation in patients undergoing radical esophagectomy for pathologic lymph node positive esophageal cancer. Radiat Oncol 2013;8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]


