Abstract
The study was aimed to evaluate oncological safety and patient satisfaction in relatively late stage breast cancer patients who was treated with skin-sparing mastectomy (SSM) followed by breast reconstruction with an extended latissimus dorsi (LD) flap. Oncological safety, postoperative complications, and cosmetic results were retrospectively analyzed in patients who underwent extended LD flap breast reconstruction following SSM between October 2011 and August 2014. A total of 62 patients who underwent 63 breast reconstructions were enrolled in the study. Local recurrence rate was 1.6% over a median follow-up of 63 months. On final aesthetic assessment, 37 reconstructions were rated excellent, 19 good, 5 fair, and 2 poor. Reconstruction-related complications occurred in 22 patients (34.9%); these patients’ satisfaction scores were significantly lower than those of patients without complications (P < .05). Five patients developed shoulder movement limitation, and 2 had minor twitching and pain in the reconstructed breast. However, these patients did not find their problems disabling and were able to live normally. SSM followed by breast reconstruction with extended LD flap can improve patients’ postoperative quality of life and is as oncologically safe as total mastectomy even in patients with tumors of relatively late stage.
Keywords: breast cancer, breast reconstruction, extended latissimus dorsi flap, skin-sparing mastectomy
1. Introduction
In recent years, breast-conserving treatment (BCT) has significantly improved esthetic outcomes for many women undergoing surgery for the treatment or prophylaxis of breast cancer.[1–3] However, numerous such women still undergo mastectomy, especially in China, where the rate of BCT is extremely low.[4,5] The disfigurement resulting from mastectomy can lead to poor self-confidence, and correcting it through breast reconstruction can improve psychosocial well-being and quality of life.[6,7] Furthermore, many studies have confirmed that breast reconstruction does not delay adjuvant therapy, increase the risk of local recurrence, or affect the ability to detect recurrence.[8,9] Thus, breast reconstruction following mastectomy should become a standard procedure in breast cancer treatment.
Patients who opt for breast reconstruction can choose between prosthetic and autologous reconstruction. However, even the best contoured breast implants are rarely able to completely match the curves of the opposite breast, and prosthetics do not age naturally, leading to an increasingly noticeable difference over time between the reconstructed and natural breasts.[10,11] Moreover, prosthetic breast reconstruction, especially in patients undergoing external radiotherapy, carries the risk of expander/implant-associated complications, such as capsular contracture, implant leak, implant infection, implant migration, and so on.[11,12] Given recent advances in anatomic knowledge and surgical techniques, autologous breast reconstruction can provide softer, cosmetically superior, more natural-appearing results than prosthetic breast reconstruction.[6] Furthermore, the use of autologous tissues can provide naturally aging breasts that need fewer subsequent revisions.[6,13] Thus, there are still a certain percentage of patients who opt for autologous breast reconstruction.
However, a breast reconstructed with autologous tissue after radical mastectomy may not match the color of the patient's skin, which may affect cosmetic results a lot.[14] Skin-sparing mastectomy (SSM) avoids this problem completely, except for patients who undergo dissection of the nipple–areola complex (NAC) followed by patching with autologous skin, which may result in a local skin color mismatch around the nipple; however, this can be rectified by nipple reconstruction and areola tattooing.[15,16] Hence, more and more patients are opting for SSM followed by autologous breast reconstruction, and its oncological safety has been proven in patients with early stage tumors.[9,17]
Owing to inadequate examination rates, many Chinese breast cancer patients are not diagnosed until the tumor is relatively advanced, with a high percentage already being in stage III.[5] Thus, the first aim of this study was to evaluate the oncological safety of autologous breast reconstruction, with particular attention to such late-stage patients. In addition, as most Chinese women have relatively small breasts, the extended latissimus dorsi (LD) flap is a popular option in China, for the reason that the flap is stable, well vascularized, and easy to be harvested as well as can provide enough tissue to reconstruct the breast without an implant. Furthermore, the dorsal oblique flap design also permit correction of inferior projection and shape in patients who underwent NAC dissection; though it also leaves an obvious donor site scar which cannot be covered by the bra. Thus, the second aim of this study was to present outcomes and patient satisfaction for extended LD flap reconstruction with dorsal oblique flap design following SSM.
