Abstract
Background:
A randomized controlled trial was performed to compare analgesic effects and adverse effects of oxycodone and sufentanil in patient-controlled intravenous analgesia (PCIA) after abdominal surgery under general anesthesia.
Methods:
Adult patients undergoing elective abdominal surgery were randomly allocated into oxycodone and sufentanil groups according to the randomization sequence. Study personnel, health-care team members, and patients were masked to the group assignment throughout the study period. Oxycodone (0.1 mg/kg for endoscopy; 0.15 mg/kg for laparotomy) or sufentanil (0.1 μg/kg for endoscopy; 0.15 μg/kg for laparotomy) was administrated at the end of surgeries. Postoperative pain was controlled using PCIA. Bolus dose was 2 mg and 2 μg for oxycodone and sufentanil group, respectively. The lockout time was 5 minutes for all patients, and there was no background infusion for oxycodone group, whereas 0.02 μg/kg/h background infusion was administrated in sufentanil group. The primary outcomes were the total analgesic doses in PCIA, effective bolus times, the length of first bolus since patients returning to ward from postanesthesia care unit (PACU), rescue analgesic rate in PACU, numeric rating scales, functional activity scores, and patients’ satisfaction scores.
Results:
A total of 200 patients were screened, and 175 patients were enrolled. Patients were randomly assigned to oxycodone (n = 87) and sufentanil (n = 88) groups. Both oxycodone and sufentanil PCIA provided adequate postoperative pain relief. Patients in oxycodone group showed a shorter consciousness recovery time after surgery. The major adverse effect in patients from oxycodone group was nausea/vomiting, whereas multiple adverse complications including nausea/vomiting, pruritus, and respiratory depression were observed in patients from sufentanil group. Patients from oxycodone group showed significantly reduced analgesic drug consumption (calculated as equivalent dose of morphine), functional activity scores, and patient satisfaction scores.
Discussion:
Compared with sufentanil PCIA, oxycodone PCIA showed better analgesic effects, lower incidence of adverse complications, and less analgesic drug consumption during postoperative pain management.
Keywords: abdominal surgery, oxycodone, patient-controlled intravenous analgesia, sufentanil
1. Introduction
Acute postoperative pain is one of the major challenges for anesthesiologists, surgeons, and patients despite the common concern and advancements in pain management strategies in recent years. In America, approximately 86% patients suffered from pain after surgery and 75% of these patients had moderate/extreme pain during the immediate postsurgical period, with 74% still experiencing these levels of pain after discharge.[1] Although there were no national data reported, Wang and Lin[2] found that 86.5% patients experienced moderate or severe pain after orthopedic surgeries in Beijing, illustrating the poor postoperative analgesia in China. Morphine is widely used in Europe and North America, sufentanil and fentanyl are more popular in China in postoperative analgesia by anesthesiologists because of their quicker onset of action and less adverse effects especially respiratory depression and postoperative nausea and vomiting.[3] Because of the shorter half-life of fentanyl and sufentanil (0.5 and 1.5 hours, respectively), continuous infusion dose is usually needed in patient-controlled intravenous analgesia (PCIA) after surgeries, which may increase the risk of respiratory depression when compared with the demand-only infusion PCIA.[4] Moreover, both fentanyl and sufentanil are potent agonists for μ-opioid receptors and have little effects on κ-opioid receptor, which has been confirmed to be the major opioid receptor involved in the visceral pain.[5,6] Therefore many patients complain about vague, diffuse, and poorly defined discomfort, although PCIA is used after abdomen surgeries.[7]
Oxycodone is a semisynthetic baine derivative opioid receptor agonist, which has been in clinical used since 1917.[8] Unlike morphine, fentanyl, and sufentanil, which are full agonists of μ-opioid receptor, oxycodone binds to both μ- and κ-opioid receptors, although its affinity to κ-opioid receptor is relatively lower.[8,9] Considering the pivotal role of κ-opioid receptors in visceral pain attenuation, it is reasonable to deduce that oxycodone may provide better acute postoperative analgesia (improved analgesia effect, higher patient satisfaction, and less side effects) when compared with pure μ-opioid receptor agonists such as fentanyl and sufentanil, which, however, is controversial in clinical practice.[10–14]
In the present study, sufentanil and oxycodone were administered as PCIA for 48 hours to patients undergoing abdominal surgeries under general anesthesia, respectively, and their analgesia effects, analgesic consumption, adverse complications, and degree of satisfaction were compared.
