Abstract
The successful therapeutic use of anti-TNF biological agents in patients with ankylosing spondylitis (AS) indicates that tumor necrosis factor-α (TNF-α) and tumor necrosis factor receptor (TNFR) genes are involved in the pathogenesis of AS. TNF-α exerts its biological activity by binding to its cell surface receptors (p55 TNF-α receptor [TNFRI, encoded by the Tumor Necrosis Factor Receptor Superfamily Member 1A (TNFRSF1A)] and p75 receptor [TNFRII, encoded by the Tumor Necrosis Factor Receptor Superfamily Member 1B (TNFRSF1B)]. TNFRSF1A and TNFRSF1B may be related to AS, but the relevant studies are still limited. Therefore, we aim to explore the association between TNFRSF1A and TNFRSF1B polymorphisms and susceptibility and short- and long-term response to anti-TNF treatment in human leukocyte antigen-B27 (HLA-B27)-positive Chinese Han patients with AS.
A total of 215 HLA-B27-positive patients with AS and 216 HLA-B27-positive matched controls were enrolled and genotyped for rs767455, rs2234649, and rs1061622. A subset of 50 AS patients was also studied for the association of these polymorphisms with the short- and long-term response to etanercept assessed by Assessment in Ankylosing Spondylitis 20 (ASAS20) and Assessment in Ankylosing Spondylitis 40 (ASAS40).
Our data showed that rs767455 was associated with the susceptibility of AS, G allele of rs767455 exhibited an association with the risk of developing AS (OR = 1.63 (1.04–2.55), P = .032). Rs1061622 polymorphism was associated with total back pain and chest expansion. Only rs1061622 was significantly associated with long-term efficacy of etanercept: the TG genotype of rs1061622 worsened ASAS20 and ASAS40 responses at 12 months (P = .021 and .041, respectively).
The results suggest that TNFRSF1A and TNFRSF1B polymorphisms were associated with susceptibility, severity, and the long-term therapeutic efficacy of etanercept of patients with AS.
Keywords: ankylosing spondylitis, single nucleotide polymorphisms, TNFRSF1A, TNFRSF1B
1. Introduction
Ankylosing spondylitis (AS) is the most genetically related disease we found. Genetic factors have been strongly implicated in the etiology of AS, and heritability, as assessed by twin studies, has been reported as high as 90% or more.[1] However, overall contribution to heritability of AS of confirmed susceptibility genes was only 25%.[2,3] There are also much genetic factors worth exploring. Our previous studies found that interleukin-33 (IL-33) serum level was elevated in patients with AS[4] and IL-33 was associated with the development of AS.[5] We also found the association of IL-1F7 gene with susceptibility to human leukocyte antigen-B27 positive ankylosing spondylitis in Han Chinese population.[6]
Tumour necrosis factor alpha (TNF-α) is a potent immune mediator and proinflammatory cytokine which plays an important role on the pathogenesis of ankylosing spondylitis. TNF-α exerts its biological activity by binding to its cell surface receptors (p55 TNF-α receptor [TNFRI, encoded by the TNFRSF1A] and p75 receptor [TNFRII, encoded by the TNFRSF1B]).[7,8]TNFRSF1A and TNFRSF1B might be candidate genes in the pathogenesis of AS, but the relevant studies are still limited. Single nucleotide polymorphisms (SNPs) in gene sequences that alter gene transcription or the encoded protein function could account for the variability in the pathogenesis of autoimmune diseases. An association between AS and rs1061622 polymorphism in TNFRSF1B gene has been reported in Taiwan.[9] A preliminary study was first reported the association of the rs2234649 polymorphism in TNFRSF1A gene and rs1061622 polymorphism in TNFRSF1B gene for AS in Mexican population.[10] In addition, many studies showed that rs767455 and rs1061622 polymorphism influenced the susceptibility and response to anti-TNF therapy in rheumatoid arthritis (RA), Crohn's disease (CD), and psoriasis (PsA),[11–13] which might be associated with AS. These SNPs above are of interest for the genetic epidemiology of AS, suggesting the necessity for further studies to test the associations with AS.