2. Patients and methods
2.1. Patients
After obtaining informed consent and institutional review board approval, we conducted a retrospective case-note study on 62 consecutive SSM patients who underwent 63 immediate or delayed breast reconstructions using the extended LD flap technique between October 2011 and August 2014. Cases of reconstruction using tissue expanders or other flaps were excluded from this study. The other exclusion criteria were as follows: distant metastasis, skin invasion, history of radiation therapy, ongoing systemic infection at the time of surgery, pregnancy, a history of preoperative systemic anticoagulant use, and a platelet count below 100,000/mL.
2.2. Surgical modalities
We performed 63 total breast reconstructions using an extended LD flap without implants; none of the patients underwent contralateral symmetrical procedure. In all cases, SSM and axillary lymph node dissection, if necessary, were performed by a highly experienced surgeon so as to preserve the submammary fold, the thoracodorsal nerve and vessels, and the LD tendon. The dorsal oblique flap design was used so that a large skin island and LD flap, including all 5 possible sources of adipose tissue, were harvested.[18] The flap was then transposed to the anterior chest area through a subcutaneous tunnel, which was slightly high in order not to disturb the lateral edge of the breast. Finally, the LD flap was secured medially to the sternum and superiorly to the pectoralis muscle fascia.
Two suction drains were placed in the donor area, with another placed under the flap before skin closure; they remained in place until the drainage was<20 mL in a 24 hours period and then removed. Patients were instructed by nurses to limit upper arm motion to 90° of abduction for the first 2 weeks after surgery in order to promote rehabilitation and reduce complications.
2.3. Outcomes
The outcomes of primary interest in this study were tumor recurrence, cosmetic results, and overall complications. Patients were considered to have developed major complications (potentially requiring surgical intervention) if they suffered at least 1 of the following: extended LD flap failure, wound disruption, hematoma requiring surgery, or skin flap necrosis or wound infection requiring surgical debridement and cover by split skin graft. Minor complications, which could be managed successfully conservatively, were considered to include hematoma, infection, seroma, fat necrosis, and wound dehiscence not requiring surgery or drainage.
We also examined outcomes with regard to shoulder movement limitation and back contour asymmetry. After surgery, patients were instructed to exercise at home starting 2 weeks postoperatively, and we assessed shoulder movement during a follow-up visit, 4 weeks postoperatively. Differences in overhead reach and range of motion between the operated-on and intact sides were evaluated. Patients who could not lift their shoulders above 90° were categorized as having shoulder-movement limitations, and those who had limitations in both abduction and flexion of the shoulder were considered disabled. Back-asymmetry analysis was performed for each patient using preoperative and postoperative digital photographs, which were evaluated by the patient herself.
2.4. Follow-up
Patients were followed-up 1 week after discharge to check on their wounds, and any complications they suffered as outpatients were recorded. After the first follow-up visit, all patients were followed up regularly in the outpatient clinic according to a standard protocol involving 5 to 6 monthly blood and imaging tests to monitor tumor recurrence status. In addition, follow-up photographs were taken at least 3 months postoperatively to help patients make objective evaluations of the shape and symmetry of the reconstructed breast. A questionnaire was given to all patients to gauge their satisfaction and the need for revision or symmetrizing procedures (Table 1).
Table 1.
Patient questionnaire.

2.5. Statistical analysis
Data were analyzed using descriptive statistics such as means, ranges, standard deviations, and proportions. Categorical data are presented as percentages. Continuous variables were compared using the unpaired Student's t-test. All statistical evaluations were performed using the SPSS for Windows package (SPSS 18.0; IBM, Armonk, NY). Results with a P value < .05 were considered statistically significant.
3. Results
3.1. Patient characteristics
A total of 63 breast reconstructions using extended LD flaps were performed in 62 patients between October 2011 and August 2014. One patient had bilateral breast reconstructions, but each mastectomy and reconstruction was performed on a separate occasion. Her 2 reconstructions were therefore included separately in the analysis. Fifty-six patients underwent immediate reconstruction, with 7 undergoing late reconstruction. The patients had an average age of 36 years (range 21–52). The average follow-up period was 63 months (range 36–70).