2. Materials and Methods
2.1. Study design
The trial was registered at www.chictr.org.cn (ChiCTR-IOR-17013515). This study was approved by the clinical research ethics committees from all 3 hospitals (2017015; 201707; 20170416). Written informed consent was obtained from each of patient, otherwise their next of kin or their legal representative. We did a randomized, double-blind, multicenter clinical trial in the departments of anesthesiology in the Second Affiliated Hospital of Xi’an Jiaotong University, Shaanxi Province Tumor Hospital, and the Second Affiliated Hospital of Xi’an Medical College, China. This study followed the Good Clinical Practice guidelines and the guidelines of the Helsinki Declaration.
2.2. Patients
Patients [aged 18–65 years, body mass index (BMI) 18–29.5 and ASA I–III] undergoing elective abdominal surgery (laparotomy or endoscopy) under general anesthesia were screened in the studies. Patients were excluded if they met any of the following criteria: preoperative history of schizophrenia, epilepsy, Parkinsonism, or myasthenia gravis; taking monoamine oxidase inhibitors within 2 weeks before the surgery; inability to communicate in the preoperative period (coma, profound dementia, or language barrier); brain injury or neurosurgery; esophageal reflux disease; the history of alcohol or analgesia drug abuse; severe hypertension (systolic blood pressure ≥180 mm Hg, diastolic blood pressure ≥110 mm Hg); severe hepatic, renal, and pulmonary dysfunction; known preoperative left ventricular ejection fraction <30%; and women in their pregnancy or lactation.
2.3. Randomization and masking
A biostatistician, who was independent of data management and statistical analysis, generated random numbers (in a 1:1 ratio) using the SAS 9.2 software (SAS Institute, Cary, NC). The results of randomization stored online (https://pan.baidu.com) until the end of the study.
During the study period, consecutively recruited patients were randomly assigned to oxycodone or sufentanil groups. Their anesthesiologist login in online and administered the study drugs according to the randomization sequence, which was marked as red color after usage. Study personnel, health-care team members, and patients were masked to the group assignment throughout the study period. In an emergency, unmasking of the treatment allocation could be requested, and the study would be terminated. These situations were documented and analyzed to reveal its potential association with the treatment.
2.4. Procedures
Patients were not given any sedative, analgesic, antiemetic, or anti-itching drugs 24 hours before the operation. Patients were fasting from solids for 6 hours and clear liquids 4 hours before the operation. After arriving the operation room, routine monitoring including electrocardiogram, pulse oximetry (SpO2), heart rate, noninvasive blood pressure (BP), and bispectral index (BIS) were established before the anesthesia.
Total intravenous general anesthesia was conducted in this study. General anesthesia was induced with 0.05 mg/kg midazolam, 0.4 μg/kg sufentanil, 0.2 mg/kg etomidate, and 0.2 mg/kg cisatracurium. During the operation, the maintenance doses of propofol and remifentanil (0.1–0.3 μg/kg/min) were adjusted to keep BIS between 45 and 60, and hemodynamic alteration between 20% compared to the baseline BP. The maintenance infusion rate of cisatracurium was 2 μg/kg/min.
Infusion of cisatracurium was terminated 30 minutes before the end of surgery, and 0.5 mg/kg ketorolac tromethamine, 0.1 mg/kg dexamethasone, and 0.1 mg/kg tropisetron were given intravenously to the patients. Oxycodone (0.1 mg/kg for endoscopy procedures; 0.15 mg/kg for laparotomy procedures) or sufentanil (0.1 μg/kg for endoscopy procedures; 0.15 μg/kg for laparotomy procedures) was administrated intravenously at the end of surgery. PCIA was given to the patients immediately at the end of surgery. Bolus dose was 2 mg and 2 μg each time for patients in oxycodone and sufentanil group, respectively. The lockout time was 5 minutes for both of groups. There was no continuous infusion for oxycodone groups, whereas 2 mL/h (0.02 μg/kg/h) continuous infusion was administrated to patients in sufentanil groups.