Currently, antitumor necrosis factor (anti-TNF) biological agents have dramatic therapeutic effect on AS and have improved tens of thousands of patients’ living quality.[14] However, 20% to 40% of patients responded poorly to these agents,[14–16] and there were still any problems with the long-term therapeutic efficacy of these agents. Recently, some studies focused on the role of genetic markers in the response to anti-TNF biological agents in AS[17,18] and these studies were still lacked in China. In addition, genetic factors have been demonstrated which was associated with severity of AS.[19,20]
Therefore, we selected these 3 previously reported TNFRSF1A and TNFRSF1B polymorphisms and determined whether there was an association with susceptibility, diseases severity, and short- and long-term efficacy of anti-TNF biological agent in HLA-B27-positive Chinese Han patients with AS.
2. Methods
2.1. Subjects
The current case–control study was approved by the Ethical Committees of Anhui Medical University (approval number: 20113092 for the case–control study, the sample size calculation was done according the previous study in Mexican individuals. Our hypothesis was that there would be 96% patients in case and 88% induvial in control are A allele, which meant a sample size of 188 in each group was needed to get power of 80% for a significance level of 5% with a 2-tailed test. All participants provided written informed consent to participate in this study. A total of 215 HLA-B27-positive patients with AS were consecutively enrolled and followed-up at the Department of Rheumatology and Immunology of the First Affiliated Hospital of Anhui Medical University in China from February 2011 to March 2013. All patients fulfilled the 1984 revised New York criteria of the American College of Rheumatology[21] and they did not receive therapy with disease-modifying antirheumatic drugs, including biologic agents. During the same time, 216 HLA-B27-positive healthy volunteers matched for age, sex, height, and weight were enrolled as healthy controls. Participants with thyroid diseases, adrenal gland diseases, other endocrine disorders, serious liver diseases or serious kidney diseases were excluded. Participants were also excluded if they had used of steroid, oestrogen, or androgen drugs in the past 3 months. The baseline characteristics of AS patients and controls are shown in Table 1.
Table 1.
Baseline characteristics of AS patients and controls.
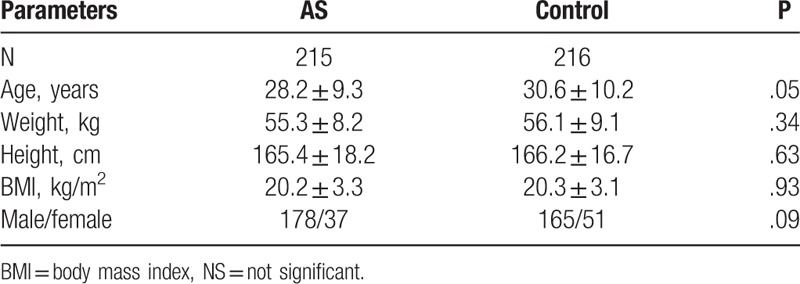
Patients were consecutively enrolled and interviewed by trained rheumatologists or investigators with a mailed questionnaire face to face. The questionnaire included patient demographic and clinic pathological characteristics data, such as age, gender, height, weight, duration of disease, HLA-B27 status, involved peripheral joint counts for tenderness and swelling, visual analog scale of overall spondylalgia, duration of morning stiffness, joint function, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI),[22] Bath Ankylosing Spondylitis Functional Index (BASFI),[23] thoracic expansion and Schober's test.