The histology of the tumors is given in Table 2. The tumor was stage 0 in 3 cases, stage I in 19 cases, stage II in 22 cases, and stage III in 19; none were stage IV. Mastectomy and axillary surgery types are also shown in Table 2. Four patients underwent adjuvant chemotherapy preoperatively; none received preoperative radiotherapy. The vast majority (n = 46, 73.0%) received postoperative chemotherapy, with the indications including tumor width > 1 cm, negativity of estrogen receptor (ER) and progesterone receptor (PR), high disease grade, lymph node involvement, capsular rupture, and young age (<40 years) at presentation. Furthermore, 21 of them received postoperative radiotherapy following chemotherapy. Hormonal treatment was required in 65.1% of cases because histological examination of the resected specimens revealed ER or PR-positivity.
Table 2.
Tumor histology and oncological procedures (n = 63).
3.2. Postoperative outcomes
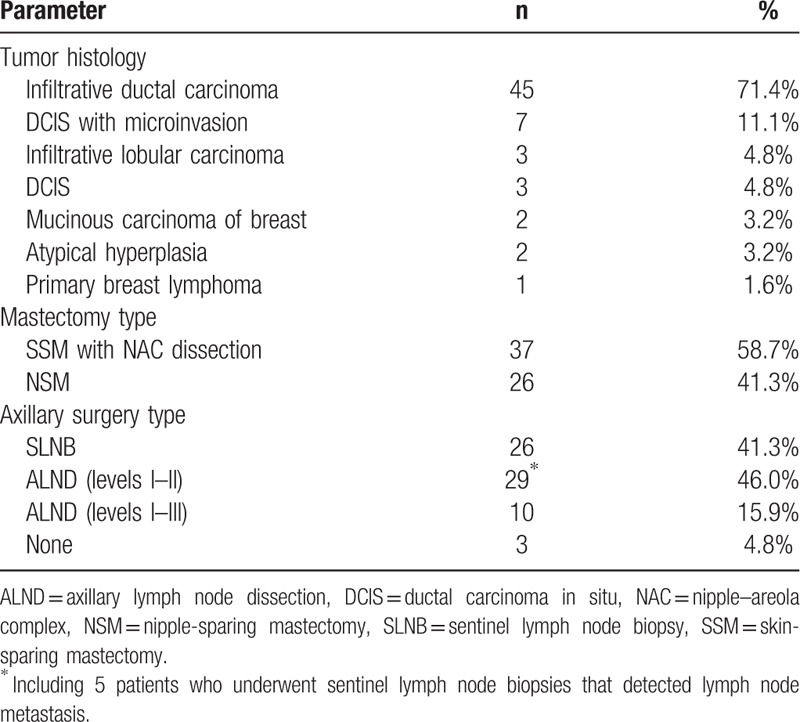
During the follow-up period, we detected only 1 local recurrence, giving a local recurrence rate of 1.6% among all patients (5.3% of stage III patients). It occurred in a 23-year-old woman with TNM stages of T3N1M0 and T1aN1M0 before and after preoperative adjuvant chemotherapy, respectively. None of the imaging tests before or after her preoperative chemotherapy showed skin invasion. Despite successful chemotherapy, she suffered skin recurrence 8 months after breast reconstruction (Fig. 1A).
Figure 1.
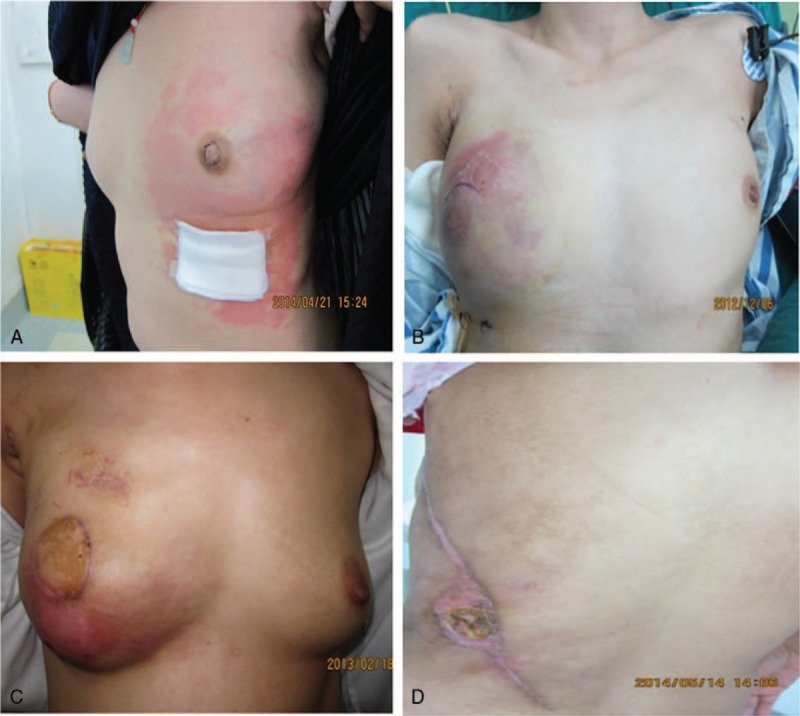
(A) Only one patient experienced local tumor recurrence. (B, C) Two patients who suffered wound infection in the reconstructed breast. (D) One patient suffered necrosis in a small area of skin in the donor site.
