Abstract
There is a need for tailored exercise recommendations to patients with Myasthenia gravis (MG). A few pilot studies have recently shown that physical exercise in accordance with general recommendations to healthy adults can be applied safely to patients with mild MG symptoms. How physical exercise affects muscle parameters and risk factors for lifestyle diseases in patients with MG is, however, only poorly known. We evaluated functional skeletal muscle parameters in 11 MG patients, before and after conducting a 12-week supervised physical therapy regimen of aerobic and resistance strength training. After the training program, parameters of the rectus femoris muscle improved: compound motor action potential (from 4.5 ± 2.6 to 5.3 ± 2.8 mV, P = .016), isometric muscle force (from 25.2 ± 4.4 to 30.2 ± 3.8 kg; P = .014), and ultrasound muscle thickness (from 19.6 ± 5.6 to 23.0 ± 3.9 mm, P = .0098) all increased. Further, physical performance based measures improved, including the 30-Second Chair Stand Test (median change +2, P = .0039) as well as the clinical MG composite score [from 3 (2–5) to 2 (0–4), P = .043]. No improvement in muscle function was observed in the biceps brachii muscle. These findings indicate that MG patients can improve their muscular functions by incorporating aerobic and resistance strength training, especially in proximal leg muscles. This is important knowledge when physical therapy is considered for this patient group, for whom no guidelines on physical exercise currently exist.
Keywords: CMAP, Myasthenia gravis, neuromuscular, physical exercise, resistance training
1. Introduction
The benefits of physical exercise for healthy individuals are well established[1] and physical inactivity is now considered one of the top five risk factors for overall mortality.[2] To reduce risks of lifestyle diseases, consensus recommendations to healthy adults are set to 150 minutes of medium-level aerobic exercise weekly, as well as strength training twice a week.[1] In accordance with this, the need for well-established exercise recommendations, tailored for individuals with Myasthenia gravis (MG), is evident. It is reasonable to assume that MG patients achieve similar benefits from physical exercise, as do healthy adults. Also, a large meta-analysis revealed that strengthening exercises in combination with aerobic exercises are likely to be effective for patients with different primary muscle disorders.[3] Still, the possible disease-specific effects of exercise need to be evaluated, especially concerning functional muscle parameters, as the disease pathology of MG involves the skeletal muscle synapse. Furthermore, the capacity to perform an exercise program could be reduced due to the myasthenic muscle fatigue.
Exercise-related research in MG is sparse and gives only vague ideas on how MG patients should perform physical exercise to obtain desirable benefits without the risk of causing disease deterioration. In the scientific literature, there are a few case reports,[4–6] some studies on respiratory muscle training[7–9] and one study comparing strength training unilaterally to the untrained contralateral side.[10] Those reports describe no major adverse effects on myasthenic fatigue, with a few exceptions.[7] In a recent pilot study, we reported that MG patients with mild disease activity (MGFA class I-II) can adhere safely to general exercise recommendations without objective or subjective disease deterioration.[11] This was supported by another recent pilot study that describes good tolerance by MG patients with generalized mild MG (MGFA class II) to either a resistance or an aerobic training regimen.[12] Some studies also suggest beneficial effects on muscle strength and function,[10–12] although this issue was not their primary focus. Consequently, there is an obvious lack of literature in the field of physical exercise and MG that hampers the possibility to individualize training programs. Individualized programs could be of particular importance to avoid development of secondary disease conditions such as lumbar spine problems, diabetes type II, cardiovascular disorders, and hypertension, which are all in part a consequence of the sedentary lifestyle that many MG patients involuntary go into.
The aim of this study was to objectively evaluate functional skeletal muscle parameters in MG patients who conducted a 12-week supervised physical therapy program, focusing on aerobic and resistance strength training, in accordance with general recommendations for healthy adults.[1] As secondary outcomes, changes in cardiovascular risk factors and self-assessed wellbeing were assessed. The primary hypothesis was that a physical exercise program based on general exercise recommendations improves muscle status in MG patients. The secondary hypothesis was that moderate aerobic and resistance strength training improves the cardiovascular risk profile and self-assessed well-being of MG patients.
2. Methods
2.1. Study design and ethical considerations
This was a prospective unblinded study, wherein each patient served as his/her own control. All subjects provided oral and written informed consent to participate in the study before they were enrolled, which was approved by the Regional Ethical Review Board, Uppsala, Sweden (Dnr 2016/144).