2.2. Therapeutic efficacy assessments of etanercept
There were 215 patients, of whom 50 (45 men and 5 women; mean age, 27.8 ± 6.9 years; mean body mass index (BMI), 20.7 ± 4.1 kg/m2) agreed to treatment with an anti-TNF biological agent (etanercept, 25 mg, subcutaneous injection, twice a week) for 3 months and sulfasalazine (2.0 g/day), and celecoxib (0.4 g/day) for another 9 months. Therapeutic improvement was measured by Assessment in Ankylosing Spondylitis Response Criteria (ASAS20) and ASAS40[24] at 3 months and 12 months.
2.3. Genotyping
The SNP rs767455 (A/G) was located in exon 1 (amino acid position +36) of the TNFRSF1A gene. The SNP rs2234649 (A/C) was located in 5′ untranslated region (UTR) (amino acid position -383) of the TNFRSF1A gene. The SNP rs1061622 (T/G) was located in exon 6 (amino acid position +196) of the TNFRSF1B gene. The genotyping of SNPs (rs767455, rs2234649 and rs1061622) was conducted by the Shanghai Biowing Applied Biotechnology Company (http://www.biowing.com.cn) using ligase detection reactions (LDRs).[5,25]
The polymorphic regions of the TNFRSF1A and TNFRSF1B genes were amplified by multiplex polymerase chain reaction (PCR). Primers for rs767455: forward primer 5′-TAGCTGTCTGGCATGGGCCTCT-3′ and reverse primer 5′-CCTACTCCAAAAGGCGGATGAA-3′; primers for rs2234649: forward primer 5′-CTTGGTGTTTGGTTGGGAGTGG-3′ and reverse primer 5′-GGGAAGAGTGAGGCAGTGTTGC-3′; primers for rs1061622: forward primer 5′-CAGAGAGGGCACACATCGTCAC-3′, and reverse primer 5′-GTGTGGACACTGGCTGGGGTAA-3′. The following experimental procedure is refer to the procedure in the article we published.[5]
2.4. Statistical methods
Statistical Product and Service Solutions (SPSS) 13.0 software was used for statistical analysis. All statistical tests were 2-tailed and a P-value < 0.05 was considered statistically significant. Variables were presented as mean ± standard deviations (SDs) and median with interquartile range (IQR) for normally distributed and un-normally distributed, respectively. Comparisons between the 2 groups were performed using 2 sample T tests for continuous measures that were normal distribution (Q–Q test was used to test the normality of data). Wilcoxon's rank sum tests were performed for continuous measures had a non-normal distribution. Pearson's chi square tests were used for categorical measures. Fisher's exact tests were used if the total sample size was smaller than 40 or the expected value was higher than 1 and smaller than 5. Odds ratio (OR) and 95% confidence interval (CI) were calculated. A test for deviation from Hardy–Weinberg equilibrium (HWE) was performed for each SNP in the control group (P > .05). Only several data were missing and we used “listwise deletion” to address the missing data.
3. Results
3.1. Association of TNFRSF1A and TNFRSF1B polymorphisms with disease susceptibility
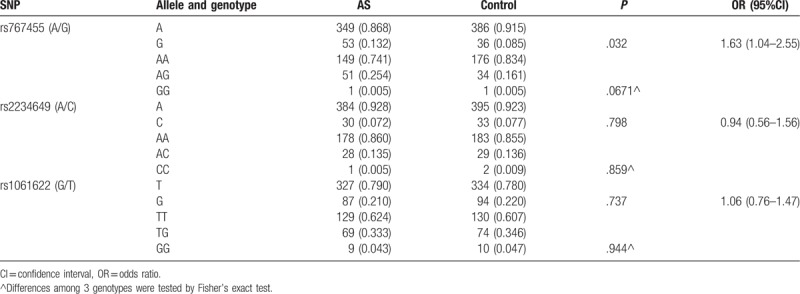
A higher frequency of G allele in rs767455 was observed in the AS group compared with the control group (13.2% vs 8.5%) in HLA-B27 positive Chinese population. The G allele of rs767455 exhibited an association with the risk of developing AS, (OR = 1.63 (1.04–2.55), P = .032), Table 2). However, there were no significant differences among the 3 genotypes of rs767455 (P = .067). There were no differences in the allele and genotype frequencies of rs2234649 and rs1061622 between patients and controls (P > .05) (Table 2).