The overall complication rate was 34.9%; complications are summarized in Table 3. There were no cases of extended LD flap loss, wound disruption, or hematoma requiring surgery. The only 2 patients who developed major complications suffered wound infections; they both underwent surgical debridements and recovered 1 month after surgery (Fig. 1B and C). Seroma was the commonest minor complication, developing in the breast or axilla in 5 patients and in the donor site in 7; they were treated with weekly aspiration in the clinic. Seven patients developed a superficial wound infection, and 2 of them suffered necrosis in a small area of skin; they were treated conservatively (Fig. 1D). Nipple and areola sloughing were reported in 1 patient; this was also counted as local skin necrosis. Fat necrosis was reported in 2 patients (3.2%), who recovered after no more than 2 weeks of aspiration in the clinic.
Table 3.
Complications after breast reconstruction (n = 63).
None of the patients were disabled with regard to shoulder movement, and only 5 showed shoulder movement limitation (2 experienced minor weakness in abduction, and 3 patients experienced minor weakness in anterior elevation). All of them were able to perform activities of daily living, as well as engage in strenuous or skilled labor, sports, or music. The remaining patients had normal shoulder joint mobility with regard to abduction, adduction, internal rotation, external rotation, and anterior and posterior elevation. Two patients had minor and occasional twitching and pain in the reconstructed breasts, but it did not significantly reduce quality of life.
3.3. Cosmetic results
The questionnaire asking patients to score their satisfaction with the reconstructed breast (Table 1) was completed at a mean 1.4 years after reconstruction (range 3 months to 2.4 years). Response rate was 100%.
Patients were asked to rate the appearance of the reconstructed breast with and without a bra, as well as its symmetry, on a scale of 10. According to the average of the 3 scores, the results might be considered excellent (9–10), good (7–8), fair (5–6), or poor (4 or less). The vast majority of patients rated their results either excellent (n = 37, 58.7%; Fig. 2A) or good (n = 19, 30.2%; Fig. 2B). Only 5 reconstructions (7.9%) were rated fair (Fig. 2C), and only 2 (3.2%; Fig. 2D) rated poor. For patients who suffered complications (which often affected breast volume, projection, or symmetry with the contralateral side), mean satisfaction scores were 8.0 (with bra), 7.3 (without bra), and 7.4 (symmetry); these were all significantly lower than the ratings given by patients who did not suffer complications (8.9 with bra, 8.4 without bra, and 8.5 for symmetry) (Fig. 3).
Figure 2.
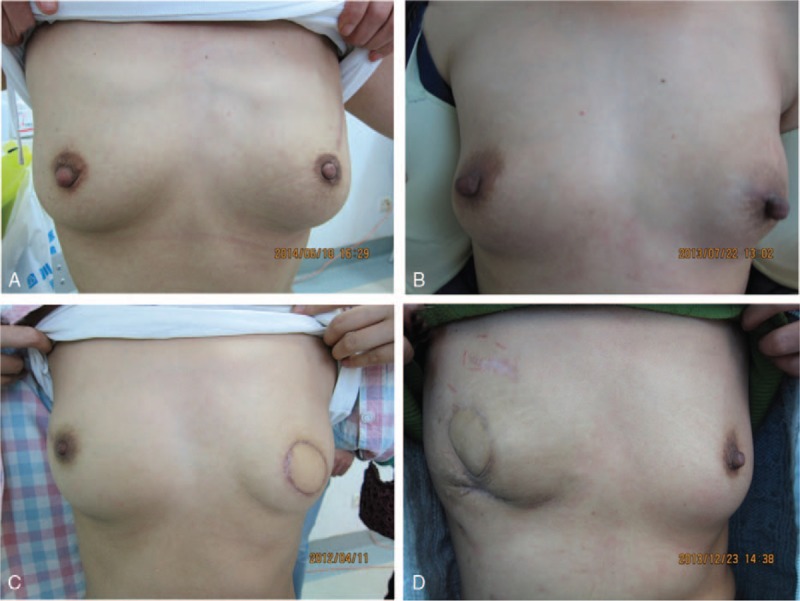
Breast reconstructions rated excellent (A), good (B), fair (C), and poor (D).