2.2. Participants
A total of 95 patients with the ICD-10 code G70.0 (MG), who were followed at the outpatient Neurology clinic of Uppsala University Hospital in Sweden during the years 2001 to 2015, were identified. Of these patients, 22 were dead. From the remaining 73 MG patients, potential subjects were identified according to the following inclusion criteria: age ≥18 years, mild to moderate MG, living <100 km from Uppsala, and no concomitant condition such as severe cardiovascular disease, other disabling disease, or pregnancy. Fifty-four eligible patients were identified and invited to participate via mail. Of these, 24 subjects agreed to participate; however, already before the screening examination, 8 patients withdrew due to lack of time. In the screening examination, 2 of the remaining 16 patients were excluded – one because of suspected heart failure and one because of misdiagnosed MG. The remaining 14 patients were confirmed to have a clinically stable disease and no concomitant condition implying risk of performing physical activity, and they were all finally included in the exercise program.
2.3. Physical activity patterns
The baseline physical activity level was measured objectively as the mean number of steps and mean sedentary time per 24 hours using an accelerometer (DynaPort MoveMonitor; McRoberts, the Hague, the Netherlands) continuously for 7 consecutive days before the training period. Mean sedentary time during waking hours was also estimated. The activity level was further evaluated by a questionnaire on physical activity habits, developed by the Swedish National Board of Health and Welfare, which was recently validated.[13] The questionnaire contains two questions: strenuous exercise habits and habits of physical activity not regarded as exercise.
2.4. Resistance strength training regimen
The physical therapy program was based on general physical exercise recommendations for healthy adults,[1] which were individually tailored by a physiotherapist with regard to intensity and resistance weights. Each exercise session lasted 90 minutes and was supervised by a research assistant. Every session consisted of aerobic, resistance strength, and balance training in a physiotherapy setting at Uppsala University Hospital two times weekly for 12 weeks. Aerobic exercise was performed on stationary bicycles, starting with a 5-minute warm-up, followed by 7 intervals of 2 minutes cycling against high load and 1-minute cycling against minimum load, and ending with a 5-minute cool-down period. The level of bicycle resistance was set, and continuously adjusted, according to the heart rate aiming for 80% of pulse maximum during the 2-minute high load periods. Typically, resistance strength training refers to strength-training activities such as weightlifting, resistant band exercises, or exercises utilizing your own body weight as resistance such as doing pushups. Seven resistance muscle exercises, that is, biceps curl, latissimus dorsi pulldown, triceps pushdown, leg curl, cable rowing, sit-ups, and leg press were carried out, each with 2 sets of 10 repetitions maximum. Increasing adjustments of muscle resistance training weights were done individually for each patient, with supervision from a physiotherapist (SSN) throughout the 12-week training period. The active training program was followed by a set of 2 balance and 6 stretching exercises. The balance and stretching exercises were not changed over time.
2.5. Outcome measures
All clinical and muscle function evaluations were made at the same time of the day before and after the training period to avoid bias due to diurnal and medication fluctuations. To avoid measuring direct responses, that is, temporary changes, of physical activity patients were not performing active physical activity on the evaluation days.
2.5.1. Clinical measures
Both the MG Composite Scale (MGC)[14] and the Quantitative Myasthenia Gravis Score (QMG)[15] were used to assess MG status and plausible disease activity before, during and after the training period. Peak expiratory flow (PEF) was evaluated separately at the same occasions and PEF% was calculated after adjustment for age, gender, and height. A board-certified neurologist (EW) performed all the MGC, QMG, and PEF examinations.
2.5.2. Isometric muscle force
Isometric muscle force was recorded with a hand-held dynamometer (HHD, model 01165; Lafayette Instrument Company, Lafayette, IN), as previously described.[16] Peak force was measured in kg during a 5-second period; the maximum force of 3 consecutive repetitions was noted. There was no rest interval between these periods. Recording was obtained from the biceps brachii (elbow flexion, dynamometer placed at the volar side of the forearm) and quadriceps muscles (knee extension, dynamometer placed at the lower part of tibia), as those muscles were involved in many of the training exercises. HHD examinations were done by EW and assessed before, during, and after the training period.
2.5.3. Motor nerve conduction studies and repetitive nerve stimulation
Motor nerve conduction studies and repetitive nerve stimulation (RNS) were performed before and after the entire training period. Unless participants reported neurological complaints in their right-sided extremities, the right biceps brachii (musculocutaneous nerve) and rectus femoris (femoral nerve) muscles were examined, as those were the muscles mainly involved in the resistance training and also are commonly involved in regular training programs. Compound motor action potential (CMAP) was chosen as a measure, as higher CMAP amplitudes have been described in proximal muscles in individuals who regularly perform high resistance muscle training than in those who do not,[16,17] and hence can be assumed to measure muscular changes in response to training. RNS was done to assess possible neuromuscular transmission failure. Motor nerve stimulation and recordings of CMAP were achieved with Key Point Classic (Alpine Biomed, Skovlunde, Denmark) as previously described.[16] Surface plate electrodes of 10 mm diameter were used as active and referential electrodes. The stimulating electrode was a surface electrode with 23 mm fixed distance between anode and cathode. The electrodes were placed according to the standardized methods at Uppsala University Hospital Clinical Neurophysiology department. A supramaximal CMAP was obtained, and the amplitude was measured from the baseline to the negative peak. RNS, at 3 Hz, was subsequently performed, with the recording and stimulating electrode in the same positions. Ten stimuli were delivered, and the decrement was calculated between the first and the fourth CMAP amplitudes. A trained biomedical technician performed all CMAP and RNS examinations.