Table 2.
Allele and genotype frequencies of 3 SNPs in AS patients and controls.
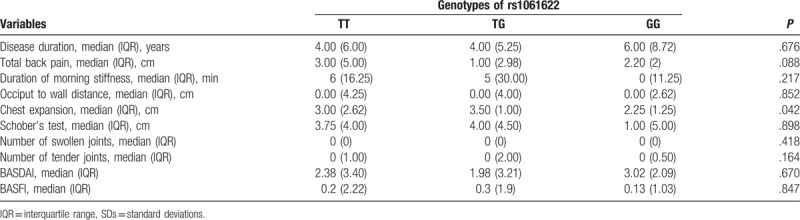
3.2. Association between rs1061622 polymorphism and disease severity
Comparison of disease severity between different genotypes was performed to test the association between rs1061622 polymorphism and disease severity (Table 3). Total back pain among 3 groups were different, although the values did not reach statistical significance (P = .088). Chest expansion of 3 groups was significantly different and the P value was .042. However, rs1061622 was found no significantly difference on disease duration, duration of morning stiffness, occiput to wall distance, Schober's test, number of swollen joints, number of tender joints, BASDAI and BASFI (P > .05). Rs2234649 and rs767455 were not analyzed because of the small sample size of GG genotype observed.
Table 3.
Association between genotypes of rs1061622 and disease severity.
3.3. Association of TNFRSF1A and TNFRSF1B polymorphisms with short- and long-term efficacy of etanercept
We determined the influence of genetic variants on the response to etanercept (Figs. 1 and 2). Only rs1061622 was significantly associated with long-term efficacy of etanercept. A higher frequency of ASAS20 response at 12 months was observed in the patients with TT/GG genotype compared with the patients with TG genotype (84.8% versus 52.9%, P = .042). A higher frequency of ASAS40 response of 12 months was observed in the patients with TT/GG genotype compared with the patients with TG genotype (66.7% vs 35.3%, P = .021). The TG genotype of rs1061622 worsened ASAS20 and ASAS40 responses etanercept at12 months.
Figure 1.
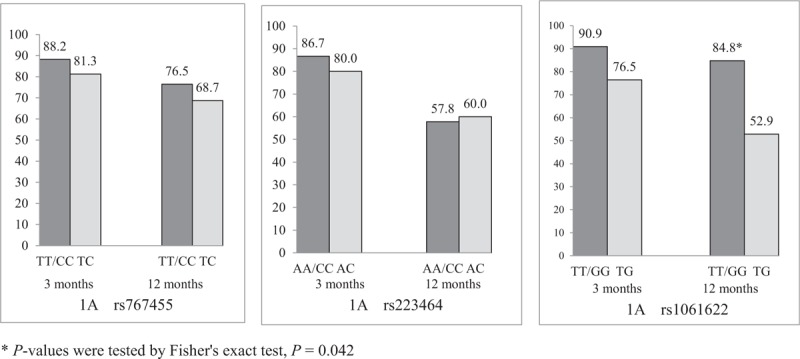
ASAS20 responses to etanercept among different genotypes of rs767455, rs223464 and rs1061622 (%). Only rs1061622 was significantly associated with long-term efficacy of etanercept. A higher frequency of ASAS20 response at 12 months was observed in the patients with TT/GG genotype compared with the patients with TG genotype (84.8% vs 52.9%, P = .042).
Figure 2.