Figure 3.
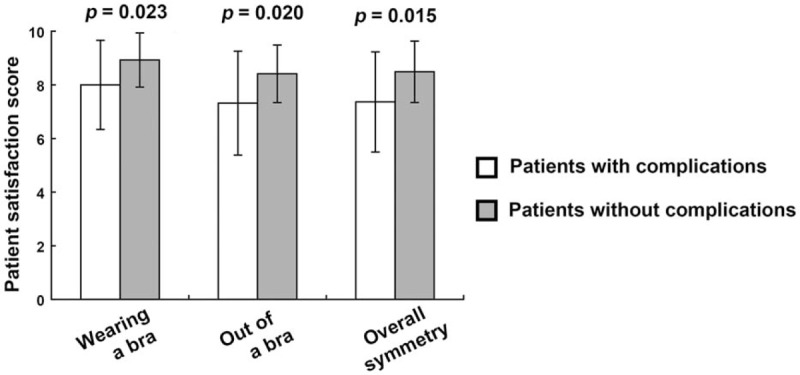
Comparison of patient satisfaction scores between patients with and without complications.
The comments given in the free-text area of the questionnaire were generally related to the patient's experience of the operation and overall treatment. Two patients commented on the donor site scar, and 2 described problems relating to twitching of the flap (which is not routinely denervated). Three patients claimed to have minor shoulder movement limitations.
4. Discussion
Effective surgical therapy and management for cancer must include not only local control of the tumor but also accurate surgical staging of disease, as well as the restoration of an acceptable cosmetic outcome in some special carcinomas. These multidimensional requirements are especially important to patients who undergo SSM followed by breast reconstruction.[11]
Freeman was the first to describe SSM, in the 1960 s; it was utilized for benign breast lesions.[19] Since then, especially in recent years, SSM, in association with biopsy site removal and even the removal of the NAC in some patients, has been introduced for treatment of malignant breast tumor patients and been proven oncologically safe for early stage patients.[20–22] One group of investigators even tested SSM for malignant breast tumors without skin invasion regardless of stage, size, or distance from the areola, finding that locoregional recurrence rates were similar for SSM and radical mastectomy.[23]
On the basis of these studies, we hypothesized that SSM followed by breast reconstruction would be oncologically safe for early breast cancer (stages 0–II) and even some patients with stage III tumors. We believe that local recurrence depends only on the biology of the tumor and is not affected by the use of SSM or reconstruction. Our results supported this hypothesis; there was only 1 case of locoregional recurrence for a rate of 1.6%, although many of the tumors in the series were relatively late stage. This case involved a stage III tumor, giving a recurrence rate of only 5.3% for tumors of that stage over an average follow-up period of 63 months, comparable to that in BCT patients with the same tumor stage over a similar follow-up period.[24] The results were comparable to those of Salhab et al,[25] who found SSM and immediate breast reconstruction to be oncologically safe even in patients with high-risk (T3 or node-positive) carcinoma. However, further studies and longer follow-up are necessary to refine the selection criteria for SSM followed by breast reconstruction.
Because SSM preserves the inframammary fold and breast envelope, it can allow greatly enhanced aesthetics for the reconstructed breast and permit excellent symmetry without the need to manipulate the opposite breast.[20,23] In China, breast reconstruction using the extended LD flap is now the standard procedure following SSM because Chinese women tend to have relatively small breasts and vascular anastomosis is not required.[26] The benefits of LD flap reconstruction, aside from superior aesthetic results, include natural aging and improved outcomes compared with prosthetic reconstruction.[27] Furthermore, the dorsal oblique flap design ensures a sufficiently large fascial component of the flap that most Chinese patients do not need the augmentation of an implant to achieve sufficient breast projection. In addition, this design leaves more tissue on the upper side of the skin island than the horizontal flap (Fig. 4A), so that after the flap is rotated 180°, there remains enough tissue to allow manipulation of projection and shape (Fig. 4B); this is particularly important for patients who have undergone NAC dissection and need donor skin to correct it. In contrast, use of the horizontal flap leaves little tissue on the upper side of the skin island (Fig. 4C), making it hard to manipulate projection and shape without an implant (Fig. 4D). In this study, the oblique flap was used for all patients, and nearly 90% of patients rated their reconstructions excellent or good after follow-up. The main disadvantage of this technique is the obvious donor site scar, with which some patients were somewhat dissatisfied.