2.5.4. Neuromuscular ultrasound
Neuromuscular ultrasound was performed before and after the training period to evaluate thickening of the muscle as an effect of physical exercise.[18,19] The thicknesses of the biceps, the rectus femoris, and the vastus intermedius muscles were measured with a stationary ultrasound device (LOGIQ S8; GE Healthcare, Milwaukee, WI) using a ML6–15 MHz linear array transducer. Patients were supine with extended legs during examination and measurements were obtained at the center of the muscle belly. To obtain the correct muscle thickness, the transducer was held perpendicular to the skin with minimal pressure, with a visible layer of ultrasound gel between the transducer and the skin on the ultrasound image, that is, the transducer had no direct contact with the skin. The position with the largest measured diameter was chosen; three consecutive images and measurements were then performed. Finally, the 3 measurements were averaged. The measurements of the biceps brachii muscle also included the brachialis muscle, as the muscle belly was measured from the superficial part of the fascia, down to the part of the fascia adjacent to the humerus. For the quadriceps muscle, the vastus intermedius muscle belly and the rectus femoris muscle belly were measured individually. Neuromuscular ultrasound examinations were performed by either CJM or JW, who have developed a uniform strategy for performing neuromuscular ultrasound.[20]
2.5.5. Physical performance based measures
Timed Up and Go (TUG), 12-Minute Walk Test (12MWT), 30-Second Chair Stand Test (30SCST), and handgrip strength test (Jamar) were assessed before and after the training period by a licensed physiotherapist. 12MWT was performed in proximity to the physiotherapy department, in a corridor where the test is usually performed in a clinical setting. It was undertaken twice on both occasions to avoid introducing a learning effect.
2.5.6. Blood sample analysis
Blood samples were collected from each patient before and after the training period. Analyses of calcium, phosphate, myoglobin, creatine kinase, creatine kinase isoenzyme MB (CKMB), C-reactive protein (CRP), interleukin (IL)-6, lactate, myoglobin, glucose, HbA1c, cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, apolipoprotein A1, and apolipoprotein B in serum or plasma were performed.
2.5.7. Blood pressure, anthropometrics and body composition
Blood pressure (mm Hg), height (m), and body weight (kg) were measured and body mass index (BMI) was calculated. The body composition variables fat-free mass, muscle mass, skeletal muscle mass, and bone mass were assessed with the dual-energy x-ray absorptiometry method using the Bioelectrical Impedance Analysis (Tanita Body Composition Analyzer SC-240 MA; Tanita, Tokyo, Japan).
2.5.8. Quality of life, fatigue, and physical activity self-assessment
Quality of life and fatigue were assessed through two questionnaires, the Myasthenia gravis Quality of Life 15 (MG-QoL15)[21] and the Fatigue Severity Score (FSS).[22] The patients were additionally asked to rate themselves on the Exercise Self-Efficacy Scale (ESES), consisting of 10 questions (maximum four points per question, higher scores indicating higher confidence) regarding self-confidence in performance of physical tasks.
2.6. Statistical analysis
Parametric data from electrophysiological and laboratory parameters are presented as mean ± SD and nonparametric clinical scores as median ± IQR. Wilcoxon signed-rank test was used to compare both parametric and nonparametric data before and after training in each patient due to the low sample size. A P value < .05 was considered significant. No post-hoc analysis was performed due to the exploratory nature of the study. The statistical analysis was performed with version 6.07 for Windows (Graph Pad software, La Jolla, CA, www.graphpad.com).