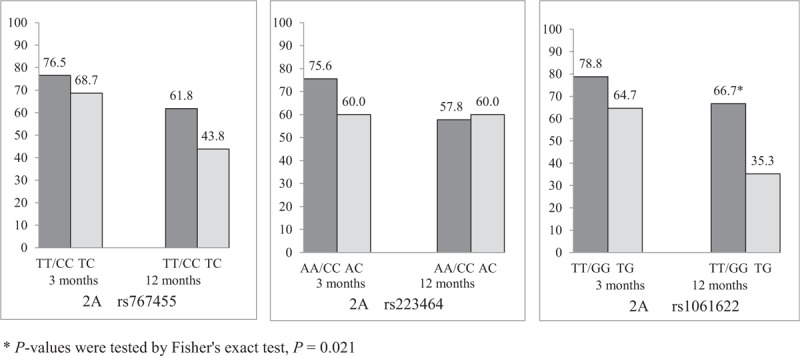
ASAS40 responses to etanercept among different genotypes of rs767455, rs223464 and rs1061622 (%). Only rs1061622 was significantly associated with long-term efficacy of etanercept. A higher frequency of ASAS40 response at 12 months was observed in the patients with TT/GG genotype compared with the patients with TG genotype (66.7% vs 35.3%, P = .021).
4. Discussion
In the present study, we examined the associations between 3 previously reported TNFRSF1A and TNFRSF1B polymorphisms and susceptibility, diseases severity, and short- and long-term efficacy of anti-TNF biological agent in HLA-B27-positive Chinese Han patients with AS. Our data showed that rs767455 was associated with the susceptibility of AS, G allele of rs767455 exhibited an association with the risk of developing AS (OR = 1.63 (1.04–2.55), P = .032). Rs1061622 polymorphism was associated with total back pain and chest expansion. In patients who underwent a 3-month-long treatment with etanercept and then a 9-month-long treatment with sulfasalazine and celecoxib, the TG genotype of rs1061622 worsened ASAS20 and ASAS40 responses to etanercept at 12 month. Thus, our data provided important insights into the effects of TNFRSF1A and TNFRSF1B polymorphisms on susceptibility, diseases severity, and short- and long-term therapeutic efficacy of etanercept of AS.
TNF-α is a potent immune mediator and proinflammatory cytokine which plays a vital role in the pathogenesis of ankylosing spondylitis. TNF-α exerts its biological activity by binding to its cell surface receptors (TNFRI and TNFRII).[1,2] A genetic polymorphism might produce conformational changes in the molecule of TNFR1, leading to modifications induced by TNF-α through mediating an abnormal signalling.[26] The studies concerned the role of TNFRSF1A and TNFRSF1B polymorphisms in AS were still insufficient and controversial.
Tung and Lu[9] study included 220 HLA-B27 positive patients and 2 controls: 140 HLA-B27 positive healthy controls and 180 HLA-B27 negative healthy controls in Taiwan. The study found that AS patients had a higher frequency of G allele in rs1061622 compared with HLA-B27 positive healthy controls and HLA-B27 negative healthy (both P < .05), but not in rs767455. Additionally, Corona-Sanchez et al[10] performed a preliminary study including 66 Mexican patients with AS and 108 gender- and age-matched controls. They found that the AA genotype of rs2234649 occurred in a higher frequency in the Mexican Mestizos AS group than controls. The A allele was also increased in AS and was a risk factor of genetic susceptibility for AS in Mexican individuals (OR = 3.48, P = .015). However, our result was opposite. We found G allele of rs767455 exhibited an association with the risk of developing AS (OR = 1.63 (1.04–2.55), P = .032). No significant association was found between SNPs (rs1061622 and rs2234649) and AS susceptibility (P > .05).
In terms of effects on AS disease severity, clinical parameters including diseases duration, smoking and lower socioeconomic status were associated with more serious disease.[27,28] Nevertheless, much of the variability in disease severity in AS remained unexplained,[29] suggesting genetic factors could also influence the disease severity. Our results indicated rs1061622 polymorphism was associated with total back pain and chest expansion. Moreover, our study provided the first evidence for the association between rs1061622 in TNFRSF1B and disease severity of AS.