Patients were transferred into postanesthesia care unit (PACU), and extubation was performed when patients recovered from anesthesia. Postoperative pain was evaluated with numeric rating scales (NRS) 5 minutes after extubation by an anesthesiologist or nurse who was unaware of patient’ allocation. Bolus dose (2 μg sufentanil or 2 mg oxycodone injection) was given to the patients immediately if NRS was >3. The procedures could be repeated 5 minutes later until NRS <4. PACU discharge was decided by the responsible intensivists according to the Steward scores.
Postoperative follow-up in wards was performed by an anesthesiologist or a nurse who was unaware of patient allocations. Postoperative cumulative analgesic doses delivered, time for the bolus administration, NRS, and adverse complications were documented. The upper limit of bolus dose was 10 mg oxycodone or 10 μg sufentanil within 1 hour. If the consumptive analgesia drugs reached the limit and patients still felt pain, responsible anesthesiologist was then contacted, and alternative rescue analgesia should be administrated and recorded. The overall satisfaction was measured as follows: very poor (1 point), poor (2 points), moderate (3 points), good (4 points), and excellent (5 points). When SpO2 was <90% and respiration rate was <10 breath/min, it was documented as respiratory depression.
2.5. Outcomes
Outcome assessment was completed by research remembers who were trained before the study and not involved in the clinical care of the patients. The primary outcomes were the total postoperative analgesic doses patient used (calculated as the equal dose of morphine), postoperative effective bolus times pressed by patients, the length of first bolus since patients returning to ward from PACU, rescue analgesic dose need in PACU, NRS scores, functional activity score (FAS), and patients’ satisfaction for PCIA. The secondary outcomes included recovery length from anesthesia after surgery, length of stay in PACU, side effects especially respiratory depression, and postoperative nausea and vomiting (PONV) and FAS.
2.6. Statistical analysis
In our preliminary study, PACU rescue analgesia rates in oxycodone and sufentanil groups were 39% and 42.11%, respectively. There was 20% to 33% decrease in VAS score with respect to previous studies,[14] so we calculated the sample size based on a mean difference of 30% in NRS score between the 2 groups with 2-tailed α = 0.05 and β = 80%, total sample size required was 56 patients (23 patients per group), calculated with the SAS software (SAS Institute Inc, Cary, NC). Considering the potential difference caused by different surgery types (endoscopy/laparotomy), patient number for each group was increased to 46. Take into account a dropout rate of approximately 25%, we planned to enroll 60 patients for each group.
Statistical analysis was performed using Graphpad Prism 6.0 software (Graphpad software Inc, La Jolla, CA). Data were presented as mean ± SD. Data were tested for Gaussian distribution (Anderson-Darling test). Numeric variables were analyzed by unpaired t test (if data were Gaussian distribution) or Wilcoxon rank-sum test (if data were not Gaussian distribution). Categorical variables were analyzed with the χ2 test. The value of P < .05 was taken as significant difference.
3. Results
As shown in Figure 1, 200 patients were screened in our study between April and November 2017; of these 175 patients were enrolled and randomly allocated into oxycodone group (n = 87) and sufentanil group (n = 88). During the study period, there were no lapses in the blinding. Anesthesia drugs were modified in 18 patients because of adverse events such as hypertension and unexpected arrhythmia or surgeon requests. The other dropout reasons included unexpected termination of PCIA after surgery due to equipment dysfunction/intravenous line problem (14 patients), patients’ allergy during procedures (5 patients), transferring into intensive care unit (3 patients), incomplete case report form (12 patients), and hemorrhea during operations (3 patients). Therefore, 63 patients in oxycodone group and 57 patients in sufentanil group were included in the final data analysis.
Figure 1.