Figure 4.
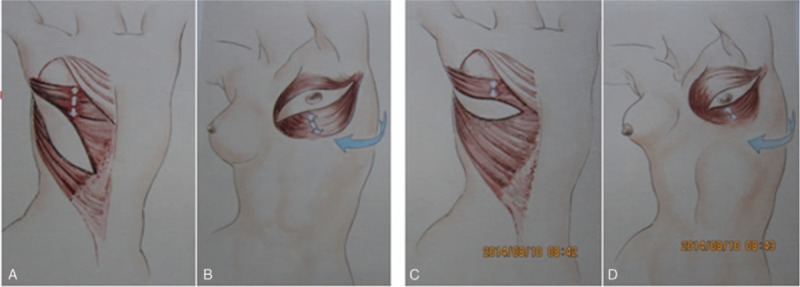
The dorsal oblique flap leaves more tissue on the upper side of the skin island, allowing better manipulation of projection and shape, compared with the horizontal flap.
SSM followed by breast reconstruction using an extended LD flap not only improves the patient's body image but also indirectly improves her health-related quality of life in its physical, social, psychic, and sexual dimensions.[12,18,27] However, we found that this technique does carry some risk of complications; although we did not observe flap necrosis or many other serious ones. Complications including wound infection, local skin necrosis, fat necrosis, and seroma often occurred in the initial postoperative period. Such complications should be minimized, as they can affect patients’ satisfaction with the reconstructed breast. Patients with skin necrosis or infection may require secondary operations or a prolonged course of wound dressings, which often lead to fibrosis and wound contraction, resulting in distorted breast shape and asymmetry. The 2 patients who rated their breast reconstruction poor in our study both suffered wound infections in the reconstructed breast. In addition, patients with complications may delay postoperative chemotherapy and radiotherapy, which may increase the risk of recurrence, although this could not be demonstrated in our study given the small sample size.
Finally, the LD flap technique may have functional consequences to the donor shoulder.[28–30] In one study using both subjective and objective assessments, one-third of patients who underwent breast reconstruction using autologous extended LD flaps suffered reduced strength, and nearly half had reduced mobility in their donor shoulder.[29] Furthermore, it has been reported that such restrictions on the mobility and strength of the donor shoulder are likely permanent.[29] However, other studies have reported a certain degree of synergistic compensation by the remaining shoulder muscles.[30] In this study, we found relatively little evidence of loss of shoulder strength after the operation. Two patients experienced minor weakness in abduction, and 3 experienced minor weakness in anterior elevation; however, all of them were able to perform activities of daily living, as well as engage in strenuous or skilled labor, sports, or music. However, active sportswomen such as swimmers, skiers, and rock climbers who depend on upper body strength and agility must consider carefully before opting for breast reconstruction with extended LD flap.
5. Conclusion
SSM followed by breast reconstruction with extended LD flap is as oncologically safe as total mastectomy for early-stage patients and even in selected stage III patients with no skin invasion. In comparison with the horizontal flap, the oblique flap design provides a considerably larger fascial component, allowing the breast to be reconstructed without an implant and permitting manipulation of its projection and shape. Thus, SSM followed by breast reconstruction with an extended oblique LD flap is a superior option with regard to both aesthetics and postoperative quality of life.
Acknowledgments
No source of support was received for this work or needs to be disclosed.
Author contributions
Contributors: DZG, ZYT, CJ, LQY, and LQ proposed the study concept and design. DZG, ZYT, CJ, LQY, and LQ performed the research and acquired the data. DZG, ZYT, and CJ analyzed and interpreted the data. ZYT, DZG, and LQY wrote the first draft, and provided a critical revision of the manuscript with important intellectual content. LQ was the study supervisor.