The high efficacy of anti-TNF biological agents has been confirmed in the clinical setting. However, 20% to 40% patients did not respond well for unknown reasons and long-term recurrence was often observed. Therefore, the identification of sensitive indicators to predict the efficacy of TNF-α antagonists was critical for improving outcomes in patients with AS. Clinical data indicated those patients with lower ages, shorter disease durations, higher disease activity, lower BASFI, lower imaging changes, higher serum amyloid protein levels, higher C-reaction protein (CRP) levels tended to respond better to initial treatment with anti-TNF biological agents.[30,31] Recent studies also revealed that genetic markers were associated with the therapeutic effects of drugs. However, these studies had primarily focused on RA rather than AS. Seitz et al[17] included patients with RA treated with etanercept, infliximab, and adalimumab for 1 year and found that patients with the TG + GG genotype of rs1061622 showed worse responses to anti-TNF biological agents at 3 months and 12 months than patients with the TT genotype. The effect of TNF-α-308 G/A was analyzed in patients with RA, psoriasis, and AS after treatment with anti-TNF biological agent for 24 weeks and assessed the disease activity with Disease Activity Score in 28 joints (DAS28) and BASDAI. They found that patients (with RA) with the AA genotype and patients (with AS) with the AG genotype had worse response to anti-TNF biological agents. In our study, we found that the TG genotype of rs1061622 worsened the long-term response to etanercept. To our knowledge, this was the first report that the TG genotype of rs1061622 was a risk factor of response to anti-TNF biological agents.
In study of RA, rs1061622 has been reported to affect the level and function of soluble TNFR p75, and consequently, influence the response to anti-TNF agent treatment.[32] Thus, we could suppose that AS patients with the TT/GG genotype of rs1061622 had better long-term responses to anti-TNF biological agents due to changes in the expression and function of soluble TNFR p75. Further studies are needed to identify genetic markers to predict the efficacy of anti-TNF biological agents.
However, there were several limitations in our study. First, a limited sample size was used in this study, especially the sample size of patients treat with etanercept. Second, some clinical and environmental factors associated with AS were not assessed. Therefore, further studies of TNFRSF1A and TNFRSF1B polymorphisms will be performed to confirm the relationship between these loci and AS in larger samples. Multivariate analyses, clinical, and environmental factors need to be taken into consideration.
5. Conclusions
Rs767455 was associated with the susceptibility of AS, G allele of rs767455 exhibited an association with the risk of developing AS (OR = 1.63 (1.04–2.55), P = .032). Rs1061622 polymorphism was first reported associated with total back pain and chest expansion. The TG genotype of rs1061622 was first reported worsened ASAS20 and ASAS40 responses to etanercept at 12 months in patients with AS. Further studies are required to confirm these findings.
Acknowledgments
We thank all patients and healthy subjects who provided the DNA and information necessary for our study. We also thank Shanghai Biowing Applied Biotechnology Company (http://www.biowing.com.cn), for valuable help with the test of SNPs. We thank all the professionals who participated in the study.
Author contributions
Data curation: Wang Xingrong.
Formal analysis: Wang Xingrong, Pan Faming.
Investigation: Liu Wen, Qi Shan.
Writing – original draft: Wang Xingrong.
Writing – review & editing: Xu Shengqian, Pan Faming, Xu jianhua.
Footnotes
Abbreviations: AS = ankylosing spondylitis, ASAS = Assessment in Ankylosing Spondylitis, BASDAI = Bath Ankylosing Spondylitis Disease Activity Index, BASFI = Bath Ankylosing Spondylitis Functional Index, CD = Crohn's disease, CI = confidence interval, CRP = C-reaction protein, DAS28 = Disease Activity Score in 28 joints, HLA-B27 = human leukocyte antigen-B27, HWE = Hardy–Weinberg equilibrium, IL-33 = interleukin-33, IQR = interquartile range, LDRs = ligase detection reactions, non-MHC = nonmajor histocompatibility complex, OR = odds ratio, PCR = polymerase chain reaction, PsA = psoriasis, RA = rheumatoid arthritis, SDs = standard deviations, SNPs = single nucleotide polymorphisms, SPSS = statistical product and service solutions, TNF-α = tumor necrosis factor-alpha, TNFR = tumor necrosis factor receptor, TNFRSF = tumor necrosis factor receptor superfamily member, UTR = untranslated region.