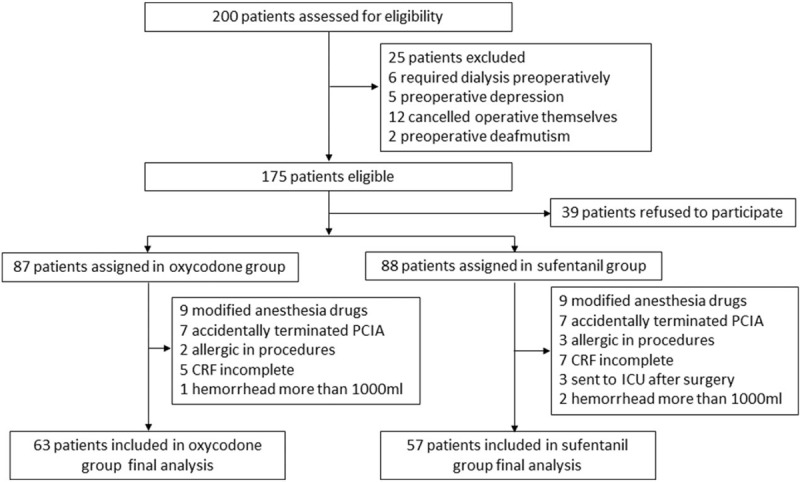
Trial profile. Data analysis included all patients in the groups to which they were randomly assigned. CRF = case report form, PCIA = patient-controlled intravenous analgesia.
There was no significant difference in demographic data including age, sex, BMI, types of procedures, length of anesthesia, length of incision, or sufentanil consumption during surgery between sufentanil and oxycodone group (P > .05, Table 1).
Table 1.
Comparison of demographic data in patients.
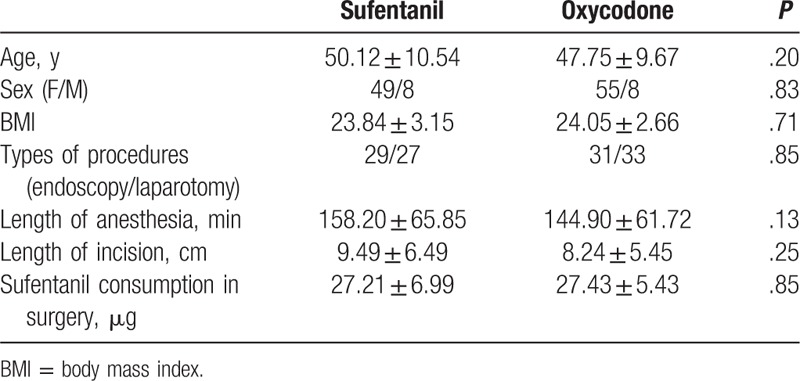
The consciousness recovery time in patients from oxycodone group was significantly shorter than that in patients from sufentanil group (P < .05, Table 2). However, there was no difference in extubation time between patients from oxycodone and sufentanil groups (P > .05). Both oxycodone and sufentanil provided adequate analgesia for most of patients in PACU (NRS < 4). For those patients need rescues analgesia, 1 bolus infusion was enough to achieve satisfied postoperative pain control, no significant difference in rescues analgesia rate in PACU was observed between oxycodone and sufentanil groups (P > .05, Table 2). There was no difference in either length between extubation and discharge from PACU, or PACU staying time between sufentanil and oxycodone groups (P > .05, Table 2). Nausea and vomiting were observed in patients from both groups, but no difference was observed (P > .05, Table 2). Pruritus and respiratory depression in PACU was only observed in sufentanil but not in oxycodone group; however, there was no statistical difference between these 2 groups (P > .05, Table 2).
Table 2.
Comparison of recovery, postoperative analgesia, and side effects in postanesthesia care unit.
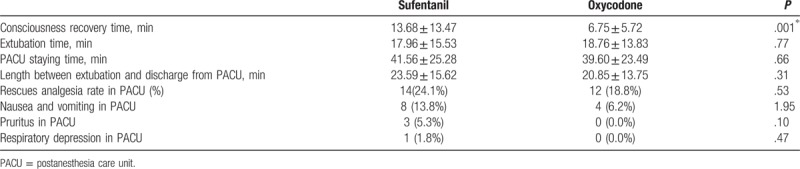
We analyzed demand bolus times, analgesic dose consumption of PCIA, and FAS after surgery in wards. The median demand bolus times in oxycodone group within 24 hours after surgery was 0.86, which increased to 2.08 in patients from sufentanil group; however, no statistical difference was observed (P > .05, Table 3). Twenty-four to 48 hours after surgery, median demand bolus times between oxycodone and sufentanil groups became similar (0.48 vs 0.45, P > .05, Table 3). Taken together, there was no statistical difference in total demand bolus times between oxycodone and sufentanil groups after surgery (P > .05, Table 3). Compared with sufentanil group, patients from oxycodone group consumed significantly less analgesic dose for PCIA when calculated as equal dose to morphine (P < .05, Table 4). Similarly, patients from oxycodone group showed higher FAS in wards when compared with that from sufentanil group (P < .05, Table 4). Patients from both groups were satisfied for the PCIA; however, the satisfaction scores in sufentanil group were significantly lower when compared with those patients from oxycodone group (P < .05, Table 4).