3. Results
Fourteen patients (60 ± 18 years old; 8 women) were included, out of whom 11 patients (60 ± 18 years old; 6 women) completed the 12-week training regimen (Table 1). Five patients had early-onset MG (EOMG; # 4, 5, 8, 10, 11) and 6 patients had late-onset MG (LOMG; # 1, 2, 3, 6, 7, 9). Eight patients were acetylcholine receptor antibody seropositive (AChR+; # 1, 2, 3, 4, 5, 7, 9, 11), 1 patient was MuSK antibody seropositive (MuSK+; #10), and 2 patients were AChR and MuSK antibody seronegative (AChR-/MuSK-; # 6 and 8). MG medications were stable during the training period, except for 3 patients who actually managed to lower their doses of cholinesterase inhibitors (CHs) during the training period. Patient characteristics, including MGC and QMG scores, current medications, and doses of CH, are presented in Table 1.
Table 1.
Patient characteristics.
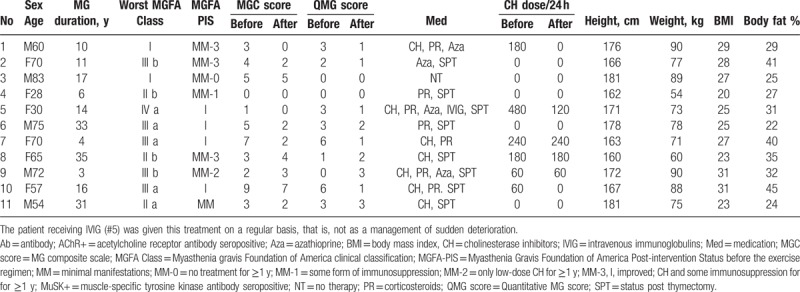
Three of the 14 initially included patients discontinued the study, 2 due to lack of time, and 1 due to work-related health problems. None of them showed any signs of clinical deterioration (according to MGC and QMG) or described other uneasiness regarding the training regimen.
3.1. Base-line physical activity patterns
Before starting the training period, the median number of steps/day was 8801 [interquartile range (IQR) 6746–9723] and the median sedentary time was 18.8 hours/24 hours (IQR 16.7–19.4) as measured by an accelerometer. When rated only for waking hours, the median sedentary time was 10.0 hours/day (IQR 9.0–10.9). Self-reported time devoted to strenuous exercise during a typical week ranged from 0 to >120 min/week (median: <30 min/week). Self-reported time devoted to physical-activity-not-regarded-as-exercise during a typical week ranged from <30 min/week to >300 min/week (median: 150–300 min/week).
3.2. Adherence to the exercise regimen
Of the 11 patients who completed the study, the participation rate (measured as attended times of maximal 24 occasions) ranged from 75% to 96% (88 ± 7%). On each occasion, the majority of the patients (72–100%) exceeded 70% of pulse maximum during the 2-minute high load periods. Ten (91%) patients increased their resistance weights in at least 4 of the 7 strength-training exercises after, as compared with before, the training period. All of the patients increased their resistance weights for the leg press exercise. Eight (73%) of the patients increased the bicycle resistance in the second half of the training regimen compared with the first half.
3.3. Outcome measures
3.3.1. Clinical myasthenic status
Disease activity (MGC and QMG) slightly decreased during the training period, indicating slightly improved MG status (Fig. 1F). None of the patients discontinued the training period due to objective or subjective deterioration. Median MGC decreased significantly by 1 point from 3 (IQR 2–5) to 2 (IQR 2–5; P = .043) and median QMG score decreased, although not significantly from 3 (IQR 0–3) to 1 (IQR 1–2; P > .05). Further, 4 of 5 patients with leg muscle fatigue on the MGC and the QMG normalized their leg muscle performance after the training regimen. Both patients who had arm muscle fatigue on the QMG improved their arm muscle performance. Intriguingly, none of the patients had arm muscle fatigue on the MGC scale before or after. Respiratory muscle function (PEF%) remained unchanged (99 ± 30 vs 96 ± 32%; P > .05). RNS did not deteriorate after the completed 12-week training program (data not shown). On the contrary, only 1 patient had an abnormal decrement after the training period compared with 4 patients before the training period.
Figure 1.