The authors have no conflicts of interest to disclose.
References
- [1].Gran JT, Ostensen M. A clinical comparison between males and females with ankylosing spondylitis. J Rheumatol 1985;12:126. [PubMed] [Google Scholar]
- [2].Reveille John D. Genetics of spondyloarthritis—beyond the MHC. Nat Rev Rheumatol 2012;8:296. [DOI] [PubMed] [Google Scholar]
- [3].Huang J, Li C. Novel non-HLA-susceptible regions determined by meta-analysis of four genomewide scans for ankylosing spondylitis. J Genet 2008;87:75. [DOI] [PubMed] [Google Scholar]
- [4].Li GX, Wang S. Serum levels of IL-33 and its receptor ST2 are elevated in patients with ankylosing spondylitis. Scand J Rheumatol 2014;42:226. [DOI] [PubMed] [Google Scholar]
- [5].Fan D, Ding N, Yang T, et al. Single nucleotide polymorphisms of the interleukin-33 (IL-33) gene are associated with ankylosing spondylitis in Chinese individuals: a case-control pilot study. Scand J Rheumatol 2014;43:374. [DOI] [PubMed] [Google Scholar]
- [6].Faming Pan, Fangfang Liao Association of IL-1F7 gene with susceptibility to human leukocyte antigen-B27 positive Ankylosing spondylitis in Han Chinese population. Clin Chim Acta 2015;411:124. [DOI] [PubMed] [Google Scholar]
- [7].Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Engl J Med 1996;334:1717. [DOI] [PubMed] [Google Scholar]
- [8].Glossop JR, Dawes PT. Polymorphism in the tumour necrosis factor receptor II gene is associated with circulating levels of soluble tumour necrosis factor receptors in rheumatoid arthritis. Arthritis Res Ther 2005;7:1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tung CH, Lu MC. Association between ankylosing spondylitis and polymorphism of tumour necrosis factor receptor II in Taiwanese patients. Scand J Rheumatol 2009;38:395. [DOI] [PubMed] [Google Scholar]
- [10].Corona-Sanchez EG, Munoz-Valle JF, Gonzalez-Lopez L, et al. −383 A/C tumor necrosis factor receptor 1 polymorphism and ankylosing spondylitis in Mexicans: a preliminary study. Rheumatol Int 2012;32:2565. [DOI] [PubMed] [Google Scholar]
- [11].Morales-Lara MJ, Canete JD. Effects of polymorphisms in TRAILR1 and TNFR1A on the response to anti-TNF therapies in patients with rheumatoid and psoriatic arthritis. Joint Bone Spine 2012;79:591. [DOI] [PubMed] [Google Scholar]
- [12].Matsukura H, Ikeda S. Genetic polymorphisms of tumour necrosis factor receptor superfamily 1A and 1B affect responses to infliximab in Japanese patients with Crohn's disease. Aliment Pharmacol Ther 2008;27:765. [DOI] [PubMed] [Google Scholar]
- [13].Gonzalez-Lara L, Batalla A. The TNFRSF1B rs1061622 polymorphism (p.M196R) is associated with biological drug outcome in Psoriasis patients. Arch Dermatol Res 2015;307:405. [DOI] [PubMed] [Google Scholar]
- [14].Pavelka K, Forejtová S. Anti-TNF therapy of ankylosing spondylitis in clinical practice. Results from the Czech national registry ATTRA. Clin Exp Rheumatol 2009;27:958. [PubMed] [Google Scholar]
- [15].Wu D, Guo YY. Efficacy of anti-tumor necrosis factor therapy for extra-articular manifestations in patients with ankylosing spondylitis: a meta-analysis. BMC Musculoskelet Disord 2015;16:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McLeod C, Bagust A. Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation. Health Technol Assess 2007;11:1. [DOI] [PubMed] [Google Scholar]