Table 3.
Comparison of patient-controlled intravenous analgesia times between groups.
Table 4.
Comparison of analgesic dose, functional activity score, and satisfaction scores between groups.

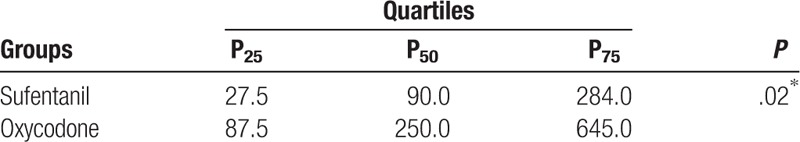
We further compared the length of first demand bolus infusion in wards since patients were discharged from PACU. Patients from oxycodone group showed much longer median length (250 minutes) when compared with patients in sufentanil group (90 minutes, P < 0.05, Table 5).
Table 5.
Comparison of first bonus analgesia in wards between groups.
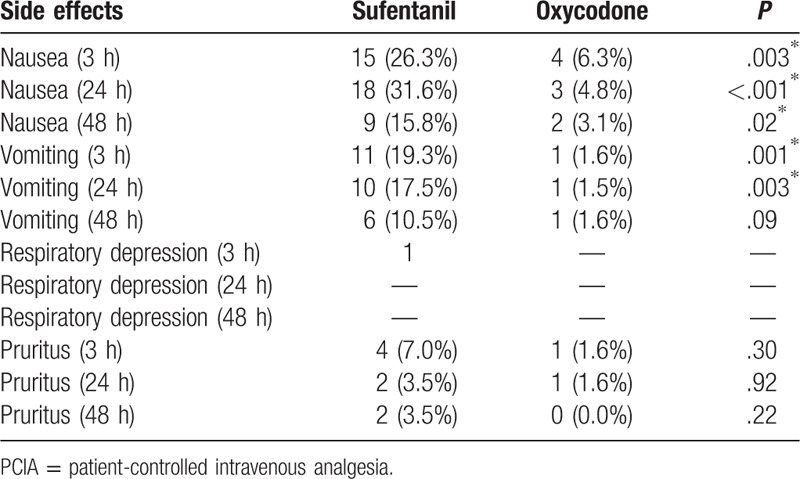
Respiratory depression was observed in 1 patient from sufentanil group within 3 hours after surgery, whereas no respiratory depression was observed in patients from oxycodone group (Table 6). Nausea and vomiting were observed in patients from all of groups, and there was a higher incidence rate in nausea and vomiting in patients from sufentanil group when compared with those patients from oxycodone group after 3, 24, and/or 48 hours after surgery (P < .05, Table 6). Pruritus was observed in patients from both sufentanil and oxycodone groups after surgery; however, we did not observe any statistical difference (P > .05, Table 6).
Table 6.
Comparison of patient-controlled intravenous analgesia adverse complications in wards between groups.