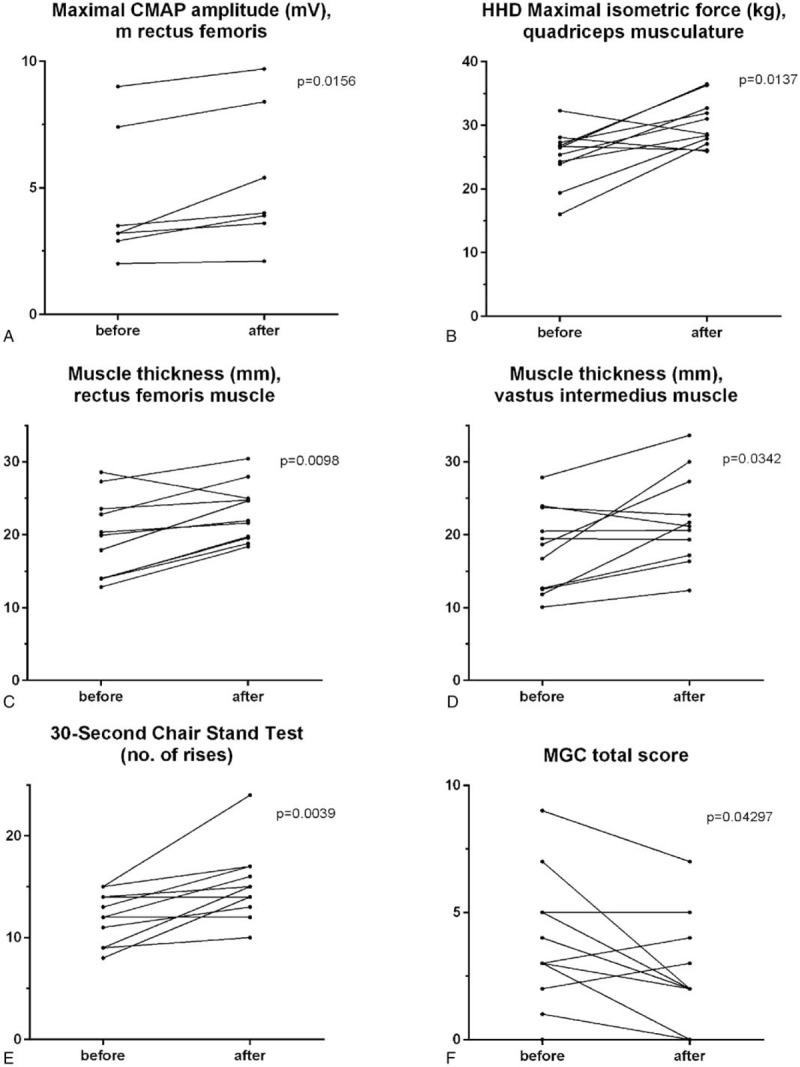
Significantly improved outcome measures of muscle status after the training period. Maximal compound motor action potential (CMAP) of the rectus femoris muscle (A) and maximal isometric force measured with a hand-held dynamometer (HHD) of the quadriceps muscle (B). Muscle thickness measured with ultrasound of the rectus femoris (C) and vastus intermedius (D) muscles. Number of rises in the 30-second Chair Stand Test (E) and Myasthenia gravis Composite (MGC) total score (F).
3.3.2. Muscle function and muscle thickness
The CMAP amplitude increased significantly after the training period in the quadriceps muscle (4.5 ± 2.6 vs 5.3 ± 2.8 mV; P = .016; Fig. 1A), but not in the biceps brachii muscle (5.5 ± 2.1 vs 4.6 ± 1.3 mV; P = .63). We found no correlation between change in CMAP amplitude and change in decrement on RNS (data not shown). Isometric muscle force (HHD) was significantly increased in the quadriceps muscle (25.2 ± 4.4 vs 30.2 ± 3.8 kg; P = .014; Fig. 1B), whereas there was no significant change in muscle force of the biceps brachii muscle (21.0 ± 6.0 vs 21.9 ± 5.6 kg; P = .58). Neuromuscular ultrasound showed a significant increase in muscle thickness for both the rectus femoris (19.6 ± 5.6 vs 23.0 ± 3.9 mm; P = .0098) and the vastus intermedius (18.0 ± 5.8 vs 22.0 ± 6.2 mm; P = .034) muscles (Fig. 1C, D). On the contrary, no significant change was seen in the biceps brachii muscle (33.3 ± 6.5 vs 32.1 ± 6.2 mm; P = .11). The physical performance based measures improved significantly regarding the 30SCST (median change +2, P = .0039; Fig. 1E). TUG, 12MWT, and Jamar remained unchanged (p > 0.05 for all).
3.3.3. Blood sample analyses
Apolipoprotein A1 increased significantly (from 1.7 ± 0.3 to 1.9 ± 0.4 g/L, P = .039). Plasma 25-hydroxyvitamin D decreased significantly (70.2 ± 11.0 vs 62.1 ± 12.5 nmol/L; P = .032). There was a tendency for lowered long-time glucose (HbA1C) from 37.8 ± 6.5 to 36.2 ± 6.7 (P = .078). No other significant changes were found for the plasma parameters CRP, IL-6, calcium, lactate, phosphate, myoglobin, creatine kinase, glucose, total cholesterol, LDL, HDL, LDL/HDL, triglycerides, Apolipoprotein B, or ApoB/ApoA1.