- [17].Seitz M, Wirthmuller U. The -308 tumour necrosis factor-alpha gene polymorphism predicts therapeutic response to TNFalpha-blockers in rheumatoid arthritis and spondyloarthritis patients. Rheumatology (Oxford) 2007;46:93. [DOI] [PubMed] [Google Scholar]
- [18].Song GG, Seo YH. Association between TNF-α (−308 A/G, −238 A/G, −857 C/T) polymorphisms and responsiveness to TNF-α blockers in spondyloarthropathy, psoriasis and Crohn's disease: a meta-analysis. Pharmacogenomics 2015;16:1427. [DOI] [PubMed] [Google Scholar]
- [19].Nossent JC, Sagen-Johnsen S. Tumor necrosis factor-alpha promoter -308/238 polymorphism association with less severe disease in ankylosing spondylitis is unrelated to serum TNF-alpha and does not predict TNF inhibitor response. J Rheumatol 2014;41:1675. [DOI] [PubMed] [Google Scholar]
- [20].Wen YF, Wei JC. rs10865331 associated with susceptibility and disease severity of ankylosing spondylitis in a Taiwanese population. PLoS One 2014;9:e104525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].van der Linden S, Valkenburg HA. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361. [DOI] [PubMed] [Google Scholar]
- [22].Garrett S, Jenkinson T. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286. [PubMed] [Google Scholar]
- [23].Calin A, Garrett S. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 1994;21:2281. [PubMed] [Google Scholar]
- [24].Sieper J, Rudwaleit M, Baraliakos X, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 2009;68:2. [DOI] [PubMed] [Google Scholar]
- [25].Pan F, Liao F. Association of IL-1F7 gene with susceptibility to human leukocyte antigen-B27 positive ankylosing spondylitis in Han Chinese population. Clin Chim Acta 2010;411:124. [DOI] [PubMed] [Google Scholar]
- [26].Glossop JR, Nixon NB. No association of polymorphisms in the tumor necrosis factor receptor I and receptor II genes with disease severity in rheumatoid arthritis. J Rheumatol 2003;30:1406. [PubMed] [Google Scholar]
- [27].Doran MF, Brophy S. Predictors of longterm outcome in ankylosing spondylitis. J Rheumatol 2003;30:316. [PubMed] [Google Scholar]
- [28].Cansu DU, Calisir C. Predictors of radiographic severity and functional disability in Turkish patients with ankylosing spondylitis. Clin Rheumatol 2011;30:557. [DOI] [PubMed] [Google Scholar]
- [29].Hamersma J, Cardon LR. Is disease severity in ankylosing spondylitis genetically determined? Arthritis Rheum 2001;44:1396. [DOI] [PubMed] [Google Scholar]
- [30].Rudwaleit M, Listing J. Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor alpha blockers in ankylosing spondylitis. Ann Rheum Dis 2004;63:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ongaro A, De Mattei M. Can tumor necrosis factor receptor II gene 676T>G polymorphism predict the response grading to anti-TNFalpha therapy in rheumatoid arthritis? Rheumatol Int 2008;28:901. [DOI] [PubMed] [Google Scholar]
- [32].Tolusso B, Sacco S. Relationship between the tumor necrosis factor receptor II (TNF-RII) gene polymorphism and sTNF-RII plasma levels in healthy controls and in rheumatoid arthritis. Hum Immunol 2004;65:1420. [DOI] [PubMed] [Google Scholar]