4. Discussion
In this study, patients undergoing endoscopy were given 0.1 mg/kg oxycodone or equal dose of sufentanil (0.1 μg/kg) at the end of surgery, similar to previous studies.[15] In our preliminary study, 0.1 mg/kg oxycodone could not provide adequate transition analgesia for patients undergoing laparotomy (data not shown), 0.15 mg/kg oxycodone or 0.15 μg/kg sufentanil was thereby administrated, which was similar with a previous study,[16] although the surgeries were different. As expected, both oxycodone and sufentanil provided satisfied transition analgesia, which was consistent with previous studies.[14–18] Interestingly, the recovery of consciousness from general anesthesia in oxycodone group was significantly quicker; however, there was no difference in extubation time and PACU staying time between oxycodone and sufentanil groups, which may be induced by discrepant understanding and practice habitation between nurses or doctors responsible in PACU, especially in different hospitals. After returning to wards, both oxycodone-PCIA and sufentanil-PCIA provided adequate postoperative pain analgesia (NRS < 4). However, patients undergoing endoscopy required less demand bolus in oxycodone group. This could be ascribed to the fact that oxycodone effectively alleviated visceral pain,[8] a type of nociceptive pain that comes from the internal organs and is usually induced by mechanical strain, spasm, ischemia, and inflammation.[19] Oxycodone binds to both μ- and κ-opioid receptors and alleviated not only somatic but also patients’ uncomfortable feeling induced by visceral pain, consequently showing higher therapeutic efficacy in acute postoperative pain after abdominal surgery. This was further confirmed by less FAS in patients from oxycodone group. After surgery, coughing is essential for keeping lungs clear, preventing pneumonia, and the acceleration of patients’ recovery. However, many patients refuse to breathe deeply and cough because of postoperative pain. In this study, patients from oxycodone group showed less hesitation for coughing due to the milder postoperative pain, demonstrating that oxycodone could provide more effective motion pain control.
The impressing part of this study was that sufentanil was associated with far more doses delivered in PCIA when compared with oxycodone, which was consistent with previous study.[14] This was ascribed to 2 possible explanations. Firstly, continuous infusion was used in patients administrated with sufentanil-PCIA, whereas patients with oxycodone-PCIA used demand-only infusion. In recently published guideline of postoperative analgesia,[4] demand-only infusion PCIA was recommended due to the potential association between adverse complications and continuous opioid infusion especially respiratory depression.[20] In China, sufentanil instead of morphine is the most commonly used opioid analgesic in PCIA; however, it is not suitable for demand-only infusion due to its short half-life of clearance. Consequently, patients needed to redose frequently. In our preliminary study, demand-only sufentanil PCIA was applied; however, the intervals between bolus analgesia were too short (approximately 1 hour), which severely interfered patients’ recovery and rest especially in the evening, consequently reduced patients’ satisfaction for PCIA. Continuous sufentanil infusion PCIA was thereby used in this study, which unavoidably increased analgesic dose consumption during PCIA. The equivalent dose converting between parenteral morphine and oxycodone is variable, with a suggested ratio of 0.65 to 1.5.[21–23] In this study, the ration of 1 was chosen on the assumption that 1 dose of oxycodone may be equianalgesic to morphine according to the recently published literatures.[23] Another possible explanation was that the analgesia effects of oxycodone could last longer than sufentanil. In this study, we compared the first bolus dose length since patients returning back to wards from PACU, and the median length in patients from oxycodone group was almost 3-fold longer than that from sufentanil group, indicating that oxycodone could provide longer intervals between bolus analgesia, thereby reducing bolus dose requirement and consumption of analgesics, consequently increased patients’ satisfaction for postoperative analgesia.
The higher sufentanil delivery dose increased adverse complications. Respiratory depression, one of the most concerned anesthesia-related adverse complications after surgery, was observed in patients from sufentanil but not oxycodone group, indicating that oxycodone may be safer than sufentanil in PCIA. This was mainly caused by less oxycodone dose consumption in PCIA in relative to sufentanil due to their different delivery patterns. However, another possible explanation was that, patients from sufentanil group may require more bolus dose because of their discomforted feeling of visceral pain, leading to the overdose of sufentanil, consequently inducing respiratory depression. The high dose consumption in sufentanil also induced higher adverse complications such as nausea and vomiting. However, in a prior study, Wang et al[14] found that oxycodone-PCIA induced similar side effects when compared with sufentanil. The difference may be induced by distinct PCIA models between these 2 studies. In Wang's study, no background infusion was used in sufentanil-PCIA, thereby reduced analgesic dose consumption, consequently showed less side effects. In another study performed in patients after cesarean section,[24] patients accepted oxycodone-PCIA showed less nausea when compared with sufentanil-PCIA, which was consistent with our study. Nausea and vomiting is a concerned issue in PCIA because it severely reduced the patients’ satisfaction for PCIA. In this study, standard preventive treatments were used and the incidence rate of nausea was approximately 35% in sufentanil groups, which is similar to previous studies.[25] However, incidence rate of nausea and vomiting in patients from oxycodone was much lower, illustrating an important advantage of oxycodone in the management of postoperative pain.