3.3.4. Blood pressure, BMI, and body composition
Blood pressure, pulse at rest, body weight, BMI, and body composition variables (fat-free mass, skeletal muscle mass, body mass) did not change significantly (data not shown).
3.3.5. Quality of life, fatigue, and ESES
The disease-specific health-related quality of life, MgQoL-15, had a tendency toward higher scores and thus improved quality of life, although not significantly [median 5 (IQR 4–11) vs median 8 (IQR 6–11); P > .05]. Further, there were no significant changes in fatigue score with FSS [median 3.1 (IQR 2.3–3.6) vs median 2.0 (IQR 1.4–4.2); P > .05] or ESES [median 33 (IQR 28–38) vs median 30 (IQR 20–36); P > .05].
4. Discussion
In this study, we found an improvement of functional muscle measures, including isometric muscle force, muscle thickness, clinical fatigue, and CMAP in proximal leg muscles of MG patients in response to a supervised 12-week physical training program. In addition, 30SCST improved significantly. Functional measures of proximal arm muscles did not display a similar improvement. Besides a slight increase in Apolipoprotein A1, no significant changes of cardiovascular risk associated anthropometrics, blood pressure, or other protein levels were observed. Neither did we observe any changes in MGQoL-15, FSS, or ESES. The training program was well tolerated and a slight, although clinically not relevant, improvement in MGC score was noted.
Like many studies of physical exercise, this study is limited by the small sample size, which also limits subgroup analyses of, for example, gender, age, and MG subtype. Therefore, we cannot rule out differences in training response between patients in each subgroup regarding EOMG and LOMG, as well as female and male patients. Furthermore, both the training intervention and the follow-up times could be regarded as short, while still being similar to most other training studies and set by practical issues such as its time-consuming properties, especially to study participants. Longer intervention and follow-up times could lead to a higher number of drop-outs and more confounding factors. A control group could be desirable, but it is a delicate challenge how to design a comparable “placebo group” in an exercise context. It would also be advantageous to have the examiners blinded to whether the subjects have been trained or not, but when there is no untrained control group, this arrangement would be hard to achieve. Nevertheless, the aim of this study was not to provide a causal link between MG and muscle changes but rather to confirm the beneficial muscle response to training in MG from previous pilot studies.
Neurophysiological or gross-structural measures of exercise effects on muscle are not extensively evaluated,[16–19,23] and when it comes to neuromuscular disease, they are basically not studied at all. CMAP and muscle thickness, as measured by ultrasound, were chosen in this study, as they have recently shown differences between trained and untrained healthy individuals,[16] where an exercise-induced effect is known to occur. Nevertheless, such an effect is not fully proven, which would have been desirable as an optimal measure variable. To our knowledge, there are no optimally validated measures and further studies are needed to improve the full understanding of muscle measurements.
The improvement of all functional outcome measures of the proximal leg muscles observed in this study is in line with the indicative improvement of clinical muscle function seen in previous studies of MG and exercise.[10–12] This is also in agreement with the muscle response seen in healthy well-trained individuals.[16] The absence of improvement in arm muscles could be regarded as somewhat puzzling, although a similar arm-leg difference was reported in one previous study.[10] Another two studies[11,12] showed at least some arm muscle improvement in response to training. There was no obvious difference in clinical or neurophysiological fatigability in arm versus leg muscle before the intervention. A larger amount of leg muscle training in the exercise regimen, due to the aerobic cycling, could be an explanation for the difference, but this would not explain the difference/nondifference in the other studies. As the quadriceps muscle is naturally more active in everyday life, we could speculate that a higher degree of arm inactivity at baseline would require a longer duration of training intervention to reach similar effects. Further, a gender-related CMAP arm-leg difference has been described, where trained healthy women have higher CMAP amplitudes in quadriceps and trained healthy men have higher CMAP amplitudes in the biceps brachii[16] than untrained individuals of the same sex. There was a slight female preponderance in this study, as well as in a previous study with similar results,[10] which could possibly contribute to the arm-leg difference in response to training.
The few and modest changes in plasma proteins, blood pressure, and anthropometrics related to cardiovascular risk could be due to a real noneffect, as different training regimens seem to affect these parameters somewhat differently according to current literature.[24] Another reason could be the short follow-up time, during which changes might not yet appear.
No changes in MGQoL-15, FSS, or ESES were seen. Importantly, though, these parameters did not deteriorate. Previous studies have indicated divergent changes in MGQoL-15 and FSS.[12] As the development of FSS was primarily based on patients with multiple sclerosis and systemic lupus erythematosus, it could be considered an unspecific tool to evaluate MG patients to whom fatigue plays a very particular role and could be of a quite different kind. As ESES scoring was high already before the training period, it could not be expected to improve much.