Conceptualization: Zhenggui Du, Qing Lv.
Data curation: Jie Chen, Quanyi Long.
Formal analysis: Jie Chen, Quanyi Long.
Methodology: Yuting Zhou.
Project administration: Qing Lv.
Software: Jie Chen.
Supervision: Qing Lv.
Validation: Qing Lv.
Visualization: Qing Lv.
Writing – original draft: Zhenggui Du, Yuting Zhou.
Writing – review & editing: Zhenggui Du.
Footnotes
Abbreviations: ALND = axillary lymph node dissection, BCT = breast-conserving treatment, DCIS = ductal carcinoma in situ, LD = latissimus dorsi, NAC = nipple–areola complex, NSM = nipple-sparing mastectomy, SLNB = sentinel lymph node biopsy, SSM = skin-sparing mastectomy.
ZD and YZ contributed equally to this study.
The authors have no conflicts of interest to disclose.
References
- [1].Immink JM, Putter H, Bartelink H, et al. Long-term cosmetic changes after breast-conserving treatment of patients with stage I-II breast cancer and included in the EORTC ’boost versus no boost’ trial. Ann Oncol 2012;23:2591–8. [DOI] [PubMed] [Google Scholar]
- [2].Hill-Kayser CE, Vachani C, Hampshire MK, et al. Cosmetic outcomes and complications reported by patients having undergone breast-conserving treatment. Int J Radiat Oncol Biol Phys 2012;83:839–44. [DOI] [PubMed] [Google Scholar]
- [3].Hernanz F, Perez-Cerdeira M, Sanchez S, et al. Cosmetic sequelae after breast-conserving treatment using conventional surgical techniques. Breast J 2013;19:342–3. [DOI] [PubMed] [Google Scholar]
- [4].Rutter CE, Park HS, Killelea BK, et al. Growing use of mastectomy for ductal carcinoma-in situ of the breast among young women in the United States. Ann Surg Oncol 2015;22:2378–86. [DOI] [PubMed] [Google Scholar]
- [5].Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol 2014;15:e279–89. [DOI] [PubMed] [Google Scholar]
- [6].Eltahir Y, Werners LL, Dreise MM, et al. Which breast is the best? Successful autologous or alloplastic breast reconstruction: patient-reported quality-of-life outcomes. Plast Reconstr Surg 2015;135:43–50. [DOI] [PubMed] [Google Scholar]
- [7].Sisco M, Johnson DB, Wang C, et al. The quality-of-life benefits of breast reconstruction do not diminish with age. J Surg Oncol 2015;111:663–8. [DOI] [PubMed] [Google Scholar]
- [8].Eck DL, McLaughlin SA, Terkonda SP, et al. Effects of immediate reconstruction on adjuvant chemotherapy in breast cancer patients. Ann Plast Surg 2015;74(suppl 4):S201–3. [DOI] [PubMed] [Google Scholar]
- [9].Liang TJ, Wang BW, Liu SI, et al. Recurrence after skin-sparing mastectomy and immediate transverse rectus abdominis musculocutaneous flap reconstruction for invasive breast cancer. World J Surg Oncol 2013;11:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Albornoz CR, Matros E, McCarthy CM, et al. Implant breast reconstruction and radiation: a multicenter analysis of long-term health-related quality of life and satisfaction. Ann Surg Oncol 2014;21:2159–64. [DOI] [PubMed] [Google Scholar]
- [11].Ho A, Cordeiro P, Disa J, et al. Long-term outcomes in breast cancer patients undergoing immediate 2-stage expander/implant reconstruction and postmastectomy radiation. Cancer 2012;118:2552–9. [DOI] [PubMed] [Google Scholar]
- [12].Venus MR, Prinsloo DJ. Immediate breast reconstruction with latissimus dorsi flap and implant: audit of outcomes and patient satisfaction survey. J Plast Reconstr Aesthet Surg 2010;63:101–5. [DOI] [PubMed] [Google Scholar]
- [13].Nanhekhan L, Vandervoort M. New approach to shaping a ptotic breast in secondary autologous breast reconstruction. Aesthetic Plast Surg 2012;36:1144–50. [DOI] [PubMed] [Google Scholar]
- [14].Gherardini G, Thomas R, Basoccu G, et al. Immediate breast reconstruction with the transverse rectus abdominis musculocutaneous flap after skin-sparing mastectomy. Int Surg 2001;86:246–51. [PubMed] [Google Scholar]