One of the limitations in this study was that the study was performed in one province of China. Previous studies have shown that there was a significant interindividual variation in the need for oxycodone for sufficient analgesia[15]; therefore, difference in the analgesia effects for postoperative pain between oxycodone and sufentanil may be narrowed. Further evaluation and study about oxycodone for PCIA should be conducted in a clinical trial across whole China to reveal the various responses to oxycodone/sufentanil PCIA in Chinese population.
In conclusion, our study demonstrated that demand-only oxycodone PCIA could provide comparable effects for postoperative pain relief compared with continuous sufentanil infusion PCIA in patients, with better motion pain control, and less cumulative analgesic dose consumption and adverse complications. We therefore concluded that oxycodone may be useful as an alternative to sufentanil PCIA after abdominal surgery.
Author contributions
LH and YS contributed to study design, project administration, data analysis, and manuscript preparation; HX, XN, SD, KD, QL, and JL contributed to study conduction and data collection; JL contributed to patients’ randomization and allocation, data input, and statistical analysis; SL contributed to the project supervision, study design and administration, data analysis, manuscript preparation, and revision.
Conceptualization: Lichun Han, Yuquan Su, Xiaoli Niu, Siyuan Li.
Data curation: Shajie Dang, Keqin Du, Jing Liu, Siyuan Li.
Formal analysis: Jing Liu, Siyuan Li.
Funding acquisition: Siyuan Li.
Investigation: Yuquan Su, Hongfei Xiong, Xiaoli Niu, Keqin Du, Quan Li, Siyuan Li.
Methodology: Lichun Han, Hongfei Xiong, Xiaoli Niu, Shajie Dang, Keqin Du, Jing Liu, Siyuan Li.
Project administration: Yuquan Su, Peng Zhang, Siyuan Li.
Resources: Lichun Han, Quan Li, Siyuan Li.
Software: Siyuan Li.
Supervision: Yuquan Su, Siyuan Li.
Validation: Jing Liu, Siyuan Li.
Visualization: Peng Zhang, Siyuan Li.
Writing – original draft: Peng Zhang, Siyuan Li.
Writing – review and editing: Lichun Han, Siyuan Li.
Footnotes
Abbreviations: BIS = bispectral index, BP = noninvasive blood pressure, ECG = electrocardiogram, FAS = functional activity score, HR = heart rate, NRS = numeric rating scales, PACU = postanesthesia care unit, PCIA = patient-controlled intravenous analgesia, SpO2 = pulse oximetry.
LH, YS, and SL contributed equally to this work.
This work is supported by National Natural Science Foundation of China (No. 31300675), and the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20130201120085).
Trial registration: Chinese Clinical Trial Registry (ChiCTR-IOR-17013515).
The authors have no conflicts of interest to disclose.
References
- [1].Gan TJ, Habib AS, Miller TE, et al. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin 2014;30:149–60. [DOI] [PubMed] [Google Scholar]
- [2].Wang ZQ, Lin JH. Related factors for pain after orthopaedic surgery: a multicenter study in Beijing. J Shanxi Med Univ 2012;43:798–802. [Chinese]. [Google Scholar]
- [3].Castro C, Tharmaratnam U, Brockhurst N, et al. Patient-controlled analgesia with fentanyl provides effective analgesia for second trimester labour: a randomized controlled study. Can J Anaesth 2003;50:1039–46. [DOI] [PubMed] [Google Scholar]
- [4].Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain 2016;17:131–57. [DOI] [PubMed] [Google Scholar]
- [5].Sengupta JN, Su X, Gebhart GF. Kappa, but not mu or delta, opioids attenuate responses to distention of afferent fibers innervating the rat colon. Gastroenterology 1996;111:968–80. [DOI] [PubMed] [Google Scholar]
- [6].Simonin F, Valverde O, Smadja C, et al. Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. EMBO J 1998;17:886–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vecchiet L, Giamberardino MA, Dragani L, et al. Pain from renal/ureteral calculosis: evaluation of sensory thresholds in the lumbar area. Pain 1989;36:289–95. [DOI] [PubMed] [Google Scholar]