Muscle fatigue is the cardinal symptom of MG, and the health risks of sedentary behavior due to this fatigue should be taken seriously, as should possible unfavorable health effects of medications and disease-associated conditions.[25] This underlines the need for improved and tailored training recommendations for MG patients, as the scientific basis for any recommendations to MG patients regarding physical exercise is still sparse. This infers a risk in having health care professionals recommend MG patients to avoid too much physical exercise. To guide a proper design of such studies, it is important to evaluate the tolerability and effects of different training strategies in MG. The findings of improved functional muscle measures in this study suggest that the disease mechanisms in MG, at least when disease activity is fairly well controlled, do not interfere with the expected improvement of muscle parameters from training. In turn, it also implies that the applied exercise program in this study can be recommended to patients who want to remain or become physically active, despite having MG.
Limitations to this study include the low number of participants, which seem to be a hurdle in intervention studies of physical activity due to the large amount of time required for the patients. Further, the short intervention time could be considered a limitation in assessing long-term effects from physical training in MG.
In conclusion, beneficial objective and subjective muscle outcomes, especially in proximal leg muscles following training, were noted. In addition, this study supports previous results on the absence of clinical deterioration in well-controlled MG patients following regular aerobic and resistance training. The advice regarding physical exercise to patients with MG has traditionally been to restrict, or even completely discourage, exercise mainly based on theoretical assumptions and lack of knowledge.[26] Considering the results of this study, the findings of exercise tolerability in recent studies, and the well-known benefits of physical exercise (most probably attributable also to individuals with MG), we advocate active recommendations of physical activity to MG patients according to the general recommendations for healthy adults. Any other approach would be untenable.
Acknowledgments
The authors are grateful to research assistant Margaretha Rostedt, who supervised the exercise regimen, and to biomedical technician Margaretha Eklöf Grindlund, who performed the CMAP measurements.
Author contributions
Conceptualization: Anna Punga, Elisabet Westerberg.
Formal analysis: Elisabet Westerberg, Anna Punga, Carl Johan Molin.
Funding acquisition: Anna Punga.
Investigation: Elisabet Westerberg, Carl Johan Molin, Johan Widenfalk, Anna Punga, Sören Spörndly Nees.
Methodology: Sören Spörndly Nees, Elisabet Westerberg, Anna Punga, Johan Widenfalk.
Project administration: Anna Punga, Elisabet Westerberg.
Supervision: Anna Punga.
Writing – original draft: Elisabet Westerberg.
Writing – review & editing: Carl Johan Molin, Anna Punga, Sören Spörndly Nees, Johan Widenfalk, Elisabet Westerberg.
Footnotes
Abbreviations: 12MWT = 12-Minute Walk Test, 30SCST = 30-Second Chair Stand Test, AChR+ = acetylcholine receptor antibody seropositive, CH = cholinesterase inhibitors, CMAP = compound motor action potential, EOMG = early-onset myasthenia gravis, ESES = Exercise Self-Efficacy Scale, FSS = Fatigue Severity Score, HHD = hand-held dynamometer, Jamar = handgrip strength test, LOMG = late onset myasthenia gravis, MG = myasthenia gravis, MGC = myasthenia gravis composite scale, MGFA = Myasthenia Gravis Foundation of America, MG-QoL15 = Myasthenia Gravis Quality of Life 15, MuSK+ = seropositive to muscle specific tyrosine kinase antibodies, PEF = peak expiratory flow, QMG = quantitative myasthenia gravis score, RNS = repetitive nerve stimulation, TUG = Timed Up and Go.
Funding/support: The study was funded by Neuroförbundet (NEURO Sweden) to EW and the Swedish Research Council (VR-523-2014-2048) to ARP.
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
None of the authors have any conflicts of interest to disclose.