- [15].Ohno Y, Noguchi M, Yokoi-Noguchi M, et al. Nipple- or skin-sparing mastectomy and immediate breast reconstruction by the “moving window” operation. Breast Cancer 2013;20:54–61. [DOI] [PubMed] [Google Scholar]
- [16].Tan BK, Chim H, Ng ZY, et al. Aesthetic design of skin-sparing mastectomy incisions for immediate autologous tissue breast reconstruction in Asian women. Arch Plast Surg 2014;41:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chattopadhyay D, Gupta S, Jash PK, et al. Skin sparing mastectomy with preservation of nipple areola complex and immediate breast reconstruction in patients with breast cancer: a single centre prospective study. Plast Surg Int 2014;2014:589068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Delay E, Gounot N, Bouillot A, et al. Autologous latissimus breast reconstruction: a 3-year clinical experience with 100 patients. Plast Reconstr Surg 1998;102:1461–78. [DOI] [PubMed] [Google Scholar]
- [19].Freeman BS. Subcutaneous mastectomy for benign breast lesions with immediate or delayed prosthetic replacement. Plast Reconstr Surg Transplant Bull 1962;30:676–82. [DOI] [PubMed] [Google Scholar]
- [20].Burdge EC, Yuen J, Hardee M, et al. Nipple skin-sparing mastectomy is feasible for advanced disease. Ann Surg Oncol 2013;20:3294–302. [DOI] [PubMed] [Google Scholar]
- [21].Garwood ER, Moore D, Ewing C, et al. Total skin-sparing mastectomy: complications and local recurrence rates in 2 cohorts of patients. Ann Surg 2009;249:26–32. [DOI] [PubMed] [Google Scholar]
- [22].Rami H, Nasr M, Suidan J, et al. Skin sparing mastectomy: a revisited technique. J Med Liban 2009;57:115–23. [PubMed] [Google Scholar]
- [23].Kim HJ, Park EH, Lim WS, et al. Nipple areola skin-sparing mastectomy with immediate transverse rectus abdominis musculocutaneous flap reconstruction is an oncologically safe procedure: a single center study. Ann Surg 2010;251:493–8. [DOI] [PubMed] [Google Scholar]
- [24].Shin HC, Han W, Moon HG, et al. Breast-conserving surgery after tumor downstaging by neoadjuvant chemotherapy is oncologically safe for stage III breast cancer patients. Ann Surg Oncol 2013;20:2582–9. [DOI] [PubMed] [Google Scholar]
- [25].Salhab M, Al Sarakbi W, Joseph A, et al. Skin-sparing mastectomy and immediate breast reconstruction: patient satisfaction and clinical outcome. Int J Clin Oncol 2006;11:51–4. [DOI] [PubMed] [Google Scholar]
- [26].Yang Y, Chen Y, Qu J, et al. The use of OK-432 to prevent seroma in extended latissimus dorsi flap donor site after breast reconstruction. J Surg Res 2015;193:492–6. [DOI] [PubMed] [Google Scholar]
- [27].Serra MP, Sinha M. Adaptation of the Hall-Findlay technique for simultaneous contralateral reduction in delayed breast reconstruction with extended latissimus dorsi flap. J Plast Reconstr Aesthet Surg 2010;63:996–1002. [DOI] [PubMed] [Google Scholar]
- [28].Kim H, Wiraatmadja ES, Lim SY, et al. Comparison of morbidity of donor site following pedicled muscle-sparing latissimus dorsi flap versus extended latissimus dorsi flap breast reconstruction. J Plast Reconstr Aesthet Surg 2013;66:640–6. [DOI] [PubMed] [Google Scholar]
- [29].Button J, Scott J, Taghizadeh R, et al. Shoulder function following autologous latissimus dorsi breast reconstruction. A prospective three year observational study comparing quilting and non-quilting donor site techniques. J Plast Reconstr Aesthet Surg 2010;63:1505–12. [DOI] [PubMed] [Google Scholar]
- [30].Forthomme B, Heymans O, Jacquemin D, et al. Shoulder function after latissimus dorsi transfer in breast reconstruction. Clin Physiol Funct Imaging 2010;30:406–12. [DOI] [PubMed] [Google Scholar]