- [8].Kokki H, Kokki M, Sjovall S. Oxycodone for the treatment of postoperative pain. Expert Opin Pharmacother 2012;13:1045–58. [DOI] [PubMed] [Google Scholar]
- [9].Nielsen CK, Ross FB, Lotfipour S, et al. Oxycodone and morphine have distinctly different pharmacological profiles: radioligand binding and behavioural studies in two rat models of neuropathic pain. Pain 2007;132:289–300. [DOI] [PubMed] [Google Scholar]
- [10].Kim NS, Lee JS, Park SY, et al. Oxycodone versus fentanyl for intravenous patient-controlled analgesia after laparoscopic supracervical hysterectomy: a prospective, randomized, double-blind study. Medicine (Baltimore) 2017;96:e6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xie K, Zhang W, Fang W, et al. The analgesic efficacy of oxycodone hydrochloride versus fentanyl during outpatient artificial abortion operation: a randomized trial. Medicine (Baltimore) 2017;96:e7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ding Z, Wang K, Wang B, et al. Efficacy and tolerability of oxycodone versus fentanyl for intravenous patient-controlled analgesia after gastrointestinal laparotomy: a prospective, randomized, double-blind study. Medicine (Baltimore) 2016;95:e4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hwang BY, Kwon JY, Kim E, et al. Oxycodone vs. fentanyl patient-controlled analgesia after laparoscopic cholecystectomy. Int J Med Sci 2014;11:658–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang N, Zhou H, Song X, et al. Comparison of oxycodone and sufentanil for patient-controlled intravenous analgesia after laparoscopic radical gastrectomy: a randomized double-blind clinical trial. Anesth Essays Res 2016;10:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kokki M, Broms S, Eskelinen M, et al. Analgesic concentrations of oxycodone—a prospective clinical PK/PD study in patients with laparoscopic cholecystectomy. Basic Clin Pharmacol Toxicol 2012;110:469–75. [DOI] [PubMed] [Google Scholar]
- [16].Cajanus K, Kaunisto MA, Tallgren M, et al. How much oxycodone is needed for adequate analgesia after breast cancer surgery: effect of the OPRM1 118A>G polymorphism. J Pain 2014;15:1248–56. [DOI] [PubMed] [Google Scholar]
- [17].Cafiero T, Di Minno RM, Sivolella G, et al. Immediate postoperative pain management in patients undergoing major abdominal surgery after remifentanil-based anesthesia: sufentanil vs tramadol. Minerva Anestesiol 2004;70:661–9. [PubMed] [Google Scholar]
- [18].Wang J, Fu Y, Zhou H, et al. Effect of preoperative intravenous oxycodone on sufentanil consumption after laparoscopic radical gastrectomy. J Opioid Manag 2016;12:181–5. [DOI] [PubMed] [Google Scholar]
- [19].Gebhart GF, Bielefeldt K. Physiology of visceral pain. Compr Physiol 2016;6:1609–33. [DOI] [PubMed] [Google Scholar]
- [20].George JA, Lin EE, Hanna MN, et al. The effect of intravenous opioid patient-controlled analgesia with and without background infusion on respiratory depression: a meta-analysis. J Opioid Manag 2010;6:47–54. [DOI] [PubMed] [Google Scholar]
- [21].Kalso E. Oxycodone. J Pain Symptom Manage 2005;29(5 suppl):S47–56. [DOI] [PubMed] [Google Scholar]
- [22].Silvasti M, Rosenberg P, Seppala T, et al. Comparison of analgesic efficacy of oxycodone and morphine in postoperative intravenous patient-controlled analgesia. Acta Anaesthesiol Scand 1998;42:576–80. [DOI] [PubMed] [Google Scholar]
- [23].Chang SH, Maney KM, Phillips JP, et al. A comparison of the respiratory effects of oxycodone versus morphine: a randomised, double-blind, placebo-controlled investigation. Anaesthesia 2010;65:1007–12. [DOI] [PubMed] [Google Scholar]
- [24].Nie JJ, Sun S, Huang SQ. Effect of oxycodone patient-controlled intravenous analgesia after cesarean section: a randomized controlled study. J Pain Res 2017;10:2649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dong CS, Zhang J, Lu Q, et al. Effect of dexmedetomidine combined with sufentanil for post- thoracotomy intravenous analgesia: a randomized, controlled clinical study. BMC Anesthesiol 2017;17:33. [DOI] [PMC free article] [PubMed] [Google Scholar]