References
- [1].Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334–59. [DOI] [PubMed] [Google Scholar]
- [2].World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva: World Health Organization; 2009. [Google Scholar]
- [3].Cup EH, Pieterse AJ, Ten Broek-Pastoor JM, et al. Exercise therapy and other types of physical therapy for patients with neuromuscular diseases: a systematic review. Arch Phys Med Rehabil 2007;88:1452–64. [DOI] [PubMed] [Google Scholar]
- [4].Scheer BV, Valero-Burgos E, Costa R. Myasthenia gravis and endurance exercise. Am J Phys Med Rehabil 2012;91:725–7. [DOI] [PubMed] [Google Scholar]
- [5].Stout JR, Eckerson JM, May E, et al. Effects of resistance exercise and creatine supplementation on myasthenia gravis: a case study. Med Sci Sports Exerc 2001;33:869–72. [DOI] [PubMed] [Google Scholar]
- [6].Lucia A, Mate-Munoz JL, Perez M, et al. Double trouble (McArdle's disease and myasthenia gravis): how can exercise help? Muscle Nerve 2007;35:125–8. [DOI] [PubMed] [Google Scholar]
- [7].Elsais A, Johansen B, Kerty E. Airway limitation and exercise intolerance in well-regulated myasthenia gravis patients. Acta Neurol Scand Suppl 2010. 12–7. [DOI] [PubMed] [Google Scholar]
- [8].Fregonezi GA, Resqueti VR, Guell R, et al. Effects of 8-week, interval-based inspiratory muscle training and breathing retraining in patients with generalized myasthenia gravis. Chest 2005;128:1524–30. [DOI] [PubMed] [Google Scholar]
- [9].Rassler B, Hallebach G, Kalischewski P, et al. The effect of respiratory muscle endurance training in patients with myasthenia gravis. Neuromuscul Disord 2007;17:385–91. [DOI] [PubMed] [Google Scholar]
- [10].Lohi EL, Lindberg C, Andersen O. Physical training effects in myasthenia gravis. Arch Phys Med Rehabil 1993;74:1178–80. [PubMed] [Google Scholar]
- [11].Westerberg E, Molin CJ, Lindblad I, et al. Physical exercise in myasthenia gravis is safe and improves neuromuscular parameters and physical performance-based measures: a pilot study. Muscle Nerve 2017;56:207–14. [DOI] [PubMed] [Google Scholar]
- [12].Rahbek MA, Mikkelsen EE, Overgaard K, et al. Exercise in myasthenia gravis: a feasibility study of aerobic and resistance training. Muscle Nerve 2017;56:700–9. [DOI] [PubMed] [Google Scholar]
- [13].Olsson SJ, Ekblom O, Andersson E, et al. Categorical answer modes provide superior validity to open answers when asking for level of physical activity: a cross-sectional study. Scand J Public Health 2016;44:70–6. [DOI] [PubMed] [Google Scholar]
- [14].Burns TM, Conaway M, Sanders DB. The MG composite: a valid and reliable outcome measure for myasthenia gravis. Neurology 2010;74:1434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bedlack RS, Simel DL, Bosworth H, et al. Quantitative myasthenia gravis score: assessment of responsiveness and longitudinal validity. Neurology 2005;64:1968–70. [DOI] [PubMed] [Google Scholar]
- [16].Molin CJ, Punga AR. Compound motor action potential: electrophysiological marker for muscle training. J Clin Neurophysiol 2016;33:340–5. [DOI] [PubMed] [Google Scholar]
- [17].Duez L, Qerama E, Fuglsang-Frederiksen A, et al. Electrophysiological characteristics of motor units and muscle fibers in trained and untrained young male subjects. Muscle Nerve 2010;42:177–83. [DOI] [PubMed] [Google Scholar]
- [18].Franchi MV, Longo S, Mallinson J, et al. Muscle thickness correlates to muscle cross sectional area in the assessment of strength training induced hypertrophy. Scand J Med Sci Sports 2018;28:846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Estes RR, Malinowski A, Piacentini M, et al. The effect of high intensity interval run training on cross-sectional area of the vastus lateralis in untrained college students. Int J Exerc Sci 2017;10:137–45. [PMC free article] [PubMed] [Google Scholar]
- [20].Molin CJ, Widenfalk J, Punga AR. High-resistance strength training does not affect nerve cross sectional area – an ultrasound study. Clin Neurophysiol Pract 2017;2:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Burns TM, Conaway MR, Cutter GR, et al. Less is more, or almost as much: a 15-item quality-of-life instrument for myasthenia gravis. Muscle Nerve 2008;38:957–63. [DOI] [PubMed] [Google Scholar]
- [22].Valko PO, Bassetti CL, Bloch KE, et al. Validation of the fatigue severity scale in a Swiss cohort. Sleep 2008;31:1601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Massey G, Evangelidis P, Folland J. Influence of contractile force on the architecture and morphology of the quadriceps femoris. Exp Physiol 2015;100:1342–51. [DOI] [PubMed] [Google Scholar]
- [24].Fikenzer K, Fikenzer S, Laufs U, Werner C. Effects of endurance training on serum lipids. Vasc Pharmacol 2018;101:9–20. [DOI] [PubMed] [Google Scholar]
- [25].Gilhus NE. Myasthenia gravis. N Engl J Med 2016;375:2570–81. [DOI] [PubMed] [Google Scholar]
- [26].Anziska Y, Sternberg A. Exercise in neuromuscular disease. Muscle Nerve 2013;48:3–20. [DOI] [PubMed] [Google Scholar]


